A Novel Highly Stable Biomass Gel Foam Based on Double Cross-Linked Structure for Inhibiting Coal Spontaneous Combustion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Gel Foam
2.3. The Process of Coating Coal with Gel Foam
2.4. Performance Test of Gel Foam
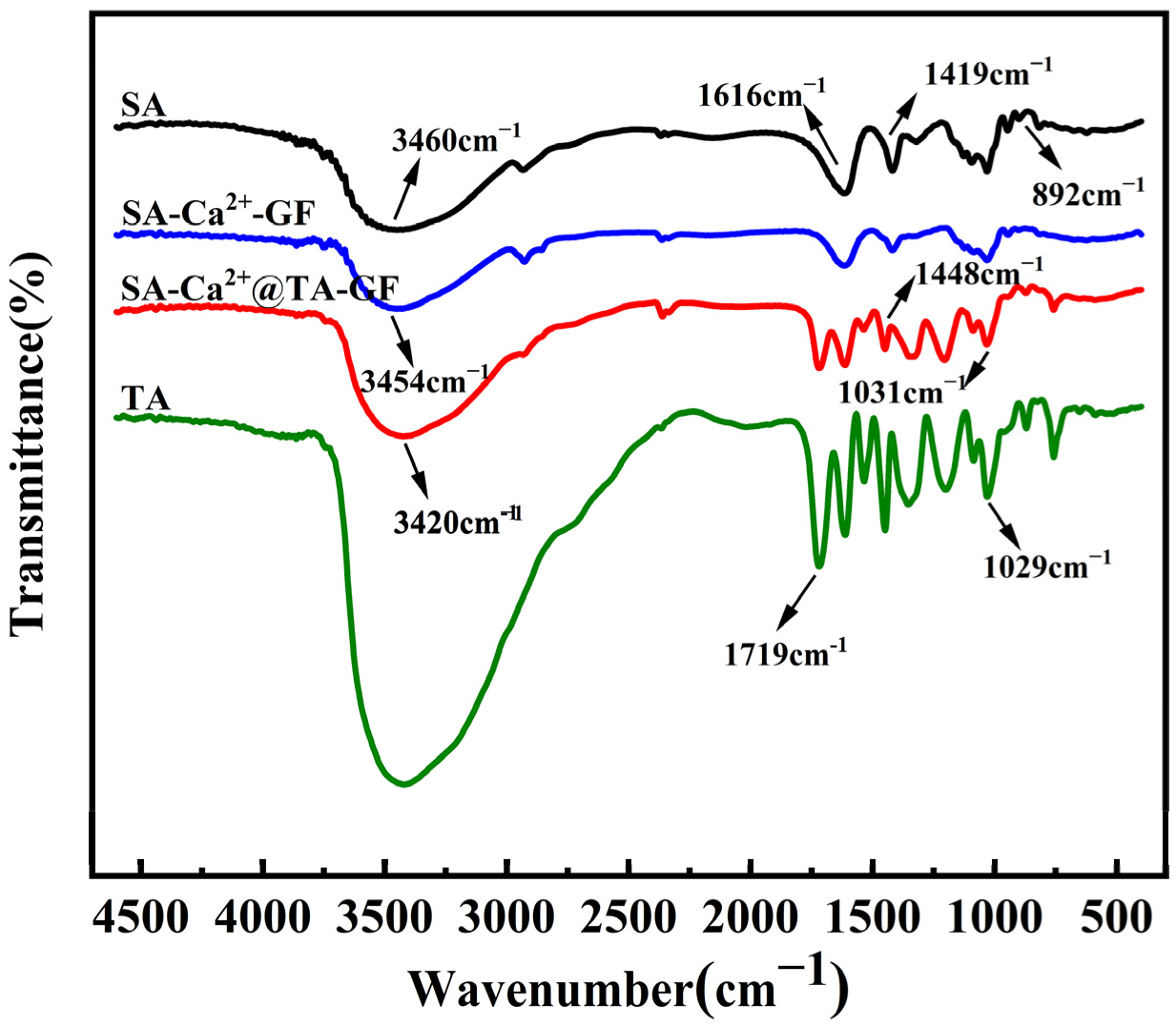
2.4.1. FT-IR Analysis
2.4.2. Gelation Time and Half-Life
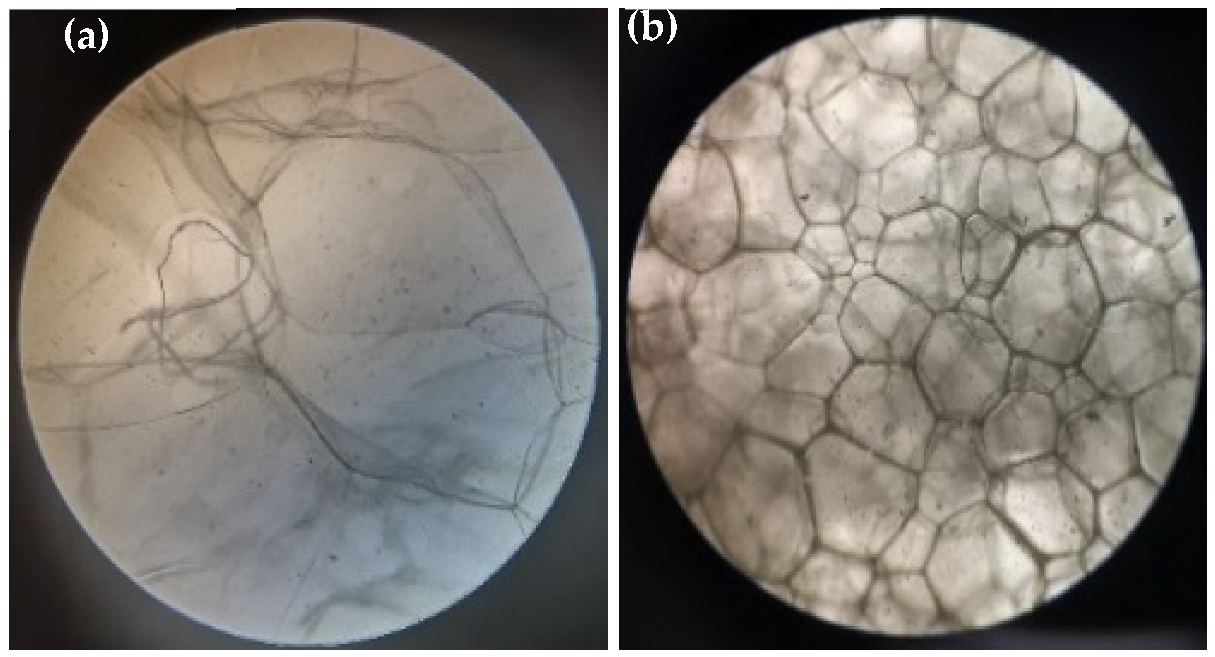
2.4.3. Micro-Morphology of Gel Foams
2.4.4. Strength of Gel Foams
2.4.5. The Inhibition Performance of SA-Ca2+@TA-GF in Coal Room Temperature Oxidation
2.4.6. The Inhibition Performance of SA-Ca2+@TA-GF in the Coal Temperature-Programmed Oxidation
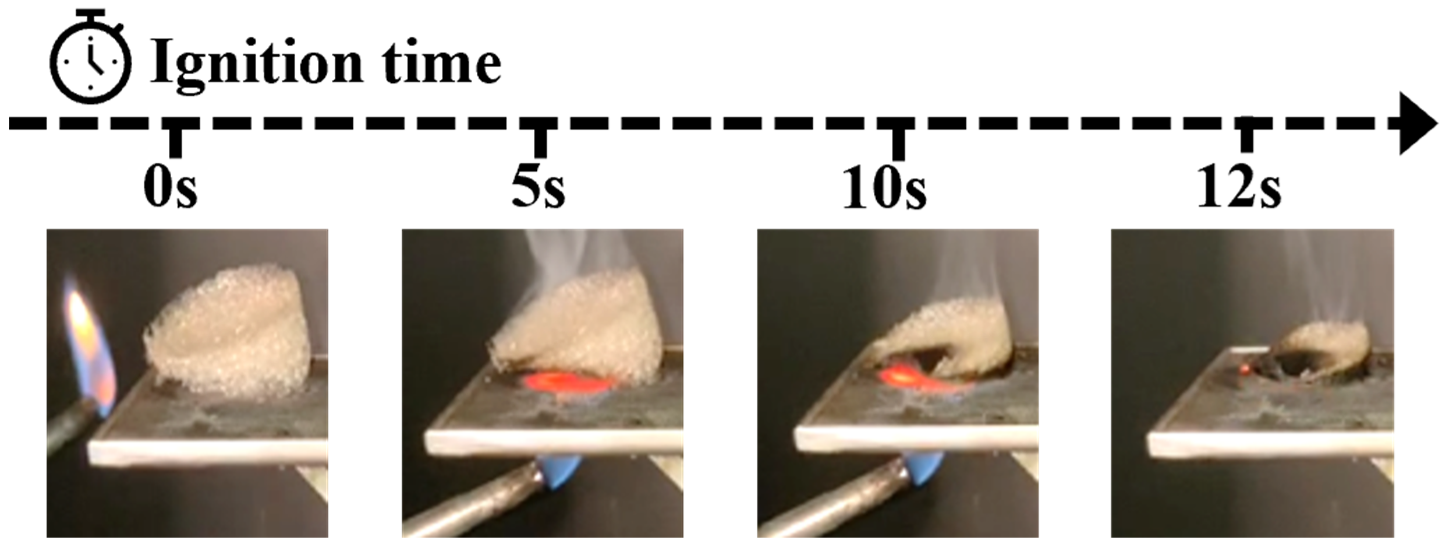
2.4.7. The Fire Extinguishing Performance of SA-Ca2+@TA-GF
2.4.8. XPS Analysis
3. Results
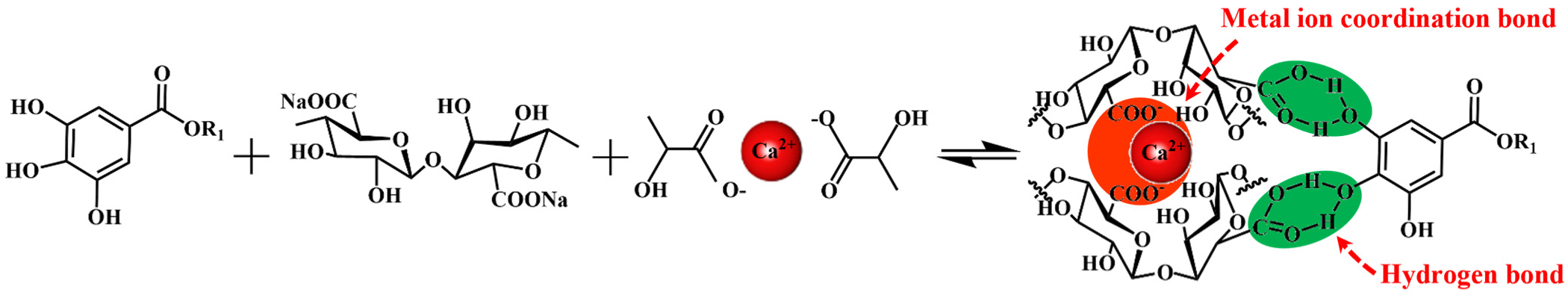
3.1. FT-IR Analysis
3.2. Stability and Micro-Morphology Analysis of SA-Ca2+@TA-GF
3.3. Mechanical Strength Analysis of SA-Ca2+@TA-GF
3.4. The Inhibition Performance of SA-Ca2+@TA-GF in Coal Room Temperature Oxidation
3.5. The Inhibition Performance of SA-Ca2+@TA-GF in Coal Temperature-Programmed Oxidation
3.6. Fire Extinguishing Performances Analysis of SA-Ca2+@TA-GF
3.7. XPS Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, J.; Deng, J.; Chen, L.; Wang, T.; Song, J.; Zhang, Y.; Shu, C.; Zeng, Q. Correlation analysis of the functional groups and exothermic characteristics of bituminous coal molecules during high-temperature oxidation. Energy 2019, 181, 136–147. [Google Scholar] [CrossRef]
- Zhao, J.; Deng, J.; Wang, T.; Song, J.; Zhang, Y.; Shu, C.; Zeng, Q. Assessing the effectiveness of a high-temperature-programmed experimental system for simulating the spontaneous combustion properties of bituminous coal through thermokinetic analysis of four oxidation stages. Energy 2019, 169, 587–596. [Google Scholar] [CrossRef]
- Yang, F.; Lu, Y.; Yan, Z.; Wang, G.; Hu, X.; Gu, W. Colloidal Particle-Stabilized Foam to Control the Coal Spontaneous Combustion: Stability Mechanism Analysis and Extinguishing Properties. Energy Fuels 2020, 34, 14822–14831. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, X.; Wu, M.; Zhao, Y.; Yu, C. Effects of different catalysts on the structure and properties of polyurethane/water glass grouting materials. Polym. Sci. 2018, 135, 46460. [Google Scholar] [CrossRef]
- Lu, Y. Laboratory Study on the Rising Temperature of Spontaneous Combustion in Coal Stockpiles and a Paste Foam Suppression Technique. Energy Fuels 2017, 31, 7290–7298. [Google Scholar] [CrossRef]
- Tian, Z.; Lu, Y.; Liu, S.; Shi, S.; Li, H.; Ye, Q. Application of Inorganic Solidified Foam to Control the Coexistence of Unusual Methane Emission and Spontaneous Combustion of Coal in the Luwa Coal Mine, China. Combust. Sci. Technol. 2019, 192, 638–656. [Google Scholar] [CrossRef]
- Shi, B.; Ma, L.; Dong, W.; Zhou, F. Application of a Novel Liquid Nitrogen Control Technique for Heat Stress and Fire Prevention in Underground Mines. J. Occup. Environ. Hyg. 2015, 12, D168–D177. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Wang, H.; Yang, J.; Liu, L. Large-area goaf fires: A numerical method for locating high-temperature zones and assessing the effect of liquid nitrogen fire control. Environ. Earth Sci. 2016, 75, 1396. [Google Scholar] [CrossRef]
- Cheng, W.; Hu, X.; Xie, J.; Zhao, Y. An intelligent gel designed to control the spontaneous combustion of coal: Fire prevention and extinguishing properties. Fuel 2017, 210, 826–835. [Google Scholar] [CrossRef]
- Qin, B.; Ma, D.; Li, F.; Li, Y. Aqueous clay suspensions stabilized by alginate fluid gels for coal spontaneous combustion prevention and control. Environ. Sci. Pollut. Res. Int. 2017, 24, 24657–24665. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, X.; Yuan, Y.; Qi, G.; Hu, X.; Li, J.; Liang, Y.; Guo, B. Study on the characteristics and mechanism of a new type of antioxidant gel foam for coal spontaneous combustion prevention. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127254. [Google Scholar] [CrossRef]
- Xi, X.; Shi, Q. Study of the preparation and extinguishment characteristic of the novel high-water-retaining foam for controlling spontaneous combustion of coal. Fuel 2021, 288, 119354. [Google Scholar] [CrossRef]
- Shi, B.; Zhou, F. Impact of heat and mass transfer during the transport of nitrogen in coal porous media on coal mine fires. Sci. World J. 2014, 2014, 293142. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Hu, X.; Cheng, W.; Wei, J.; Zhao, Y.; Shen, L. Fire prevention and control using gel-stabilization foam to inhibit spontaneous combustion of coal: Characteristics and engineering applications. Fuel 2020, 264, 116903. [Google Scholar] [CrossRef]
- Wu, M.; Liang, Y.; Zhao, Y.; Wang, W.; Hu, X.; Tian, F.; He, Z.; Li, Y.; Liu, T. Preparation of new gel foam and evaluation of its fire extinguishing performance. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127443. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, Y. Preparation, Properties and Application of Gel Materials for Coal Gangue Control. Energies 2022, 15, 557. [Google Scholar] [CrossRef]
- Wang, G.; Yan, G.; Zhang, X.; Du, W.; Huang, Q.; Sun, L.; Zhang, X. Research and development of foamed gel for controlling the spontaneous combustion of coal in coal mine. J. Loss Prev. Process Ind. 2016, 44, 474–486. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, W.; Wang, M.; Qi, G.; He, Z.; Li, J. Preparation and properties of self-assembled gels based on electrostatic interactions: Applications for N2/CO2 adsorption and inhibition. Fuel 2021, 302, 121053. [Google Scholar]
- Gao, C.; Liu, M.; Chen, J.; Zhang, X. Preparation and controlled degradation of oxidized sodium alginate hydrogel. Polym. Degrad. Stab. 2009, 94, 1405–1410. [Google Scholar] [CrossRef]
- Wang, H.; Gong, X.; Miao, Y.; Guo, X.; Liu, C.; Fan, Y.; Zhang, J.; Niu, B.; Li, W. Preparation and characterization of multilayer films composed of chitosan, sodium alginate and carboxymethyl chitosan-ZnO nanoparticles. Food Chem. 2019, 283, 397–403. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, K.; Hu, J.; Zhang, Y.; Dai, Y.; Xia, F. Role of a high calcium ion content in extending the properties of alginate dual-crosslinked hydrogels. J. Mater. Chem. A 2020, 8, 25390–25401. [Google Scholar] [CrossRef]
- Wu, L.; Qi, G.; Lu, W.; He, Z.; Li, J.; Li, J.; He, M. Study on Preparation and Performance of Calcium Carbide Slag Foam for Coal Mine Disaster Reduction and CO2 Storage. Colloids Surf. A Physicochem. Eng. Asp. 2020, 606, 125322. [Google Scholar] [CrossRef]
- Fan, H.; Wang, J.; Jin, Z. Tough, Swelling-Resistant, Self-Healing, and Adhesive Dual-Cross-Linked Hydrogels Based on Polymer-Tannic Acid Multiple Hydrogen Bonds. Macromolecules 2018, 51, 1696–1705. [Google Scholar] [CrossRef]
- Zhou, Y.; Tawiah, B.; Noor, N.; Zhang, Z.; Sun, J.; Yuen, R.K.K.; Fin, B. A facile and sustainable approach for simultaneously flame retarded, UV protective and reinforced poly(lactic acid) composites using fully bio-based complexing couples. Compos. Part B Eng. 2021, 215, 108833. [Google Scholar] [CrossRef]
- Xu, R.; Ma, S.; Lin, P.; Yu, B.; Zhou, F.; Liu, W. High Strength Astringent Hydrogels Using Protein as the Building Block for Physically Cross-linked Multi-Network. ACS Appl. Mater. Interfaces 2018, 10, 7593–7601. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Vatankhah-Varnoosfaderani, M.; Zhou, J.; Li, Q.; Sheiko, S. Weak Hydrogen Bonding Enables Hard, Strong, Tough, and Elastic Hydrogels. Adv. Mater. 2015, 27, 6899–6905. [Google Scholar] [CrossRef]
- Han, C.; Nie, S.; Liu, Z.; Liu, S.; Zhang, H.; Li, J.; Zhang, H.; Wang, Z. A novel biomass sodium alginate gel foam to inhibit the spontaneous combustion of coal. Fuel 2022, 314, 122779. [Google Scholar] [CrossRef]
- Qin, B.; Jia, Y.; Lu, Y.; Li, Y.; Wang, D.; Chen, C. Micro fly-ash particles stabilized Pickering foams and its combustion-retardant characteristics. Fuel 2015, 154, 174–180. [Google Scholar] [CrossRef]
- Tang, L.; Wang, G.; Wang, E.; Li, X.; Cheng, Q. The fire inhibition characteristics of composite inert gas and its application potential analysis. Energy Sources Part A Recovery Util. Environ. Eff. 2021, 1–12. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, D.; He, R.; Li, N.; Zhou, H.; Wang, Y.; Song, Y.; Zhi, K.; Teng, Y.; Liu, Q. Microstructural characteristics of Shengli lignite during low-temperature oxidation and promotion effect of iron species. Fuel 2019, 255, 115830. [Google Scholar] [CrossRef]
- Hou, Y.; Zhong, X.; Ding, Y.; Zhang, S.; Shi, F.; Hu, J. Alginate-based aerogels with double catalytic activity sites and high mechanical strength. Carbohydr. Polym. 2020, 245, 116490. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Hu, X.; Cheng, W.; Bian, S.; Zhao, Y.; Wu, M.; Xue, D.; Li, Y.; Lu, W.; Wang, P. Study of resource utilization and fire prevention characteristics of a novel gel formulated from coal mine sludge (MS). Fuel 2020, 267, 117261. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, D.; Cai, J.; Liu, W.; Yan, N.; Qiu, X. Robust Conductive Hydrogel with Antibacterial Activity and UV-Shielding Performance. Ind. Eng. Chem. Res. 2020, 59, 17867–17875. [Google Scholar] [CrossRef]
- Li, T.; Hu, X.; Zhang, Q.; Zhao, Y.; Wang, P.; Wang, X.; Qin, B.; Lu, W. Poly(acrylic acid)-chitosan @ tannic acid double-network self-healing hydrogel based on ionic coordination. Polym. Adv. Technol. 2020, 31, 1648–1660. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Yang, Y.; Zhang, X. Study on the generation of active sites during low-temperature pyrolysis of coal and its influence on coal spontaneous combustion. Fuel 2019, 241, 283–296. [Google Scholar] [CrossRef]
- Wang, D.; Xin, H.; Qi, X.; Dou, G.; Qi, G.; Ma, L. Reaction pathway of coal oxidation at low temperatures: A model of cyclic chain reactions and kinetic characteristics. Combust. Flame 2016, 163, 447–460. [Google Scholar] [CrossRef]
- Li, J.; Lu, W.; Kong, B.; Cao, Y.; Qi, G.; Qin, C. Mechanism of Gas Generation during Low-Temperature Oxidation of Coal and Model Compounds. Energy Fuels 2019, 33, 1527–1539. [Google Scholar] [CrossRef]
- Xue, D.; Hu, X.; Cheng, W.; Wu, M.; Shao, Z.; Li, Y.; Zhao, Y.; Zhang, K. Carbon dioxide sealing-based inhibition of coal spontaneous combustion: A temperature-sensitive micro-encapsulated fire-retardant foamed gel. Fuel 2020, 266, 117036. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, X.; Gao, Y.; Zhang, Y.; Li, Z.; Wang, H.; Shi, X. Experimental study on the compound system of proanthocyanidin and polyethylene glycol to prevent coal spontaneous combustion. Fuel 2019, 254, 115610. [Google Scholar] [CrossRef]
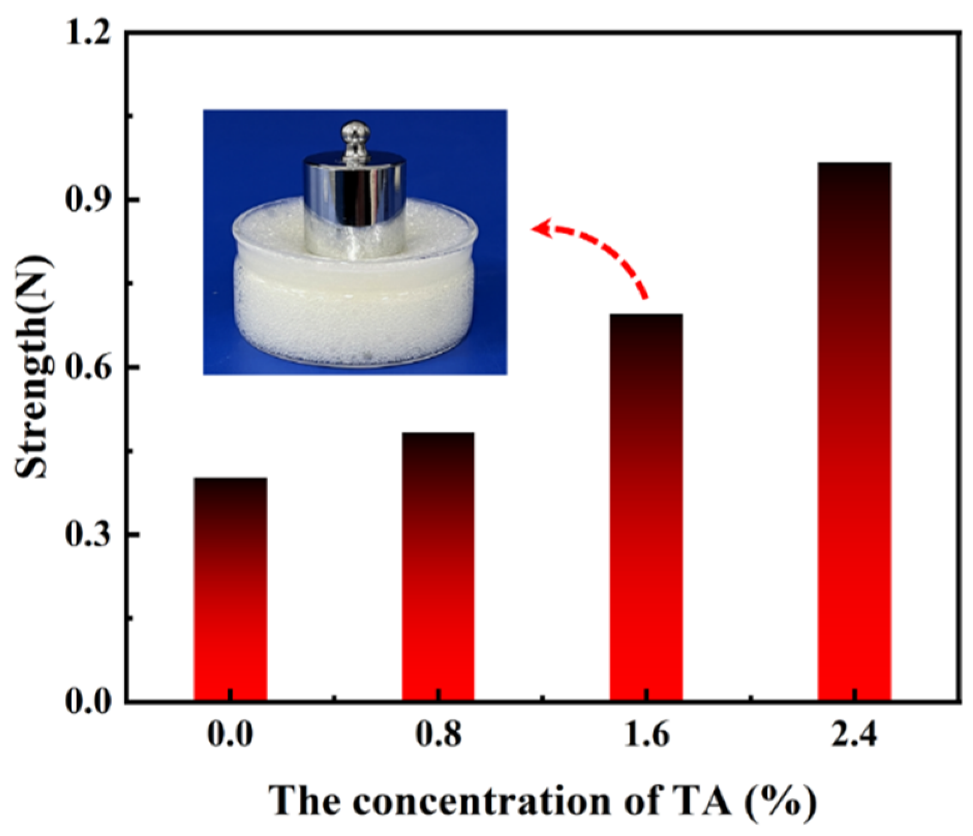
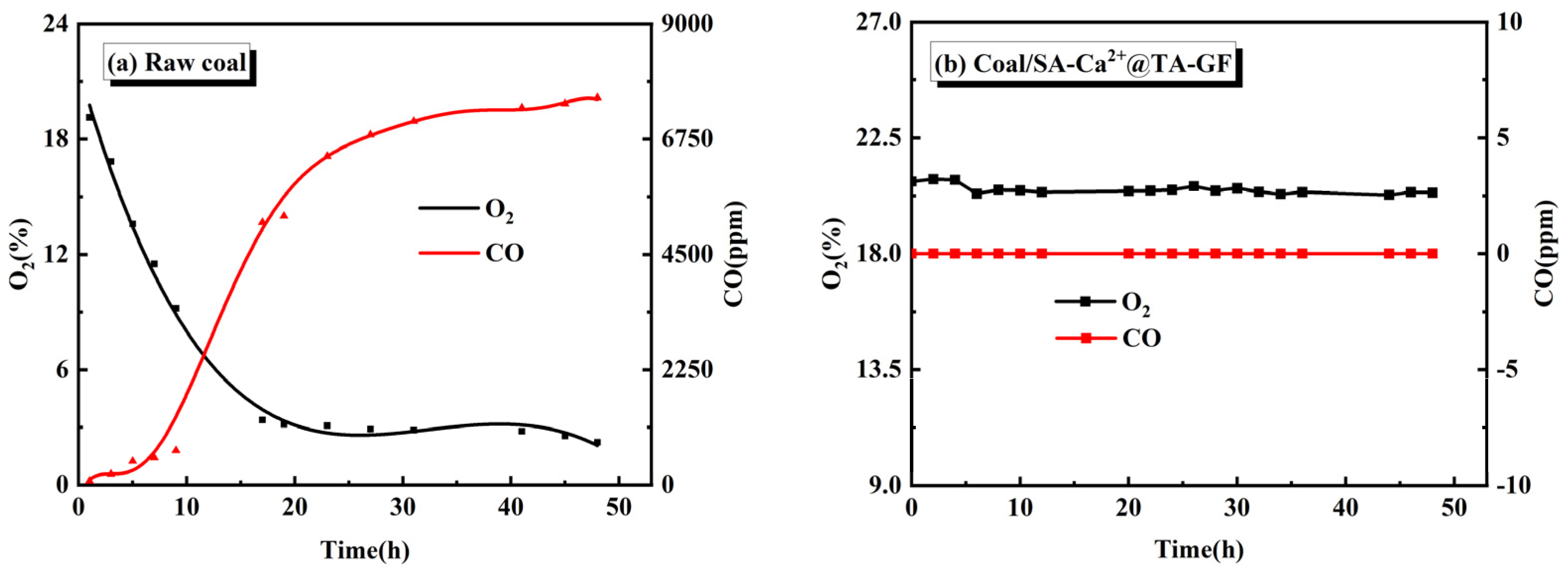

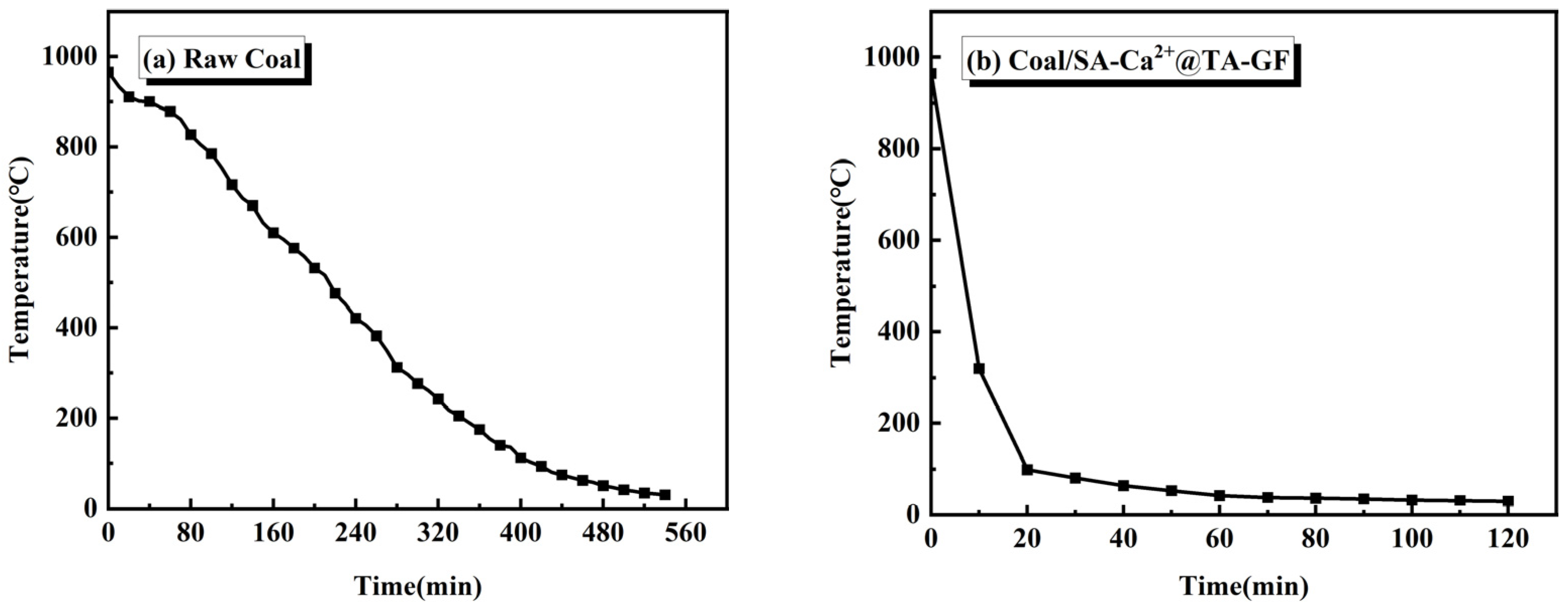

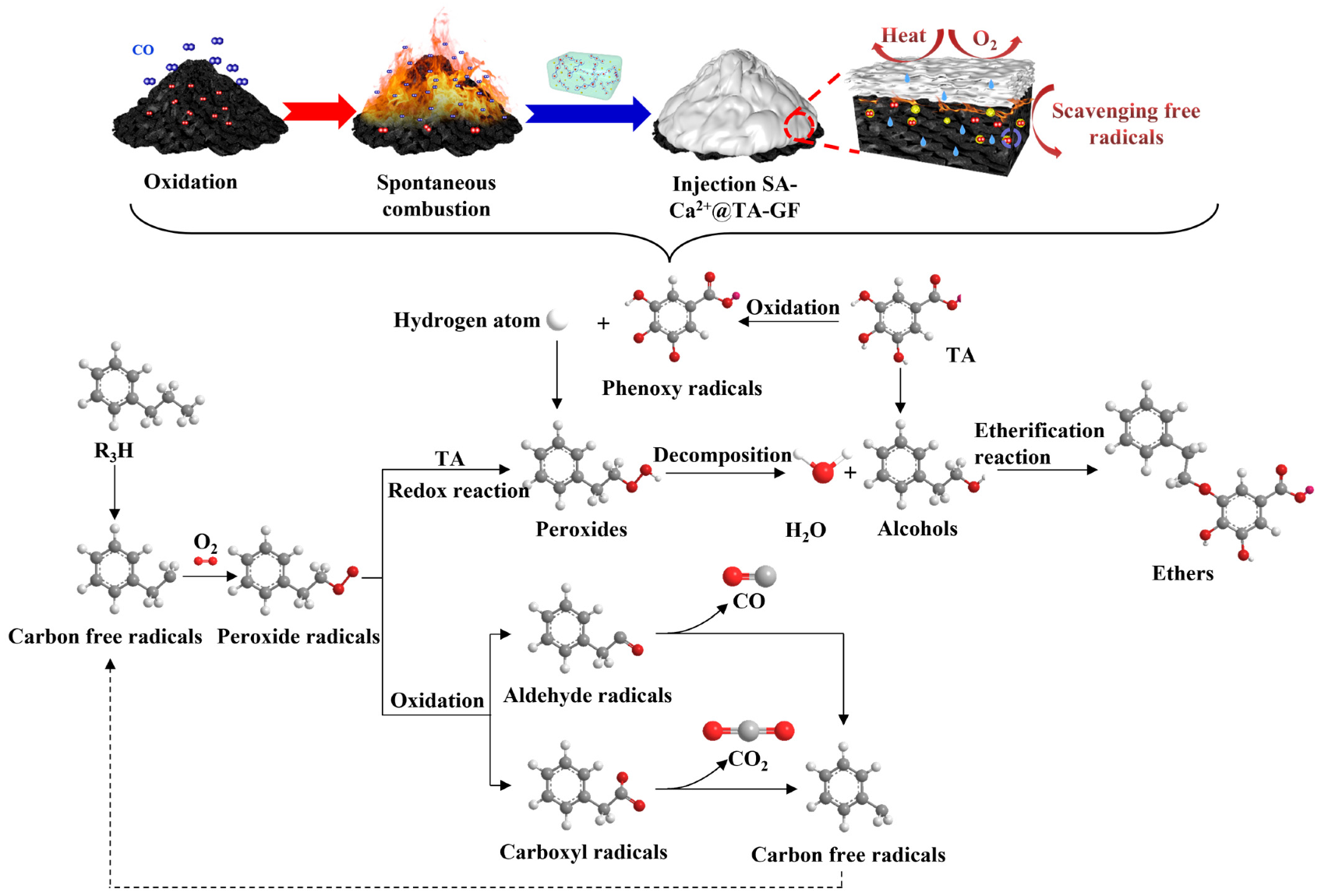
| SA/wt% | CL/wt% | CFA/wt% | TA/wt% | Gelation Time/min | Half-Life/d |
|---|---|---|---|---|---|
| 0.4 | 0.05 | 0.3 | 0 | No gelation | 0.4 |
| 0.05 | 0.3 | 0.8 | 20 | 20 | |
| 0.05 | 0.3 | 1.6 | 10 | 30 | |
| 0.05 | 0.3 | 2.4 | 1 | 35 |
| Samples | Temperature/°C | Carbon Forms (Content/%) | ||||
|---|---|---|---|---|---|---|
| C-C/C-H | C*-C* | C-O | C=O | –COO | ||
| Raw Coal | 30 | 62.21 | 12.74 | 8.23 | 4.23 | 12.59 |
| 110 | 61.09 | 14.09 | 8.37 | 5.61 | 10.84 | |
| 200 | 58.95 | 14.33 | 8.64 | 6.47 | 11.62 | |
| Coal/SA-Ca2+@TA-GF | 30 | 47.11 | 12 | 19.78 | 6.73 | 14.37 |
| 110 | 51.62 | 13.53 | 18.08 | 5.20 | 11.56 | |
| 200 | 52.14 | 14.97 | 17.51 | 5.08 | 10.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, C.; Nie, S.; Liu, Z.; Yang, J.; Zhang, H.; Zhang, H.; Li, J.; Wang, Z. A Novel Highly Stable Biomass Gel Foam Based on Double Cross-Linked Structure for Inhibiting Coal Spontaneous Combustion. Energies 2022, 15, 5207. https://doi.org/10.3390/en15145207
Han C, Nie S, Liu Z, Yang J, Zhang H, Zhang H, Li J, Wang Z. A Novel Highly Stable Biomass Gel Foam Based on Double Cross-Linked Structure for Inhibiting Coal Spontaneous Combustion. Energies. 2022; 15(14):5207. https://doi.org/10.3390/en15145207
Chicago/Turabian StyleHan, Chao, Shibin Nie, Zegong Liu, Jinian Yang, Hong Zhang, Haoran Zhang, Jiayi Li, and Zihan Wang. 2022. "A Novel Highly Stable Biomass Gel Foam Based on Double Cross-Linked Structure for Inhibiting Coal Spontaneous Combustion" Energies 15, no. 14: 5207. https://doi.org/10.3390/en15145207
APA StyleHan, C., Nie, S., Liu, Z., Yang, J., Zhang, H., Zhang, H., Li, J., & Wang, Z. (2022). A Novel Highly Stable Biomass Gel Foam Based on Double Cross-Linked Structure for Inhibiting Coal Spontaneous Combustion. Energies, 15(14), 5207. https://doi.org/10.3390/en15145207





