Hydrogen Storage in Geological Formations—The Potential of Salt Caverns
Abstract
:1. Introduction
2. Hydrogen as an Energy Carrier
3. Experience in Underground Storage
4. Underground Geological Formations with the Potential to Hydrogen Storage
- Natural water-bearing reservoirs (aquifers);
- Abandoned underground mines;
- Depleted gas and oil fields;
- Rock caverns being excavated using conventional mining techniques;
- Man-made salt caverns [22].
Salt Caverns
| Location | Dimensions | Capacity [m3] | Geology | Pressure Conditions [MPa] | Additional Information | Ref. |
|---|---|---|---|---|---|---|
| Simulated cavern | Thickness: min 30 m; depth: 30 m. | 565,000 | Salt formation density 2200 kg/m3; salt formation specific heat 840 J/kgK; thermal conductivity 5.24 W/mK. | High porosity and permeability. | [59] | |
| Germany | Thickness: 280 m; Height × diameter: 150 × 20 m. | 300,000 | Precambrian to quaternary salt rocks (layers of 400–2000 m). | 4.6–7.2 | Heat condition: <100 °C, lack of water, high porosity and permeability. | [20] |
| UK (Cheshire salt basin—NW England) | Thickness: 250 m; depth: 600–1200 m; height × diameter: 60–80 × 80–100 m. | 100,000–300,000 | Various proportions of halite, anhydrite, gypsum, K-Mg minerals and other minerals. Minerals occur as an admixture in rock salt beds: anhydrite, gypsum, carnallite, kainite, langbeinite, bischofite, polyhalite, sylvite, kieserite, clay, minerals, quartz. Salt layer: 400/500–2000 m. | Low porosity and permeability. | [60] | |
| SW Poland | Thickness: 150–1800 m; depth: 1000–2000 m. | 730,800 | Upper Permian salt deposits. | 7.4–23.8 | Good viscoplastic behavior, low porosity and permeability, lack of water. | [33] |
| Rogóźno Poland | Thickness: max 196.3 m; height × diameter: 300 × 49 m | 32,000 | Clay-sulphate (gypsum—anhydrite). | 8–10 | [21] | |
| Lubień Poland | Thickness: max 893 m | Sulphate (gypsum-anhydrite) | 8–10 | [21] | ||
| China | Depth: 750–1250 m | 200,000 | The cavern section—argillaceous rock salt and mudstone interlayers (glauberite mudstone, anhydrite mudstone, clay shales, silty mudstone). | 6–16 | Low porosity and permeability. | [58] |
| China, Jiangsu province, Jitan salt mine | Depth: 900–1100m, height × diameter: 85 × 73 m | 210,000 | Cretaceous to tertiary lacustrine bedded salt rocks. Caprock and interlayer including: glauberite, gypsum, anhydrite, siltstone. | Very low porosity and permeability. In situ vertical stress of 21–25 Mpa. | [8] |
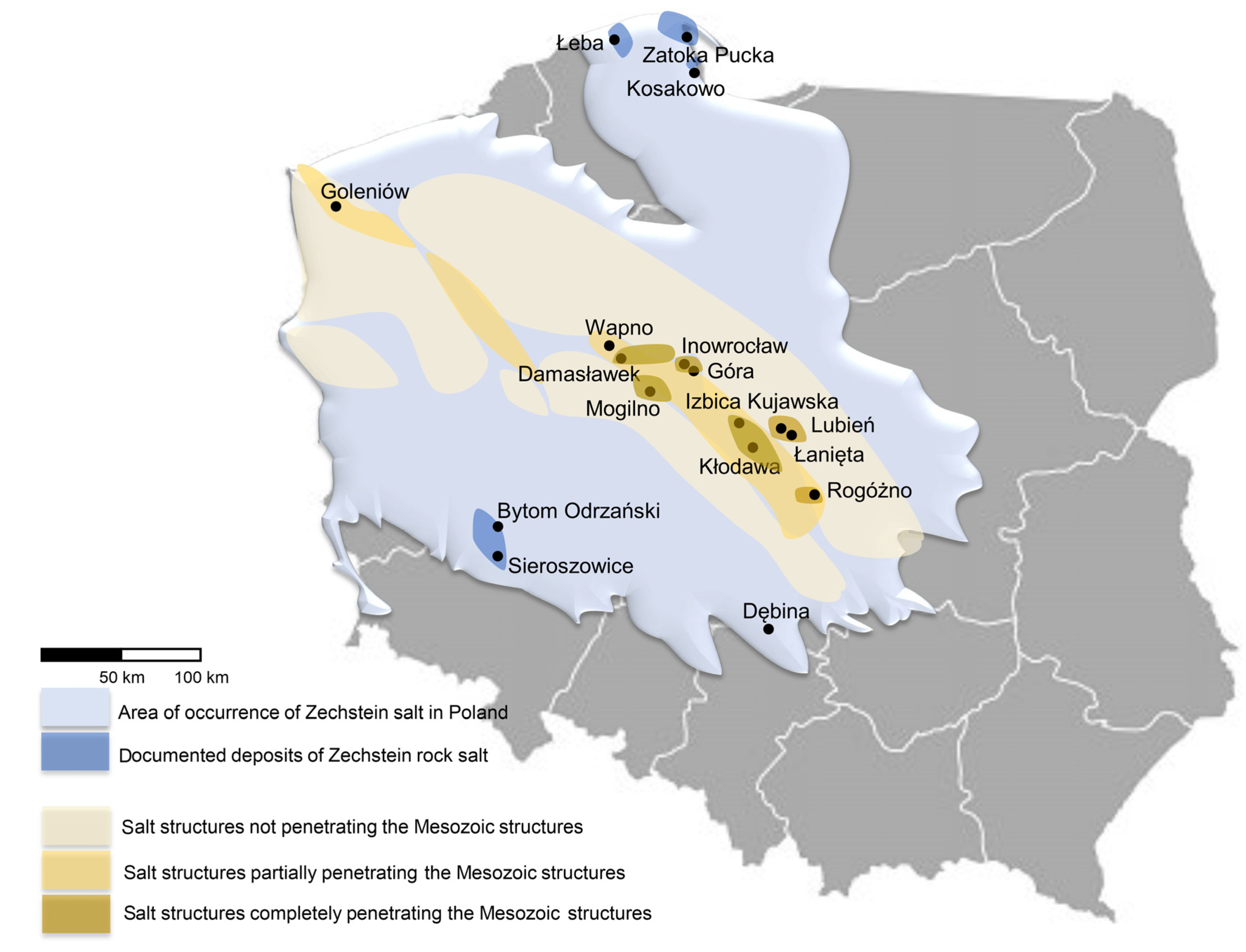
5. Potential of Underground Hydrogen Storage in Salt Caverns—Examples from Europe and Poland
6. Summary
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Główny Urząd Statyystyczny Ochrona Środowiska 2020. Anal. Stat. 2020, 161–162. Available online: https://stat.gov.pl/files/gfx/portalinformacyjny/pl/defaultaktualnosci/5484/1/21/1/ochrona_srodowiska_2020.pdf (accessed on 26 March 2021).
- Horowitz, C.A. Paris Agreement. Int. Leg. Mater. 2016, 55, 740–755. [Google Scholar] [CrossRef]
- European Commission. The European Green Deal; COM(2019) 640; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- Wind Energy in Europe 2020 Statistics and the Outlook for 2021–2025. Available online: https://windeurope.org/intelligence-platform/product/wind-energy-in-europe-in-2020-trends-and-statistics/ (accessed on 7 April 2021).
- Główny Urząd Statystyczny Energia ze Źródeł Odnawialnych w 2018 r. 2019; pp. 1–5. Available online: https://stat.gov.pl/files/gfx/portalinformacyjny/pl/defaultaktualnosci/5485/10/2/1/energia_ze_zrodel_odnawialnych_w_2018.pdf/ (accessed on 26 March 2021).
- European Commission. A Hydrogen Strategy for a Climate-Neutral Europe; COM(2020) 301; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Crotogino, F. Larger Scale Hydrogen Storage; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128034408. [Google Scholar]
- Liu, W.; Zhang, Z.; Chen, J.; Jiang, D.; Wu, F.; Fan, J.; Li, Y. Feasibility evaluation of large-scale underground hydrogen storage in bedded salt rocks of China: A case study in Jiangsu province. Energy 2020, 198, 117348. [Google Scholar] [CrossRef]
- Tarkowski, R. Underground hydrogen storage: Characteristics and prospects. Renew. Sustain. Energy Rev. 2019, 105, 86–94. [Google Scholar] [CrossRef]
- Polska Strategia Wodorowa do Roku 2030 r. z Perspektywą do 2040 r. Available online: https://www.teraz-srodowisko.pl/media/pdf/aktualnosci/9801-Projekt-Polskiej-Strategii-Wodorowej-do-roku-2030-z-perspektywa-do-2040-r.pdf (accessed on 20 March 2021).
- Ceran, B. Multi-criteria comparative analysis of clean hydrogen production scenarios. Energies 2020, 13, 4180. [Google Scholar] [CrossRef]
- European Commission. Europe’s Moment: Repair and Prepare for the Next Generation; COM(2020) 456; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Hobein, B.; Krueger, R. Hydrogen and Fuel Cells–Fundamentals, Technologies and Applications; Stolten, D., Ed.; Wiley-VCH Verlag: Weinheim, Germany, 2010. [Google Scholar]
- Andersson, J.; Grönkvist, S. Large-scale storage of hydrogen. Int. J. Hydrogen Energy 2019, 44, 11901–11919. [Google Scholar] [CrossRef]
- Surygała, J. Wodór Jako Paliwo; Wydawnictwo Naukowo-Technologiczne: Warsaw, Poland, 2008. [Google Scholar]
- Mazloomi, K.; Gomes, C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Wolf, E. Large-Scale Hydrogen Energy Storage. Electrochem. Energy Storage Renew. Sources Grid Balanc. 2015, 129–142. [Google Scholar] [CrossRef]
- Gabrielli, P.; Poluzzi, A.; Kramer, G.J.; Spiers, C.; Mazzotti, M.; Gazzani, M. Seasonal energy storage for zero-emissions multi-energy systems via underground hydrogen storage. Renew. Sustain. Energy Rev. 2020, 121, 109629. [Google Scholar] [CrossRef]
- Kruck, O.; Crotogino, F.; Prelicz, R.; Rudolph, T. Overview on all Known Underground Storage Technologies for Hydrogen. J. Pet. Sci. Eng. 2013, 124, 132–136. [Google Scholar]
- Lord, A.S.; Kobos, P.H.; Borns, D.J. Geologic storage of hydrogen: Scaling up to meet city transportation demands. Int. J. Hydrogen Energy 2014, 39, 15570–15582. [Google Scholar] [CrossRef] [Green Version]
- Tarkowski, R.; Czapowski, G. Salt domes in Poland–Potential sites for hydrogen storage in caverns. Int. J. Hydrogen Energy 2018, 43, 21414–21427. [Google Scholar] [CrossRef]
- Crotogino, F.; Schneider, G.S.; Evans, D.J. Renewable energy storage in geological formations. Proc. Inst. Mech. Eng. Part A J. Power Energy 2018, 232, 100–114. [Google Scholar] [CrossRef]
- Muhammed, N.S.; Haq, B.; Al Shehri, D.; Al-Ahmed, A.; Rahman, M.M.; Zaman, E. A review on underground hydrogen storage: Insight into geological sites, influencing factors and future outlook. Energy Reports 2022, 8, 461–499. [Google Scholar] [CrossRef]
- Caglayan, D.G.; Weber, N.; Heinrichs, H.U.; Linßen, J.; Robinius, M.; Kukla, P.A.; Stolten, D. Technical potential of salt caverns for hydrogen storage in Europe. Int. J. Hydrogen Energy 2020, 45, 6793–6805. [Google Scholar] [CrossRef]
- Rosen, M.A.; Koohi-Fayegh, S. The prospects for hydrogen as an energy carrier: An overview of hydrogen energy and hydrogen energy systems. Energy Ecol. Environ. 2016, 1, 10–29. [Google Scholar] [CrossRef] [Green Version]
- Chromik, M. Koncepcja magazynowania nadwyżek energii elektrycznej w postaci wodoru w kawernach w złożach soli kamiennej w Polsce–wstępne informacje. Przegląd Solny 2016, 12, 11–18. [Google Scholar]
- Cipriani, G.; Di Dio, V.; Genduso, F.; La Cascia, D.; Liga, R.; Miceli, R.; Ricco Galluzzo, G. Perspective on hydrogen energy carrier and its automotive applications. Int. J. Hydrogen Energy 2014, 39, 8482–8494. [Google Scholar] [CrossRef]
- Johnston, B.; Mayo, M.C.; Khare, A. Hydrogen: The energy source for the 21st century. Technovation 2005, 25, 569–585. [Google Scholar] [CrossRef]
- Kanezaki, T.; Narazaki, C.; Mine, Y.; Matsuoka, S.; Murakami, Y. Effects of hydrogen on fatigue crack growth behavior of austenitic stainless steels. Int. J. Hydrogen Energy 2008, 33, 2604–2619. [Google Scholar] [CrossRef]
- Edwards, P.P.; Kuznetsov, V.L.; David, W.I.F. Hydrogen energy. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2007, 365, 1043–1056. [Google Scholar] [CrossRef]
- Kothari, R.; Singh, D.P.; Tyagi, V.V.; Tyagi, S.K. Fermentative hydrogen production-An alternative clean energy source. Renew. Sustain. Energy Rev. 2012, 16, 2337–2346. [Google Scholar] [CrossRef]
- Lord, A.S. Overview of Geologic Storage of Natural Gas with an Emphasis on Assessing the Feasibility of Storing Hydrogen; Sandia National Laboratories (SNL): Albuquerque, NM, USA; Livermore, CA, USA, 2009; Volume 28, p. 975258. [Google Scholar]
- Lankof, L.; Tarkowski, R. Assessment of the potential for underground hydrogen storage in bedded salt formation. Int. J. Hydrogen Energy 2020, 45, 19479–19492. [Google Scholar] [CrossRef]
- Li, L.; Khorsandi, S.; Johns, R.T.; Dilmore, R.M. CO2 enhanced oil recovery and storage using a gravity-enhanced process. Int. J. Greenh. Gas Control 2015, 42, 502–515. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Lashgari, H.R.; Wu, K.; Sepehrnoori, K. CO2 injection for enhanced oil recovery in Bakken tight oil reservoirs. Fuel 2015, 159, 354–363. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef] [Green Version]
- Aminu, M.D.; Nabavi, S.A.; Rochelle, C.A.; Manovic, V. A review of developments in carbon dioxide storage. Appl. Energy 2017, 208, 1389–1419. [Google Scholar] [CrossRef] [Green Version]
- Donadei, S.; Schneider, G.S. Compressed Air Energy Storage in Underground Formations; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128034408. [Google Scholar]
- Michalski, J.; Bünger, U.; Crotogino, F.; Donadei, S.; Schneider, G.S.; Pregger, T.; Cao, K.K.; Heide, D. Hydrogen generation by electrolysis and storage in salt caverns: Potentials, economics and systems aspects with regard to the German energy transition. Int. J. Hydrogen Energy 2017, 42, 13427–13443. [Google Scholar] [CrossRef]
- Hagemann, B.; Rasoulzadeh, M.; Panfilov, M.; Ganzer, L.; Reitenbach, V. Mathematical modeling of unstable transport in underground hydrogen storage. Environ. Earth Sci. 2015, 73, 6891–6898. [Google Scholar] [CrossRef] [Green Version]
- Jain, I.P. Hydrogen the fuel for 21st century. Int. J. Hydrogen Energy 2009, 34, 7368–7378. [Google Scholar] [CrossRef]
- Zivar, D.; Kumar, S.; Foroozesh, J. Underground hydrogen storage: A comprehensive review. Int. J. Hydrogen Energy 2020, 46, 23436–23462. [Google Scholar] [CrossRef]
- Tarkowski, R.; Uliasz-Misiak, B.; Tarkowski, P. Storage of hydrogen, natural gas, and carbon dioxide–Geological and legal conditions. Int. J. Hydrogen Energy 2021, 46, 20010–20022. [Google Scholar] [CrossRef]
- Emami-Meybodi, H.; Hassanzadeh, H.; Green, C.P.; Ennis-King, J. Convective dissolution of CO2 in saline aquifers: Progress in modeling and experiments. Int. J. Greenh. Gas Control 2015, 40, 238–266. [Google Scholar] [CrossRef]
- Omrani, S.; Mahmoodpour, S.; Rostami, B.; Salehi Sedeh, M.; Sass, I. Diffusion coefficients of CO2–SO2–water and CO2–N2–water systems and their impact on the CO2 sequestration process: Molecular dynamics and dissolution process simulations. Greenh. Gases Sci. Technol. 2021, 11, 764–779. [Google Scholar] [CrossRef]
- Buzek, F.; Onderka, V.; Vančura, P.; Wolf, I. Carbon isotope study of methane production in a town gas storage reservoir. Fuel 1994, 73, 747–752. [Google Scholar] [CrossRef]
- Šmigáň, P.; Greksák, M.; Kozánková, J.; Buzek, F.; Onderka, V.; Wolf, I. Methanogenic bacteria as a key factor involved in changes of town gas stored in an underground reservoir. FEMS Microbiol. Lett. 1990, 73, 221–224. [Google Scholar] [CrossRef]
- Panfilov, M. Underground and Pipeline Hydrogen Storage; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; ISBN 9781782423621. [Google Scholar]
- Beckingham, L.E.; Winningham, L. Critical Knowledge Gaps for Understanding Water-Rock-Working Phase Interactions for Compressed Energy Storage in Porous Formations. ACS Sustain. Chem. Eng. 2020, 8, 2–11. [Google Scholar] [CrossRef]
- Gregory, S.P.; Barnett, M.J.; Field, L.P.; Milodowski, A.E. Subsurface microbial hydrogen cycling: Natural occurrence and implications for industry. Microorganisms 2019, 7, 53. [Google Scholar] [CrossRef] [Green Version]
- Blanco, H.; Faaij, A. A review at the role of storage in energy systems with a focus on Power to Gas and long-term storage. Renew. Sustain. Energy Rev. 2018, 81, 1049–1086. [Google Scholar] [CrossRef]
- Ontras Sichere Verwahrung der Schachtanlage Burggraf-Bernsdorf. Available online: https://www.ontras.com/fileadmin/Dokumente_Unternehmen/Broschuere_Burggraf-Bernsdorf.pdf (accessed on 30 May 2022).
- Tengborg, P.; Johansson, J.; Durup, J.G. Storage of Highly Compressed Gases in Underground Lined Rock Caverns-More than 10 Years of Experience. In Proceedings of the the World Tunnel Congress, Iguassu Falls, Brazil, 9–15 May 2014; pp. 1–7. [Google Scholar]
- Papadias, D.D.; Ahluwalia, R.K. Bulk storage of hydrogen. Int. J. Hydrogen Energy 2021, 46, 34527–34541. [Google Scholar] [CrossRef]
- Langmi, H.W.; Engelbrecht, N.; Modisha, P.M.; Bessarabov, D. Hydrogen storage. In Electrochemical Power Sources: Fundamentals, Systems, and Applications. Hydrogen Production by Water Electrolysis; Smolinka, T., Garche, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 455–486. [Google Scholar]
- Cedigaz Underground Gas Storage in the World–2020 Status. Available online: https://www.cedigaz.org/underground-gas-storage-in-the-world-2020-status/ (accessed on 7 April 2021).
- Lin, L.; Tian, Y.; Su, W.; Luo, Y.; Chen, C.; Jiang, L. Techno-economic analysis and comprehensive optimization of anon-sitehydrogen refuelling station system using ammonia: Hybrid hydrogen purification with both high H2purity and high recovery. Sustain. Energy Fuels 2020, 4, 3006–3017. [Google Scholar] [CrossRef]
- Liu, W.; Chen, J.; Jiang, D.; Shi, X.; Li, Y.; Daemen, J.J.K.; Yang, C. Tightness and suitability evaluation of abandoned salt caverns served as hydrocarbon energies storage under adverse geological conditions (AGC). Appl. Energy 2016, 178, 703–720. [Google Scholar] [CrossRef]
- Barajas, P.; Civan, F. Effective modeling and analysis of salt-cavern natural-gas storage. SPE Prod. Oper. 2014, 29, 51–60. [Google Scholar] [CrossRef]
- Cyran, K.; Kowalski, M. Shape modelling and volume optimisation of salt caverns for energy storage. Appl. Sci. 2021, 11, 423. [Google Scholar] [CrossRef]
- Xing, W.; Zhao, J.; Hou, Z.; Were, P.; Li, M.; Wang, G. Horizontal natural gas caverns in thin-bedded rock salt formations. Environ. Earth Sci. 2015, 73, 6973–6985. [Google Scholar] [CrossRef]
- Leitner, C.; Neubauer, F.; Urai, J.L.; Schoenherr, J. Structure and evolution of a rocksalt-mudrock-tectonite: The haselgebirge in the Northern Calcareous Alps. J. Struct. Geol. 2011, 33, 970–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crotogino, F.; Donadei, S.; Bünger, U.; Landinger, H. Large-Scale Hydrogen Underground Storage for Securing Future Energy Supplies. In WHEC 2010 Parallel Sessions Book 4 Storage System/Policy Perspectives, Initiatives and Co-operations, Proceedings of the 18th World Hydrogen Energy Conference, Essen, Germany, 16–21 May 2010; Research Center Jülich: Julich, Germany, 2010; Volume 78, p. 10. [Google Scholar]
- Liu, W.; Pei, P. Evaluation of the Influencing Factors of Using Underground Space of Abandoned Coal Mines to Store Hydrogen Based on the Improved ANP Method. Adv. Mater. Sci. Eng. 2021, 2021, 7506055. [Google Scholar] [CrossRef]
- Cyran, K. Insight into a shape of salt storage caverns. Arch. Min. Sci. 2020, 65, 363–398. [Google Scholar] [CrossRef]
- Ozarslan, A. Large-scale hydrogen energy storage in salt caverns. Int. J. Hydrogen Energy 2012, 37, 14265–14277. [Google Scholar] [CrossRef]
- Warren, J.K. Evaporites: Sediments, Resources and Hydrocarbons, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 7, ISBN 978-3-540-32344-0. [Google Scholar]
- Plaat, H. Underground gas storage: Why and how. In Underground Gas Storage: Worldwide Experiences and Future Development in the UK and Europe; Evans, D.J., Chadwick, R.A., Eds.; Geological Society: London, UK, 2009; Volume 313, pp. 25–37. [Google Scholar]
- Li, J.; Shi, X.; Yang, C.; Li, Y.; Wang, T.; Ma, H. Mathematical model of salt cavern leaching for gas storage in high-insoluble salt formations. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Wang, T.; Yang, C.; Ma, H.; Daemen, J.J.K.; Wu, H. Safety evaluation of gas storage caverns located close to a tectonic fault. J. Nat. Gas Sci. Eng. 2015, 23, 281–293. [Google Scholar] [CrossRef]
- Cosenza, P.; Ghoreychi, M.; Bazargan-Sabet, B.; de Marsily, G. In situ rock salt permeability measurement for long term safety assessment of storage. Int. J. Rock Mech. Min. Sci. 1999, 36, 509–526. [Google Scholar] [CrossRef]
- Luboń, K.; Tarkowski, R. Numerical simulation of hydrogen injection and withdrawal to and from a deep aquifer in NW Poland. Int. J. Hydrogen Energy 2020, 45, 2068–2083. [Google Scholar] [CrossRef]
- Bauer, S.; Pichler, M. Underground Sun Storage. Energ. Wasser-Prax. 2017, 8, 64–69. [Google Scholar]
- NortH2. Available online: https://www.north2.eu/ (accessed on 28 June 2022).
- SeaH2Land. Available online: https://seah2land.nl/en (accessed on 28 June 2022).
- HyPSTER. Available online: https://hypster-project.eu/ (accessed on 28 June 2022).
- Cornot-Gandolphe, S. Status of Global Coal Markets and Major Demand Trends in Key Regions. Available online: https://www.ifri.org/en/publications/etudes-de-lifri/status-global-coal-markets-and-major-demand-trends-key-regions (accessed on 28 June 2022).
- Zeljas, D. Magazyny gazu ziemnego w cechsztynskich formacjach solnych elementem bezpieczenstwa energetycznego polski. Prz. Geol. 2020, 68, 824–832. [Google Scholar]
- Czapowski, G. Prospects of hydrogen storage caverns location in the upper permian (Zechstein) stratiform rock salts in Poland–Geological Valuation. Biul.-Panstw. Inst. Geol. 2019, 477, 21–54. [Google Scholar] [CrossRef]
- Laskowska, T. Underground gas storage in salt caverns. Polish Mark. 2012, 9, 28–29. [Google Scholar]
- Król, K.; Kuśnierz, B. Bezzbiornikowe Magazynowanie Substancji w Górotworze–Techniczne i Prawne Aspekty Działalności. AGH Drill. Oil Gas 2019, 36, 5–18. [Google Scholar] [CrossRef]
- Tarkowski, R. Perspectives of using the geological subsurface for hydrogen storage in Poland. Int. J. Hydrogen Energy 2017, 42, 347–355. [Google Scholar] [CrossRef]

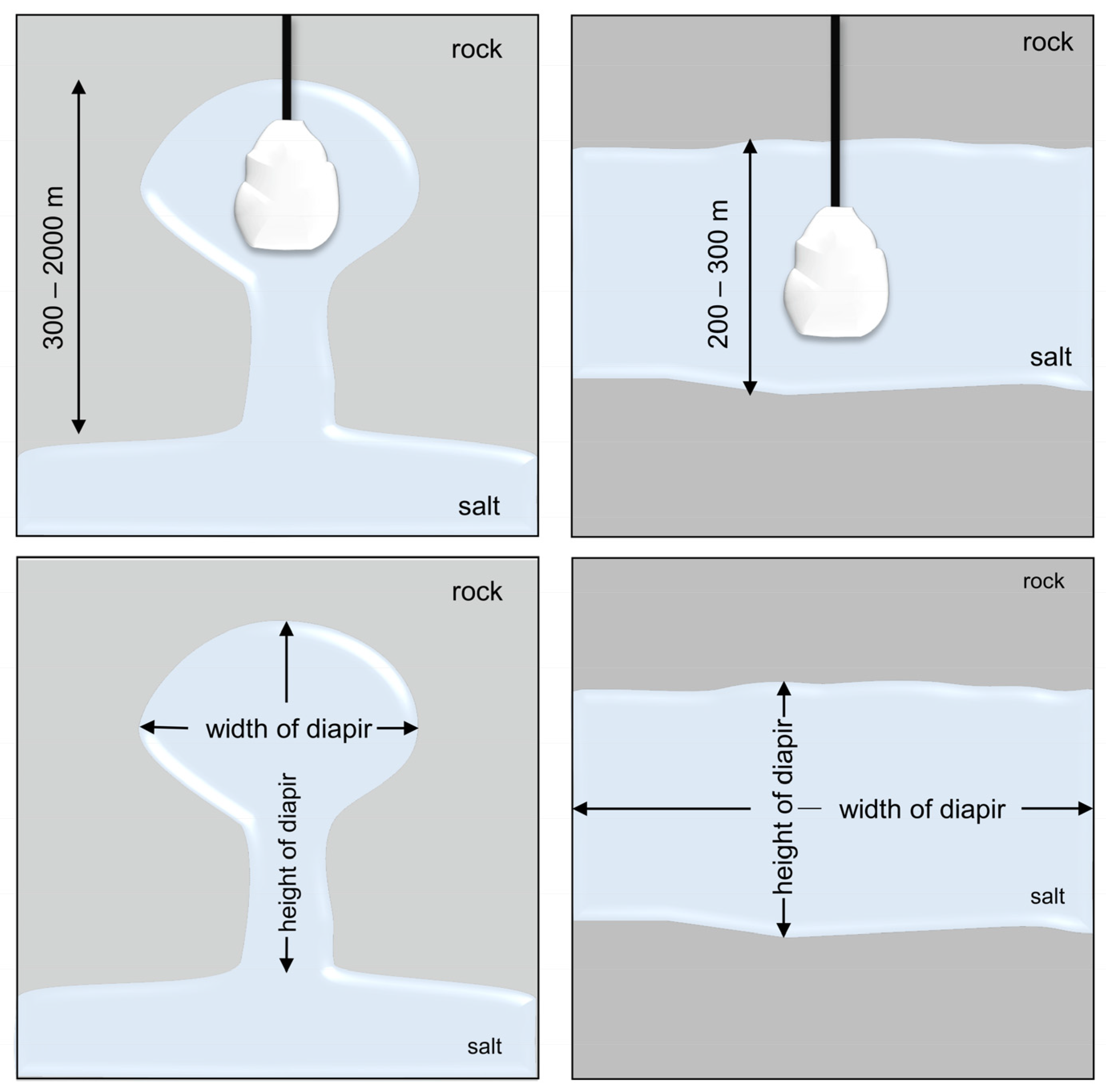
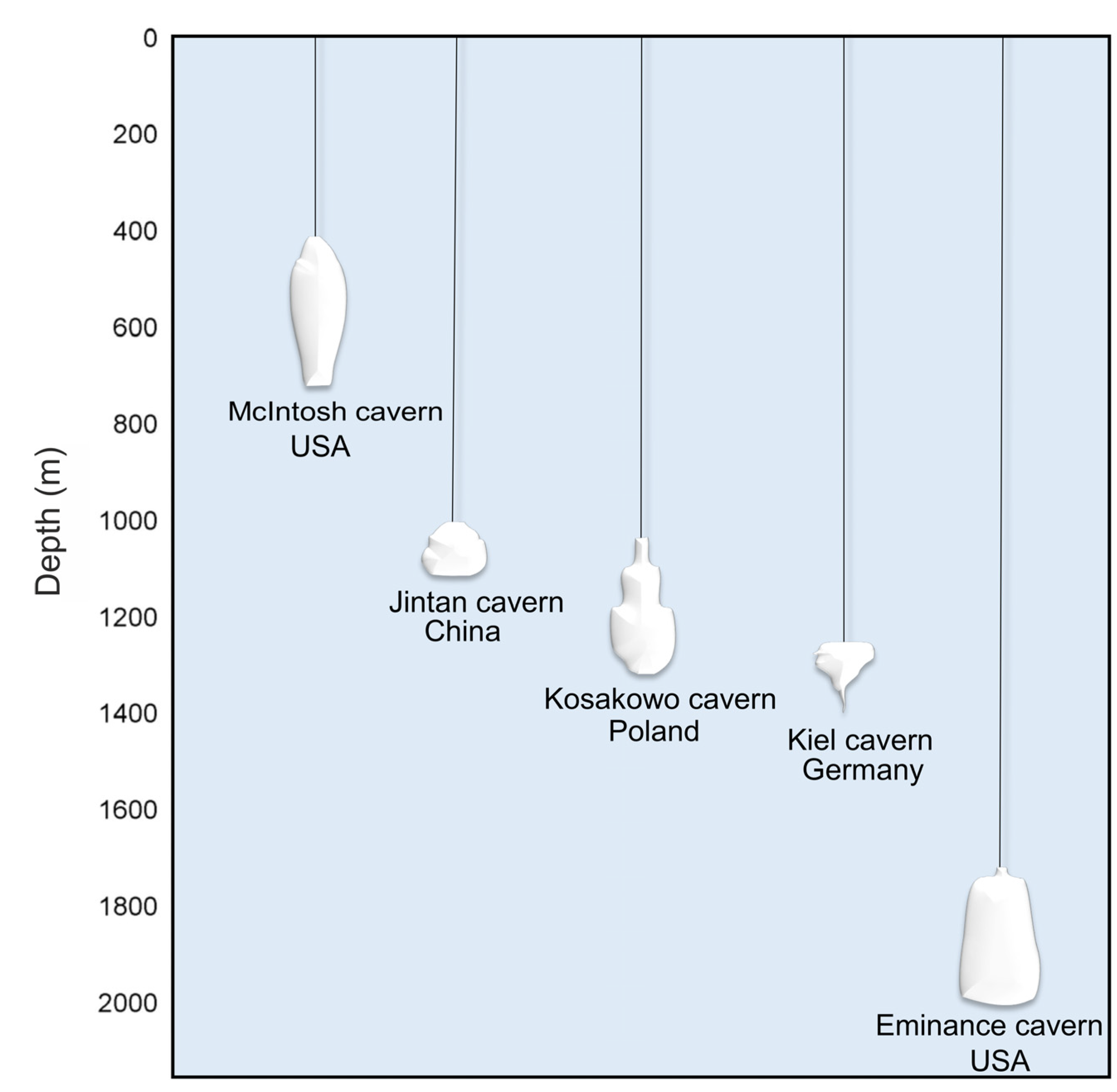

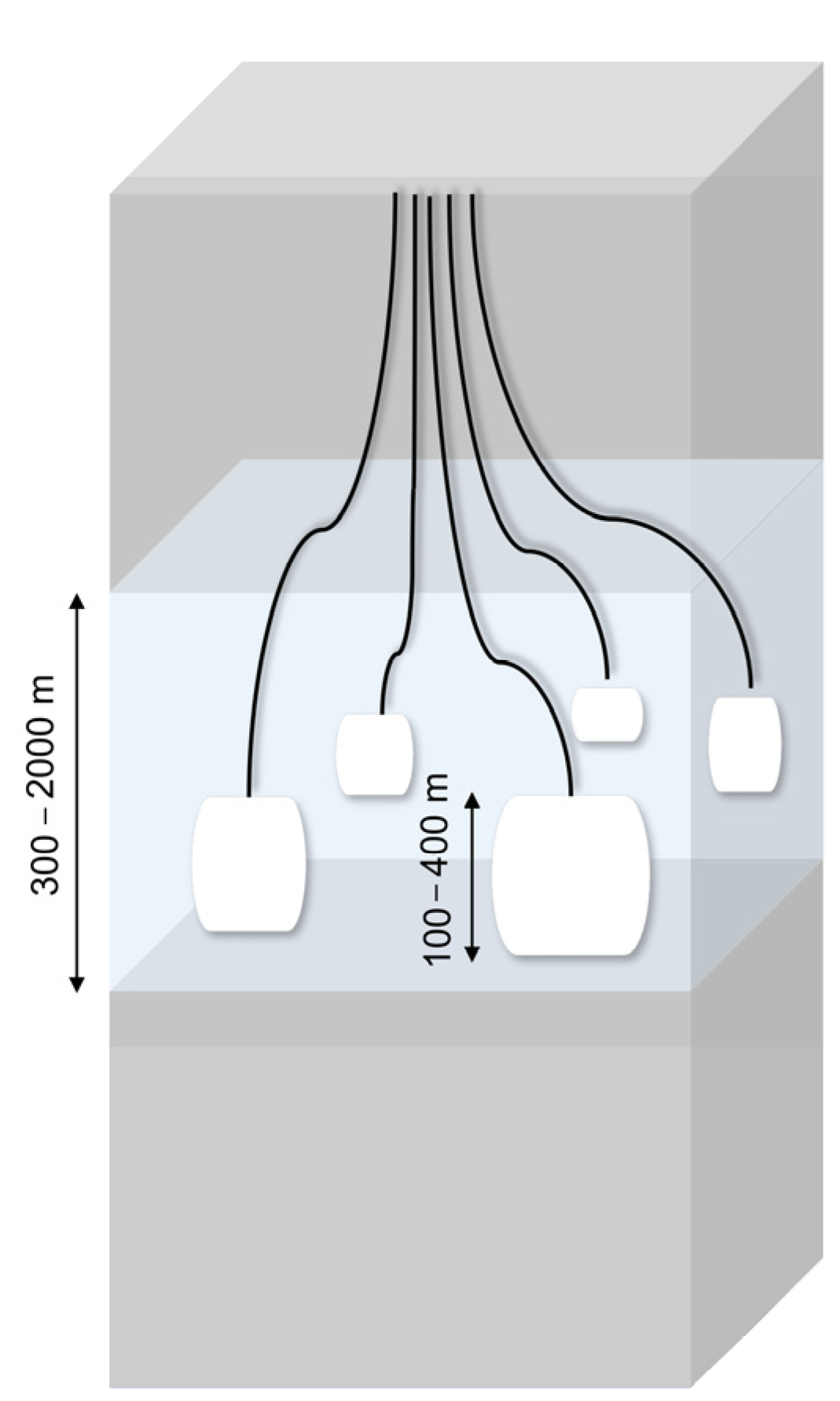
| Storage Technology | General | Advantages | Disadvantages |
|---|---|---|---|
| Gaseous hydrogen storage in pressure tanks | Above- (lower pressure, lower hydrogen density) and underground (higher pressure, higher hydrogen density) options | Large-scale hydrogen storage | Relatively high investments and operational costs (gas .compressors), the possibility of hydrogen losses, operational safety (high pressure). |
| Storage of liquid hydrogen | Storage in cryo tanks, hydrogen at a temperature of −253 °C | Hydrogen storage on a large scale. The high density of hydrogen at atmospheric pressure. | High investment and operational costs (high energy demand for liquefaction), hydrogen losses (evaporation of liquid hydrogen). |
| Sorption of hydrogen on materials with high active surface | Examples of adsorbents: carbon nanotubes, zeolites, metal-organic frameworks (MOFs) | Storage under mild conditions. | Little experience in comparison with storage of compressed gaseous or liquid hydrogen, small-scale storage. |
| Chemical storage in metal hydrides | Hydrogen directly bonded to a metal atom or a part of a complex ion bonded to a metal atom. Hydrogen release via thermo- or hydrolysis | Usually quite high gravimetric hydrogen storage capacity. | Little experience in comparison with storage of compressed gaseous or liquid hydrogen. Thermal dehydrogenation usually requires relatively high temperature and/or pressure and results in solid waste production. Intermetallic hydrides are expensive. |
| Chemical storage in chemical hydrides | Chemical hydrides—compounds with non-metallic elements, e.g., methanol, formic acid | Simplified transport, storage, heat, and mass transfer compared to metal hydrides (as chemical hydrides are typically liquids under standard conditions). High gravimetric storage density | Expensive catalysts and relatively high temperature in some dehydrogenation processes |
| Property | Hydrogen | Methane | Gasoline |
|---|---|---|---|
| Density under standard conditions (kg/m3) | 0.082 | 0.657 | 751 |
| Viscosity under standard conditions (Pa s) | 0.89 × 10−5 | 1.1 × 10−5 | ~0.39 × 10−3 |
| Gravimetric energy density (MJ/kg) | 120–143 | 50–55.5 | 44–47.3 |
| Volumetric energy density (MJ/L) | 0.0170 | 0.0378 | 34.2 |
| Diffusion coefficient in water at 25 °C (m2/s) | 5.13 × 10−9 | 1.85 × 10−9 | _ |
| Flammability limits (vol.% in air) | 4–75 | 5.3–15 | 1–7.6 |
| Octane number | >130 | 125 | 87 |
| Auto ignition temperature (°C) | 585 | 540 | 246–280 |
| Teesside (UK) | Clemens (US) | Moss Bluff (US) | Spindletop (US) | |
|---|---|---|---|---|
| Salt formation | Bedded salt | Salt dome | Salt dome | Salt dome |
| Operator | Sabic Petroleum | ConocoPhilips | Praxair | Air Liquide |
| Commissioned (year) | 1972 | 1983 | 2007 | 2017 |
| Volume (m3) | 210,000 (3 × 70,000) | 580,000 | 566,000 | 906,000 |
| Average cavern depth (m) | 365 | 1000 | 1200 | 1340 |
| Pressure range (bar) | 45 | 70–137 | 55–152 | 68–202 |
| Net energy stored (GWh) | 27 | 81 | 123 | 274 |
| Possible working gas capacity H2 (103 t) | 0.83 | 2.56 | 3.72 | Information not available |
| Geological Formation | General | Advantages | Disadvantages | Utilization | Ref. |
|---|---|---|---|---|---|
| Aquifers | Porous and permeable rock formations (typically sandstones or carbonate rocks), containing fresh or saline water, | Availability—common in all sedimentary basins, often located near energy consumers such as large cities. High capacity. | Geological structure usually unknown—considerable investment costs, time-consuming exploration works. Possible gas losses due to the presence of water, biological and chemical reactions. | Natural gas and carbon dioxide storage (Belgium, Denmark, Norway, France, and Germany), when neither depleted gas, oil fields nor caverns are available. | [19,20] |
| Depleted gas and oil fields | Permeable sandstones or carbonate rocks. | The existence of surface and subsurface infrastructure. Geological structure usually well known—lower investment costs. The gas remained in depleted gas fields can serve as a cushion gas. | The presence of hydrocarbon residues in oil fields reduces hydrogen purity. The possibility of chemical reactions (e.g., conversion of hydrogen to methane) and hydrogen dissolution in the oil—losses of hydrogen. | Natural gas storage. | [7,21] |
| Abandoned mines | Reservoirs after excavation of deposits, | Existing infrastructure. Plenty of abandoned mines in various types of geological formations across the Europe. | Designed and constructed with the intention of natural resources extraction not storage of a gas—safety problems. | Only rare examples of abandoned mines conversion into gas storage are known. Natural gas storage in Belgium, USA, and Germany (closed now due to high cost associated with the sealing of the shafts). | [19,32,52] |
| Rock caverns | Massive, homogenous rocks (e.g., metamorphic crystalline rocks such as granite and gneiss) with minimal structural or textural weaknesses, great mechanical stability, and low permeability. | Lined rock caverns have suitable parameters for gas storage. | Necessity of additional sealing provided by groundwater management or lining of the host rock—additional investment costs. | Liquid hydrocarbons storage in Europe (mostly in Scandinavia because of favorable geological structures) since 1950s. The natural gas cavern in Czech Republic (Háje) and Sweden (Skallen). | [19,22,53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Małachowska, A.; Łukasik, N.; Mioduska, J.; Gębicki, J. Hydrogen Storage in Geological Formations—The Potential of Salt Caverns. Energies 2022, 15, 5038. https://doi.org/10.3390/en15145038
Małachowska A, Łukasik N, Mioduska J, Gębicki J. Hydrogen Storage in Geological Formations—The Potential of Salt Caverns. Energies. 2022; 15(14):5038. https://doi.org/10.3390/en15145038
Chicago/Turabian StyleMałachowska, Aleksandra, Natalia Łukasik, Joanna Mioduska, and Jacek Gębicki. 2022. "Hydrogen Storage in Geological Formations—The Potential of Salt Caverns" Energies 15, no. 14: 5038. https://doi.org/10.3390/en15145038
APA StyleMałachowska, A., Łukasik, N., Mioduska, J., & Gębicki, J. (2022). Hydrogen Storage in Geological Formations—The Potential of Salt Caverns. Energies, 15(14), 5038. https://doi.org/10.3390/en15145038







