Abstract
The aim of the study was to estimate the effectiveness of ultrasonic coagulation aiding. The effect of ultrasound exposure alone and associated systems (ultrasound exposure/coagulant) on the contamination of natural water was examined. The evaluation of the test results was based on changes in indicators, such as TOC, color, turbidity, and electrokinetic potential. Three different coagulants were used in the tests of associated systems. The tests included basic processes related to volumetric coagulation, such as agitation, flocculation, and sedimentation. Sonication of water samples was carried out at a constant frequency of 22 kHz, variable vibration amplitude of 8–16 μm, and an exposure time of 1–5 min. The most efficient removal of organic contaminants from the water tested was achieved at a maximum amplitude of A = 16 μm, with effectiveness reaching 29% (TOC). In the tests of the associated systems, the effect of ultrasound exposure on the removal of water turbidity (an increase in the effectiveness of 25–35%) was generally greater than that on water color (8–21%). This relationship reflects the differentiated effect of ultrasonic energy on colloids of different stability. In removing turbidity, ultrasound exposure had the most favorable effect on aluminum sulfate. In respect of color, a better result was obtained using the modified coagulant. The possibility of reducing the coagulant dose confirmed the aiding effect of ultrasound. In the coagulation process, ultrasound exposure has a positive effect on the course of flocculation and the sedimentation of suspensions. In addition to the reduction in the doses of chemical reagents, it also leads to the modification of the post-coagulation sludge structure.
1. Introduction
There are many years worth of research conducted on the application of ultrasonic technology in various fields of science. Particularly extensive research is carried out in disciplines related to environmental engineering [1,2]. The observed effects of ultrasonic energy confirm the possibility of reducing the amount of chemical reactants introduced into the environment. This is an important ecological and economic factor in the assessment of the method of conducting modern technological processes [3,4]. Beneficial effects were confirmed in sludge dewatering, wastewater treatment, and water treatment [5,6,7].
The processes used in water treatment require large amounts of chemical reactants, which, in addition to negative side effects on water quality, also cause the formation of a significant volume of sludge. At the same time, with increasing pollution of captured waters and stricter quality criteria for treated water, a further increase in the amount of chemicals added is observed.
For this reason, natural materials are increasingly taking up space in the area of water purification research. Natural materials are widely recognized by the scientific community in water remediation research as high-potential substrates used for many purposes [8]. Optimization studies are also being carried out on the use of natural coagulants, mainly vegetables (e.g., Moringa oleifera), for water treatment (mainly turbidity removal) [9].
In light of these problems, the coagulation process requires special attention. The increase in doses of coagulants, lime, or other reaction regulators, and the increasingly frequent need to add flocculants cause, on the one hand, an increase in financial outlays for water treatment and, on the other hand, ecological problems related to the introduction of these substances into the environment, including post-coagulation sediments, amounting to 0.1–0.7% of the volume of treated water [10]. In practice, as a method of removing colloidal impurities, coagulation is realized by adding chemical coagulants into the treated water. Destabilization can be intensified by the interactions influencing the reduction in the electrokinetic potential of colloids, dehydration of hydrophilic particles, or degradation of protective colloids. Using the aiding-medium accelerates the development and sedimentation of flocs, increases the specific surface of flocs, decreases the negative effect of low temperatures, broadens the optimal pH range, and helps reduce the coagulant dose [10].
The problem of intensifying coagulation is reflected in legal regulations in many countries. In general, however, it is considered that the criteria followed when choosing the method used for this purpose should include maximizing turbidity removal and reducing TOC (at a lower dose of the coagulant), obtaining minimum amounts of remaining coagulant and sludge, and minimizing operating costs.
The active effects of ultrasound exposure in liquid media are the basis for the application of ultrasonic energy as a method of intensification of water purification processes, e.g., disinfection, coagulation, and adsorption [11,12,13,14]. Ultrasonic irradiation appears to be an effective method for the destruction of organic contaminants in water and wastewater. The studies evaluated the sonochemical degradation of a variety of chemical contaminants, such as chlorinated hydrocarbon, pesticides, phenols, and pharmaceuticals. Ultrasound exposure causes their transformation into short-chain acids, CO2,, and inorganic ions as the final product [15,16,17,18,19,20,21]. The results of previous studies conducted in this field, however, mostly concerned pure substances and deionized water. They focused on the kinetics of reactions and their mechanisms. However, few studies reported the effects observed in the presence of other organic and inorganic substances constituting water impurities and affecting sonochemical transformations. Therefore, research is increasingly being conducted in the natural water environment, as demonstrated in other studies. A review of the findings of these studies reveals the possibilities of water purification and supporting selected unit processes by using ultrasound, e.g., adsorption or ultrafiltration. It is confirmed that ultrasonic energy intensifies the desorption of impurities from adsorbents filling the filter columns. The beneficial effects of ultrasound exposure were also observed in the regeneration of filtration membranes. Particular attention is still paid to the removal of biological contaminants (bacteria, fungi, and algae), humic substances (humic and fulvic acids), and halogenated derivatives, as well as other compounds, i.e., iron from water. In research on the removal of humic acids, Chemat et al. confirmed two types of effects of ultrasound exposure (at f = 20 kHz): direct degradation during cavitation by oxidizing particles (hydroxyl radicals), and fragmentation (breakdown) causing the formation of numerous reactive particles, leading to more effective mineralization of oxidized products [7,22,23,24,25,26].
It is believed that the coagulation aiding is connected to the processes of separation, hydrodynamic gravity, and electrokinetic phenomena. Coagulation in the ultrasonic field is also accelerated by the degradation of protective organic colloids due to sonochemical oxidation. The effect of aiding the coagulation of hydrophilic impurities is also caused by the degradation of hydrative layers by the destructive forces accompanying ultrasonic cavitation. However, it is known that besides the electrostatic and van der Waals′ forces, the course of polar coagulation of hydrophilic colloids also depends on the hydration forces [2,7].
In the analysis of the coagulation process, ultrasound (80 kHz) in the US/O3 system was used in combination with commonly used coagulants. The authors confirmed an increase in the efficiency of the process for the removal of natural organic matter, turbidity, and TOC from surface water [25].
Many problems require further research, including, among others, the impact of ultrasonic technology on the degradation of mixed organic substances or industrial wastewater [7]. Previous studies confirmed that recycling pre-sonicated sludge from drinking water treatment can improve the quality of coagulated water by reducing the content of natural organic matter. An increase in the efficiency of removing hydrophobic acids and fractions of 3–30 kDa (humic substances) was achieved, but the presence of substances with a molecular weight of <3 kDa was increased [27].
Furthermore, no studies evaluated the effectiveness of the ultrasonic method using parameters that offer the possibility of the combined effect of sonochemical and coagulation interactions on colloidal impurities. The problem of ultrasound application in the coagulation process can be considered as an independent or combined system, i.e., with the addition of chemical coagulants, assuming the supportive effect of ultrasound. The beneficial effect of ultrasonic phenomena on the removal of colloids is, to a large extent, determined by wave intensity and the properties of the medium. It is therefore important to determine the dependence of the technological effects of the tested method (based on changes in coagulation efficiency indicators, such as turbidity and color) on the parameters characterizing the ultrasonic field and the composition of the purified water. The intensity of the ultrasonic field is the basic quantity that, along with the technical parameters characterizing the ultrasonic generator (vibration frequency, power, efficiency, and diameter of the sonotrode) in combination with the exposure time, determines the conditions for conducting the process in terms of the ultrasound “dose”. These parameters affect the costs of processes involving ultrasound [28].
The aim of the study was to evaluate the effectiveness of ultrasonic energy as a factor supporting the removal of colloidal impurities from water using three different coagulants. When using ultrasound exposure in the process of coagulation, it is expected to reduce the doses of coagulants. The study used ultrasound at a frequency of 22 kHz and at a low intensity (3–10 W/cm2). In addition to the comparative analysis of the effectiveness of supporting the coagulation process, observation of the flocculation phase, the course of sedimentation of suspensions, and the structure of the post-coagulation sediment was carried out.
2. Materials and Methods
The studied river water was characterized by a high level and high variability of organic pollution. The effect of ultrasound on the removal of organic compounds was investigated at water parameters: TOC = 75–153 mgC/L; DOC = 74–151 mgC/L; color C = 50–52 mg/L; and COD = 10.8–41.5 mg/L.
In the second part of the study on coagulation combination systems, the tests were carried out for surface river water with the following parameters: turbidity T = 25.9–29.4 NTU; color C = 25–50 mgPt/L; pH = 7.0–7.5; Ox = 7.0–7.3 mg/L; and BOD5 = 7–16 mg/L.
The interaction of ultrasound exposure with various coagulants was also compared, including aluminum sulfate (Al), the modified coagulant–basic aluminum polychlorosulfate (WAC®HB), and ferric chloride (Fe). In the systems with ultrasound, reduced doses (50% d) were used. Sonication of the tested water samples was carried out using a UD-20 (180 W) ultrasonic disintegrator with an immersion concentrator. Ultrasonic energy generated by a sandwich transducer was supplied to the tested medium through a probe (1.8 cm in diameter). Water samples (1.01) were exposed to the ultrasonic field with the following parameters:
- -
- constant frequency, f = 22 kHz ± 1.65 kHz
- -
- vibration amplitude, A = 8–16 µm
- -
- exposure time, t = 1–5 min
The intensity (I) of the acoustic wave was calculated from the formula [2]:
- S—area of the surface that the wave passes through (cm2)
- E—acoustic energy (J)
- t—sonication time (s)
- N—acoustic power (W)
Acoustic wave intensity of infinitesimal amplitude in a medium with acoustic resistance ρ·c is given by the formula [2]:
- ρ—medium density
- c—wave propagation speed
- A—vibration amplitude
- ω—circular frequency (ω = 2πf)
- f—frequency
As can be seen from the Formula (2), the field strength of a given medium can be increased by increasing the frequency or amplitude of vibrations. The first method is to move to the ultrasound range in which large acoustic energy is obtained at a small amplitude. A change in the vibration amplitude at a given frequency results from a change in sound power. Increasing the vibration amplitude is associated with an increase in power, which simultaneously causes an increase in acoustic energy and the intensity of the ultrasonic field, as demonstrated in (1). For a field of a certain intensity, obtained at a given ultrasound frequency and vibration amplitude, the amount of acoustic energy introduced into the medium increases with the exposure time [2,7].
The intensity of the ultrasonic field within the tested range of amplitudes was 3–10 W/cm2. The TOC and DOC were determined using a Multi N/C 2100S Analytic Jena (with autosampler) analyzer. Turbidity was determined using a HACH 2100N IS Turbidimeter. The electrokinetic potential was determined using a Zeta Plus dzetameter (Brookhaven Instruments).
In the first part (the effect of ultrasound on water pollution), each measurement was conducted in raw water (Xo) and after sonication (X). The results (US system) are given as efficiency calculated from the formula:
EUS = (1 − X/Xo) 100%
In the associated coagulation systems, the increase in efficiency (ΔE) was evaluated by comparing the associated system (US/C) with the system without ultrasound exposure (C).
ΔET,C = EUS/C − EC%
EUS/C—effectiveness of lowering water parameters (turbidity T, color C) in the associated system (US/C), coagulant—50% optimal dose.
EC—effectiveness of lowering water parameters (T, C) in the coagulant system (C), 50% optimal dose.
Tests and observations were conducted for technological systems with volumetric coagulation (fast agitation—1 min, flocculation—30 min, and sedimentation—60 min). The surface water was treated by two alternative methods, using ultrasound exposure + coagulant (US/C system) and coagulant (C system), respectively. Comparison of the effects of coagulation and the course of processes in both systems made it possible to determine the way in which the part of ultrasound in the destabilization of colloids influences the subsequent effects related to the flocculation and sedimentation of post-coagulation suspensions.
During the observation of the process in the systems associated with each coagulant, the following parameters were determined:
- -
- the time of first flocs formation
- -
- the size of forming flocs
- -
- the shape and porosity of flocs surface
- -
- the time of suspension sedimentation (compared to the sedimentation time according to the given time scheme −60 min)
For the systems with aluminum sulfate, microscopic observations of the structure of post-coagulation sludge were also carried out. A Polam L-211 microscope with instrumentation for images in transmitted light was used. The images were taken with a Minolta X-300s camera at 100× magnification.
3. Results and Discussion
3.1. Ultrasound Exposure (US System)
The water tested was characterized by increased turbidity and high color. At a determined pH value, increased oxygen consumption and intensive color usually correlate with considerable contents of humic acids, mainly in the form of soluble substances. In the tests, changes in the contents of organic compounds present in water were found to occur due to the ultrasonic field exposure. The distinct effect of the ultrasonic field as defined by the vibration amplitude is noted in the test results. For a sonication time of t = 5 min., the effect of the amplitude in the examined process is shown in Figure 1. The above results confirm the reduction in the content of both total and dissolved carbon in water. Increasing the vibration amplitude in the range of A = 8–14 μm, which increases the ultrasonic field intensity, resulted in slight changes in the indices studied. Only at the maximum amplitude of A = 16 μm was a noticeable reduction in both indices of organic contamination obtained. The percentage values of the effectiveness of the sonication process show that the maximum effect is considerable, amounting to ca. 30%, and being similar irrespective of the type of index examined.
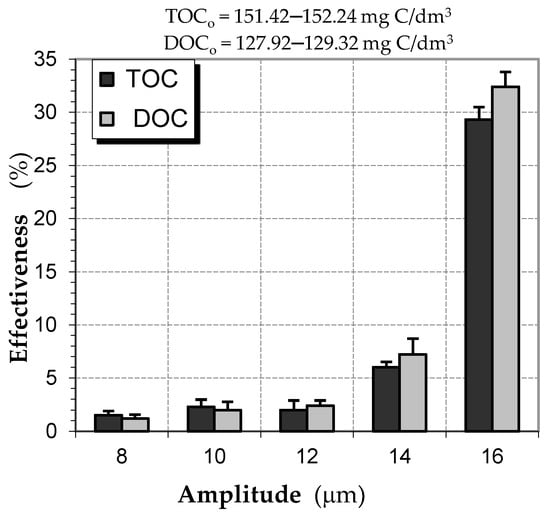
Figure 1.
Effectiveness of decreasing the examined indices of organic contamination using the ultrasonic field (tus = 5 min).
A clear increase in the effect at the maximum value of the above parameter suggests that further tests need to be carried out using a larger range of vibration amplitude. A higher amplitude of vibrations increases the intensity of the ultrasonic field. This result is concurrent with a reduction in water color (above 15%) and oxygen consumption, which indicates oxidation processes occurring during ultrasonic exposure (Figure 2).
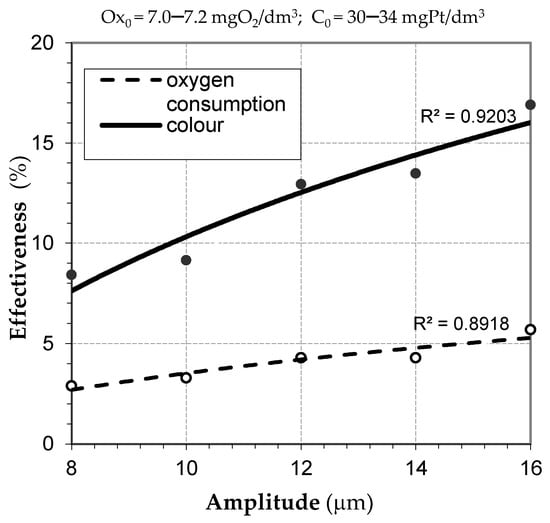
Figure 2.
Effectiveness of decreasing water color and oxygen consumption using ultrasound exposure (tus = 5 min).
Therefore, the convergence of the results confirms the most advantageous conditions of water treatment using ultrasound exposure in the case of high organic contamination.
Other authors [24] achieved a reduction in TOC of 24.5 to 34.9% (20 kHz, 42 W/cm2) after 10 min of sonication. Previous studies on HA sono-oxidation with H2O2 [22] found efficiency of 40% (10 W/cm2) for TOC after 60 min of sonication. More favorable results in the present study may be due to the fact that the tests were carried out for natural water rather than the HA solution examined in previous studies. The study [29], which examined ultrasonic pre-treatment (300 kHz, 30 min) before ozonation of HA, found no significant improvements in the efficiency of ozonation. A TOC reduction of 36% was achieved in the US/O3 process. Furthermore, changes in the chemical structure of the macromolecules of HA were observed, as they led to the formation of oxidation by-products with lower molecular weight.
In numerous studies on ultrasonic NOM removal, the effectiveness is determined by TOC reduction. However, most of the cited studies were conducted on HA solutions and for various ultrasonic parameters. It is impossible to compare the effectiveness of the process to real conditions, i.e., natural waters. This research direction is recommended for the optimization of ultrasonic technology for technical systems [30].
3.2. The Associated Systems (Ultrasound Exposure/Coagulant)
Coagulation examinations of used combined methods were carried out simultaneously for three selected coagulants in water with the same physicochemical parameters. This made it possible to compare the effects of ultrasonic energy used to support the coagulation process. The parameters of ultrasound were A = 8 μm and t = 1 ÷ 5 min, and specific amounts of coagulants were used, i.e., an optimal dose of 100% and a reduced dose of 50%. The obtained results show an increased effect of supporting coagulation by increasing the time of exposure in the ultrasonic field (Figure 3). Of the three associated systems, the lowest remaining turbidity (3.6 NTU) was recorded after coagulation with a reduced dose of aluminum sulfate (20 mg/L–50%d) preceded by water sonication over 5 min. The combined effect was better than at a full dose of 40 mg/L (without support).
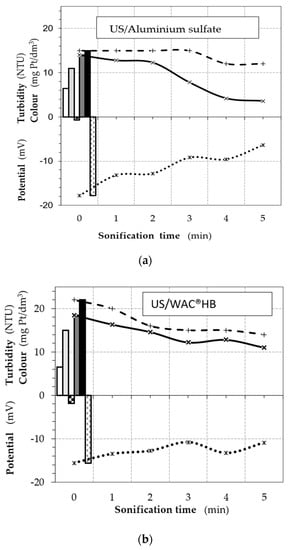
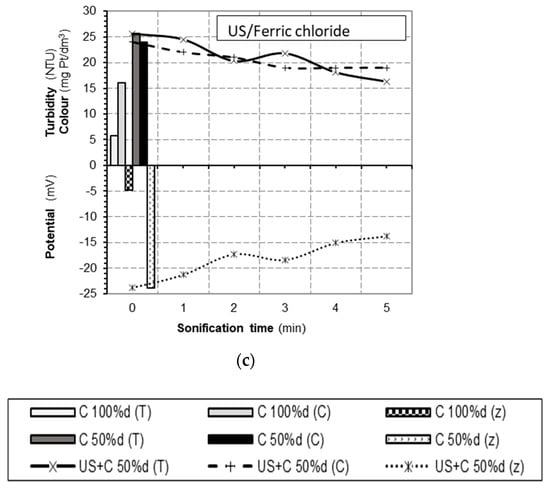
Figure 3.
Changes in color (C), turbidity (T), and electrokinetic potential ζ(z) depending on the sonication time in the coagulation-associated systems, ultrasound exposure + coagulant US/C (water with initial turbidity of 29.5 NTU and initial color of 38 mg Pt/L): (a) US/Al; (b) US/WAC®HB; and (c) US/Fe.
The ultrasound exposure slightly reduced the color, but only for longer sonication of the colloidal systems. The benefit of the combined effect on the colloids that cause water color was therefore determined by the high efficiency of aluminum sulfate. The use of ultrasound in combination with WAC®HB (a polymerized coagulant) intensified the removal of turbidity and, to a large extent, also color (already after 2-min exposure). However, the turbidity of the remaining water was higher than at a 100% dose. Coagulation using ferric chloride (at pH = 6.0) allowed for the obtaining of an additional reduction in the color and water turbidity, but not at a level reached at an optimal dose (60 mg/L). Additional filtration of water samples, only for the system with aluminum sulfate (US + C(Al)), resulted in coagulation with turbidity remaining at an acceptable level (1.0 NTU). This indicator was exceeded for the remaining coagulants.
A decrease in the potential ζ of colloidal particles with an increase in the time of ultrasonic exposure was observed in tests of all three coagulants. This effect was correlated with a gradual reduction in turbidity, confirming the association of destabilization, mainly of hydrophobic colloids, with the effectiveness of their removal. Supporting the process, in the case of organic hydrophilic colloids, occurred with a much lower effect, e.g., for the system using aluminum sulfate, by a maximum of 3 degrees Hazen, regardless of the significantly reduced electrokinetic potential of the particles (up to about −7 mV for t = 5 min).
Taking into account the basic criterion of the assessment of sonication as an agent aiding the removal of colloidal contaminants from water, the increase in the effectiveness of coagulation using aluminum sulfate, ferric chloride, and the modified WAC®HB compound was confirmed (Figure 4). The effect of ultrasound exposure on the removal of water turbidity (25–35%) was generally higher than for water color (8–21%). This relationship reflects the differentiated effect of ultrasonic energy on colloids with different stability. In removing turbidity, ultrasound exposure was most favorable when using aluminum sulfate. In respect of color, a better result was obtained for the modified coagulant. The obtained possibility of lowering the coagulant dose confirmed the aiding effect of ultrasound exposure.
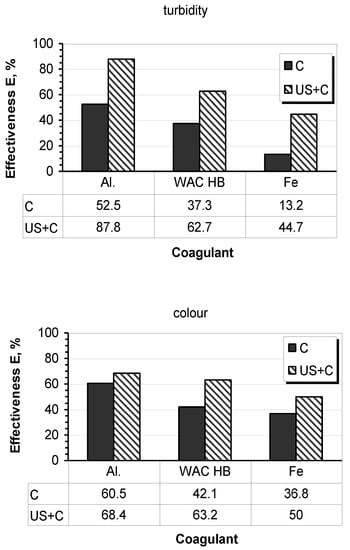
Figure 4.
The effectiveness of coagulation in the studied systems aided by ultrasound exposure (8 μm, 5 min).
In a similar study of natural water [25], ultrasound-assisted coagulation (80 kHz, 200 W/cm2) used aluminum sulfate, polymerized coagulant, and iron chloride. The intensification of the process was carried out in the US/O3 system. Results confirm a reduction in TOC of 46% and turbidity of 33% for US/O3, and a reduction in TOC of 75% and turbidity of 50% (US/O3) for the coagulant.
In the assessment of the effectiveness of aiding agents supported by reducing the required coagulant dose, the results of macroscopic and microscopic observations of the flocculation and sedimentation processes are also important. They mostly concern the flocculation starting time and suspension sedimentation capability. The advantageous results may have an effect on the size of the equipment designed.
It was observed during the experiments that in the systems with additional sonication, flocs were formed earlier than in the case of using the coagulant alone (Figure 5). This effect became more evident in the coupled systems (US/C(Al) and US/C(Fe)). The time difference was 2–4 min for the coagulation with aluminum sulfate and 2 min for ferric chloride. Combining ultrasound exposure with the WAC®HB, the flocculation starting point was almost unnoticeable. The first flocs in the compared systems were formed at a similar time, i.e., shortly after fast agitation ended. It was found that this happened in the 3rd minute of the flocculation time set in the technological system (30 min). This effect is characteristic of coagulation occurring when using polymerized compounds, where the bridging mechanism strongly intensifies the flocculation process. In the systems with conventional coagulants, the flocculation process started only within the 5th to 10th minute of flocculation, taking place later when using ferric chloride.
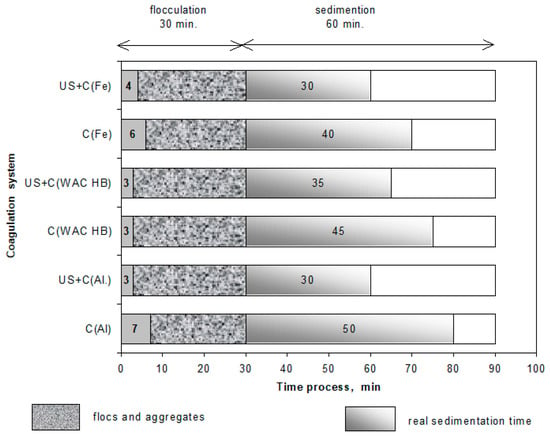
Figure 5.
The effect of ultrasound exposure (8 μm, 5 min) and coagulants on the flocculation process and sedimentation of suspensions.
The size of flocs formed during flocculation was different for the three coagulants studied. Smaller flocs were formed after using aluminum sulfate and ferric chloride (1.0 ÷ 1.5 mm), and larger (reaching up to 2.25 mm in size), more uniform flocs with a more developed surface were formed after using the polymerized WAC®HB compound. By contrast, the observations did not confirm any distinct changes in their size or shape compared to the additional ultrasound exposure. However, it was found that the density of flocs formed after using the aided systems was higher, which also improved the kinetics of the sedimentation of the flocs formed. This implied the possibility of shortening the sedimentation time used in the methodology adopted in the tests by 20 min.
The post-coagulation deposits formed after sedimentation, irrespective of the coagulation method (with or without ultrasound exposure) applied, did not differ in their macroscopic characteristics from the classical mineral–organic sludge that is classified as a dispersive coherent system with a uniform floccular structure. The sludge was highly hydrated and finely dispersed. A noticeable difference was observed (Figure 6) in the macroscopic image of the sludge structure (after coagulation with aluminum sulfate).
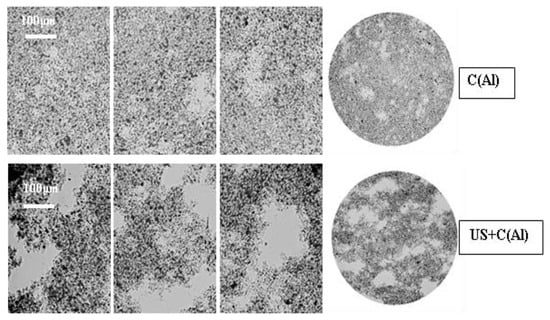
Figure 6.
Post-coagulation sludge structure after using aluminum sulfate (Al): without ultrasound exposure (C(Al)) and with ultrasound exposure (US/C(Al)).
The analysis of the examples of images reveals that as a result of the prior ultrasonic exposure, a distinct shift in the sludge phases occurred in the obtained sludge. The liquid phase forms large areas of free water (easy to remove), while the solid phase (coagulant flocs and contaminant particles) occurs in the form of separated developed agglomerates. The dispersed particles in the sludge formed after using the coagulant alone are embedded in the compact clusters of sludge obtained after coagulation aided with ultrasound exposure.
Ultrasound exposure applied in the coupled systems tested can thus be considered an agent to aid the coagulation of water contaminants. At the same time, it has a favorable effect on dewatering of post-coagulation sediments.
4. Conclusions
The research on the effect of single ultrasound exposure leads to the following conclusions:
- The ultrasonic effect of the reduction in the contents of organic compounds in water depends on vibration amplitude, which determines the ultrasonic field intensity. The most favorable removal of organic contaminants from the water tested was achieved at a maximum amplitude of the ultrasonic field, i.e., A = 16 μm (10 W/cm2), which amounted to EUS = 29% (TOC) and 32.5% (DOC).
- An increase in vibration amplitude, resulting in an increase in the intensity of the ultrasonic field, improves the effectiveness of the removal of humic compounds, which determine water color (maximum result was above 15%). The reduction in the tested water oxygen consumption caused by ultrasound exposure confirms the effect of sonochemical oxidation reactions, and is one of the causes of decreasing organic contamination indices.
The research on the effect of the use of associated systems of ultrasound exposure/coagulant leads to the following conclusions:
- The effect of ultrasound exposure on the removal of water turbidity (increase efficiency ΔE = 25–35%) was generally higher than for water color (ΔE = 8–21%) for tested coagulants (50%d).Using ultrasound (3 W/cm2, 5 min) and aluminum sulfate (20 mg/L, 50%d) yielded an increase in coagulant efficacy by 35% for turbidity and only 8% for color. For comparison, in a system without ultrasound exposure, the effectiveness was 52% and 60%, respectively. Better effectiveness of ultrasound interaction, regardless of the stability of the removed colloids, was found for the polymerized coagulant WAC®HB; in removing water color, the increase in effectiveness was over 20%. In the case of iron chloride, an increase in the effectiveness of turbidity removal was greater than 30%. However, apart from a small effect in color reduction (13%), coagulation at pH6 required a 2-step pH correction.Therefore, for the other two coagulants, recognized criteria should be taken into account in assessing their beneficial interaction with ultrasound exposure: effectiveness (which depends on the parameters of the water being coagulated), economic aspect (cost of coagulants and other reactants), and ecological aspect (effects of introducing chemicals into the environment and amount of sludge generated).This relationship reflects the different effects of ultrasonic energy on colloids of different stability. The obtained possibility of lowering the coagulant dose confirmed the aiding effect of ultrasound exposure.
- The application of ultrasound exposure before adding the coagulant accelerates the moment of floc formation (by up to 4 min) and shortens the sedimentation time. This effect may be a consequence of ultrasonic phenomena that provide better conditions for the coagulation mechanisms associated with the colloid destabilization phase (a confirmed decrease in the electrokinetic potential).
- The use of ultrasound exposure in coagulation systems with aluminum sulfate had a beneficial effect on the structure of sludge post-coagulation. This modification provides better conditions for obtaining water in subsequent sludge dewatering processes.
Compared to conventional coagulation using chemical coagulants, the ultrasonic method reduces the amount of chemicals introduced into the environment by reducing the amount of sludge formed. The use of ultrasound exposure in this study of water treatment by coagulation confirmed the effect of reducing the dose of chemical coagulants, and more favorable operating parameters of the flocculation–sedimentation process. In addition, the modification of the structure of sediments formed after the use of ultrasound exposure and aluminum sulfate, provides better conditions for their dewatering. These findings have both economic and ecological implications.
Author Contributions
Conceptualization, L.S. and E.S.-M.; methodology, L.S.; software, E.S.-M.; validation, E.S.-M.; formal analysis, L.S.; investigation, L.S. and E.S.-M.; resources, E.S.-M.; data curation, E.S.-M.; writing—original draft preparation, L.S. and E.S.-M.; writing—review and editing, L.S. and E.S.-M.; visualization, E.S.-M.; supervision, L.S.; project administration, L.S.; funding acquisition, L.S. and E.S.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the statute subvention of the Czestochowa University of Technology, Faculty of Infrastructure and Environment, BS-PB-400/301/22.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Neis, U. Ultrasound in Water, Wastewater and Sludge Treatment. Water 2000, 21, 36–39. [Google Scholar]
- Śliwiński, A. Ultrasounds and Their Applications; Scientific and Technical Publishing House: Warsaw, Poland, 2001. (In Polish) [Google Scholar]
- Mason, T.J.; Petrier, C. Ultrasound Processes, Advanced Oxidation Processes for Water and Wastewater Treatment; IWA Publishing: London, UK, 2004; pp. 185–208. [Google Scholar]
- Patel, V.K.; Sen, D.J.; Patel, H.U.; Patel, C.N. Sonochemistry: The Effect of Sonic Waves on Chemical Systems. J. Chem. Pharm. Res. 2010, 2, 573–580. [Google Scholar]
- Wolny, L.; Wolski, P. Ultrasounds Energy as an Agent of Polyelectrolyte Modification Prior to Sewage Sludge Conditioning. Energies 2021, 14, 6165. [Google Scholar] [CrossRef]
- López, M.V.; Sosa, L.E.A.; Moeller-Chávez, G.E.; Sosa, A.R.; Neumann, P.; Vidal, G. Evaluation of the Ultrasound Effect on. Treated Municipal Wastewater. Environ. Technol. 2018, 40, 3568–3577. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.; Vieira, C.L.Z.; Wolfson, J.M.; Pingtian, G.; Huang, S. Review on the Treatment of Organic Pollutants in Water by Ultrasonic Technology. Ultrason. Sonochem. 2019, 55, 273–278. [Google Scholar] [CrossRef]
- Md Anawar, H.; Chowdhury, R. Remediation of Polluted River Water by Biological, Chemical, Ecological and Engineering Processes. Sustainability 2020, 12, 7017. [Google Scholar] [CrossRef]
- Patchaiyappan, A.; Devipriya, S.P. Cost Effective Technologies for Solid Waste and Waste Water Treatment; Application of Plant-Based Natural Coagulants in Water Treatment; Chapter 5; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Nawrocki, J. Water Treatment. Physical, Chemical and Biological Processes; Parts I and II; Scientific Publishing House: Warsaw, Poland, 2010. (In Polish) [Google Scholar]
- Mason, T.J.; Joyce, E.; Phull, S.S.; Lorimer, J.P. Potential Uses of Ultrasound in the Biological Decontamination of Water. Ultrason. Sonochem. 2003, 10, 319–323. [Google Scholar] [CrossRef]
- Naddeo, V.; Landi, M.; Belgiorno, V.; Napoli, R.M.A. Wastewater Disinfection by Combination of Ultrasound and Ultraviolet Irradiation. J. Hazard. Mater. 2009, 168, 925–929. [Google Scholar] [CrossRef]
- Kusiak, M.; Okoniewska, E.; Stępniak, L.; Stańczyk-Mazanek, E. The Effect of Ultrasounds on the Effectiveness of Organic Compounds Adsorption from Water. Pol. J. Environ. Studies 2011, 20, 195–200. [Google Scholar]
- Bernardo, E.C.; Fukuta, T.; Fujita, T.; Ona, E.P.; Kojima, Y.; Matsuda, H. Enhancement of Saccharin Removal from Aqueous Solution by Activated Carbon Adsorption with Ultrasound Treatment. Ultrason. Sonochem. 2006, 13, 13–18. [Google Scholar] [CrossRef]
- Borea, L.; Naddeo, V.; Shalaby, S.M.; Zarra, T.; Belgiorno, V.; Abdalla, H.; Shaban, A.M. Wastewater Treatment by Membrane Ultrafiltration Enhanced with Ultrasound: Effect of Membrane Flux and Ultrasonic Frequency. Ultrasonics 2017, 83, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Goel, M.; Hongqiang, H.; Majumdar, A.S.; Ray, M.B. Sonochemical Decomposition of Volatile and Non-Volatile Organic Compounds—a Comparative Study. Water Res. 2004, 38, 4247–4261. [Google Scholar] [CrossRef] [PubMed]
- Collings, A.F.; Gwan, P.B. Ultrasonic Destruction of Pesticide Contaminants in Slurries. Ultrason. Sonochem. 2010, 17, 1–3. [Google Scholar] [CrossRef]
- Kim, I.; Hong, S.; Hwang, I.; Kwon, D.; Kwon, J.; Huang, C.P. TOC and THMFP Reduction by Ultrasonic Irradiation in Wastewater Effluent. Desalination 2007, 202, 9–15. [Google Scholar] [CrossRef]
- Cui, M.; Jang, M.; Cho, S.H.; Elena, D.; Khim, J. Enhancement in Mineralization of a Number of Natural Refractory Organic Compounds by the Combined Process of Sonolysis and Ozonolysis (US/O3). Ultrason. Sonochem. 2011, 18, 773–780. [Google Scholar] [CrossRef]
- Schemer, H.; Narkis, N. Sonochemical Removal of Trihalomethanes from Aqueous Solutions. Ultrason. Sonochem. 2005, 12, 495–499. [Google Scholar] [CrossRef]
- Naddeo, V.; Belgiorno, V.; Ricco, D.; Kassinos, D. Degradation of Diclofenac during Sonolysis, Ozonation and their Simulta Neous Application. Ultrason. Sonochem. 2009, 16, 790–794. [Google Scholar] [CrossRef]
- Chemat, F.; Teunissen, P.G.M.; Chemat, S.; Bartels, P.V. Sono-Oxidation Treatment of Humic Substances in Drinking Water. Ultrason. Sonochem. 2001, 8, 247–250. [Google Scholar] [CrossRef]
- Mahvi, A.H.; Maleki, A.; Rezaee, R.; Safari, M. Reduction of Humic Substances in Water by Application of Ultrasound Waves and Ultraviolet Irradiation. Iran. J. Environ. Health. Sci. Eng. 2009, 6, 233–240. [Google Scholar]
- Naddeo, V.; Belgiorno, V.; Napoli, R. Behaviour of Natural Organic Matter during Ultrasonic Irradiation. Desalination 2007, 210, 175–182. [Google Scholar] [CrossRef]
- Setareh, P.; Khezri, S.M.; Hosseini, H.; Pirsaheb, M. Coupling Effect of Ozone/Ultrasound with Coagulation for Improving NOM and Turbidity Removal from Surface Water. J. Water Process Eng. 2020, 37, 101340. [Google Scholar] [CrossRef]
- Stępniak, L.; Kępa, U.; Stańczyk-Mazanek, E. The Research on the Possibility of Ultrasound Field Application in Iron Removal of Water. Desalination 2008, 223, 180–186. [Google Scholar] [CrossRef]
- Zhou, Z.W.; Yang, Y.; Li, X.; Li, P.; Zhang, T.; Lv, X.; Liu, L.; Dong, J.; Zheng, D. Optimized Removal of Natural Organic Matter by Ultrasound-Assisted Coagulation of Recycling Drinking Water Treatment Sludge. Ultrason. Sonochem. 2018, 48, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Mahamuni, N.N.; Adewuyi, Y.G. Advanced Oxidation Processes (AOPs) Involving Ultrasound for Waste Water Treatment: A Review with Emphasis on Cost Estimation. Ultrason. Sonochem. 2010, 17, 990–1003. [Google Scholar] [CrossRef] [PubMed]
- Alfonso-Muniozguren, P.; Ferreiro, C.; Richard, E.; Bussemaker, M.; Lombraña, J.I.; Lee, J. Analysis of Ultrasonic Pre-Treatment for the Ozonation of Humic Acids. Ultrason. Sonochem. 2021, 71, 105359. [Google Scholar] [CrossRef] [PubMed]
- Sillanpaa, M.; Ncibi, M.C.; Matilainen, A. Advanced Oxidation Processes for the Removal of Natural Organic Matter from Drinking Water Sources: A Comprehensive Review. J. Environ. Manag. 2018, 208, 56–76. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).