Study of Mass Loss and Elemental Analysis of Pine Wood Pellets in a Small-Scale Reactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Properties of Pine Wood Pellets
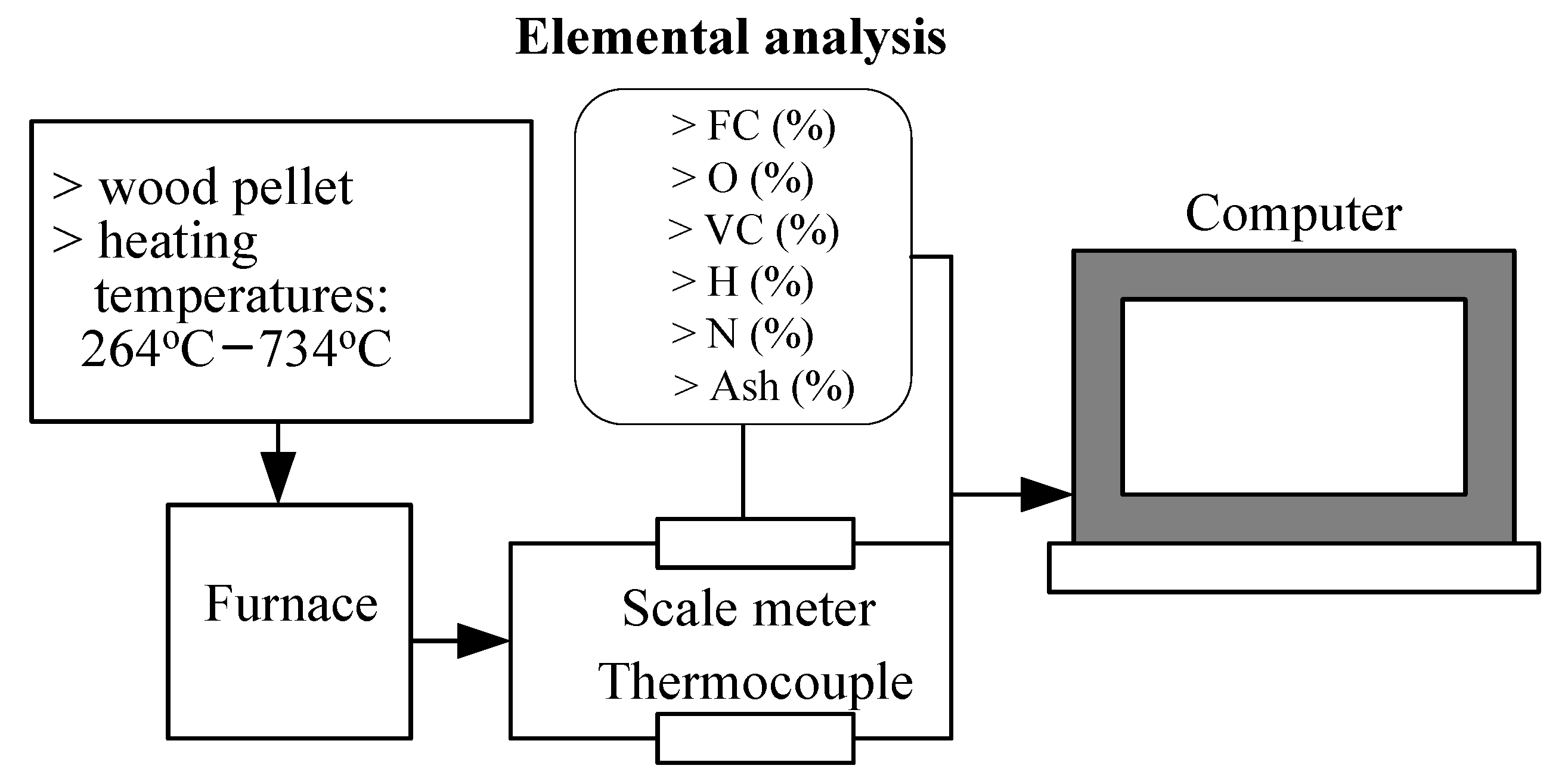
2.2. Experimental Apparatus and Procedure
3. Results and Discussion
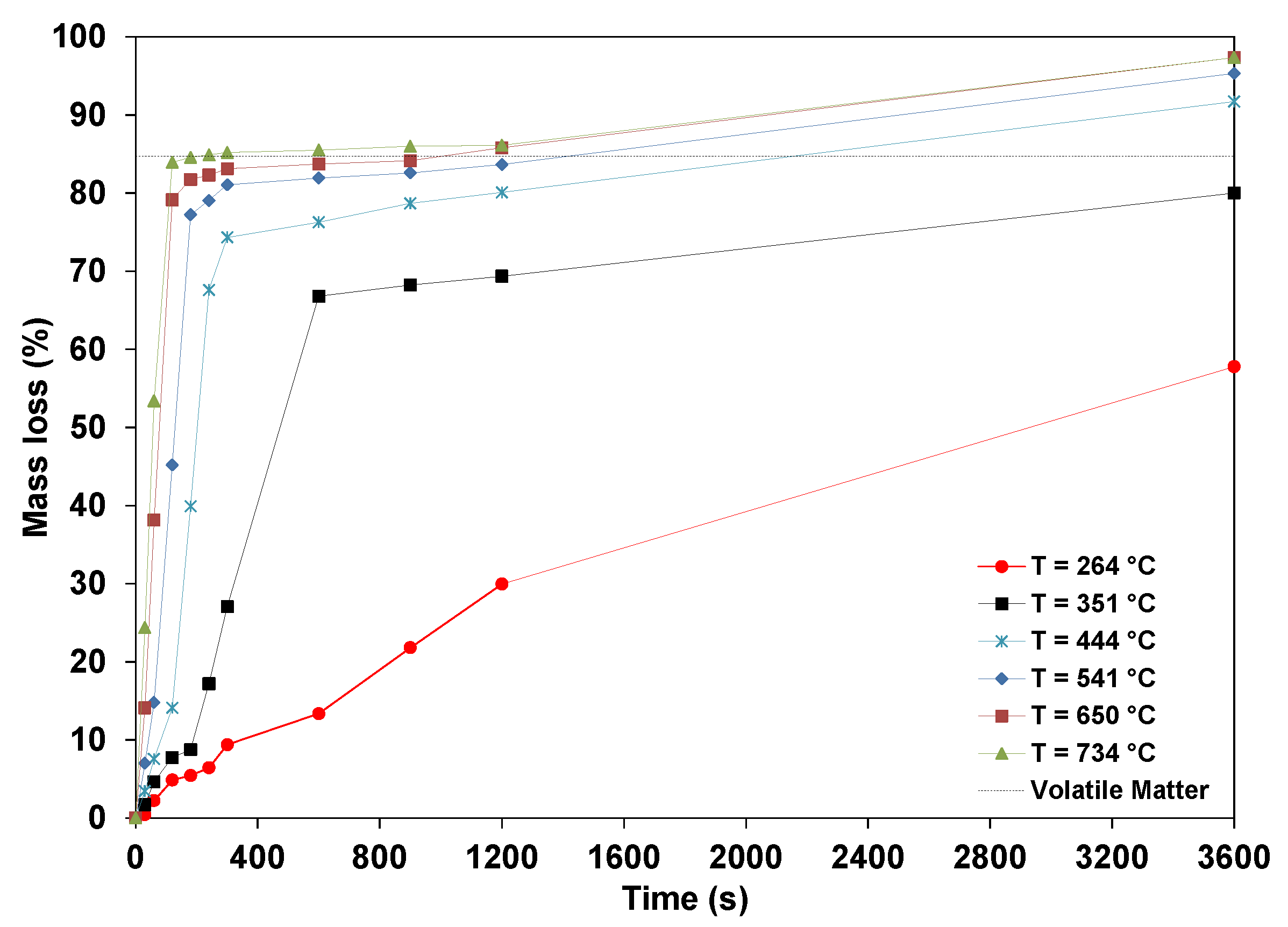
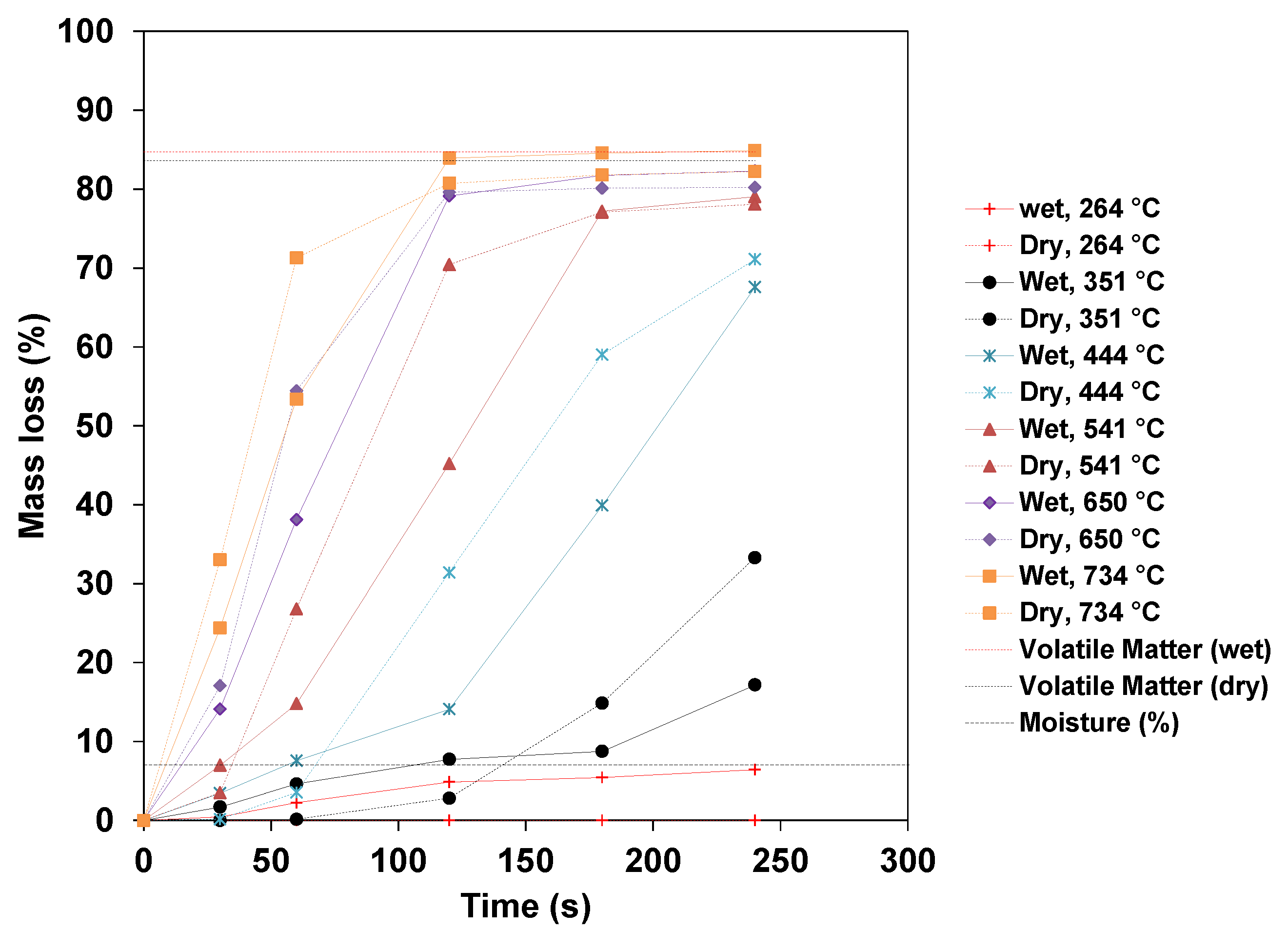
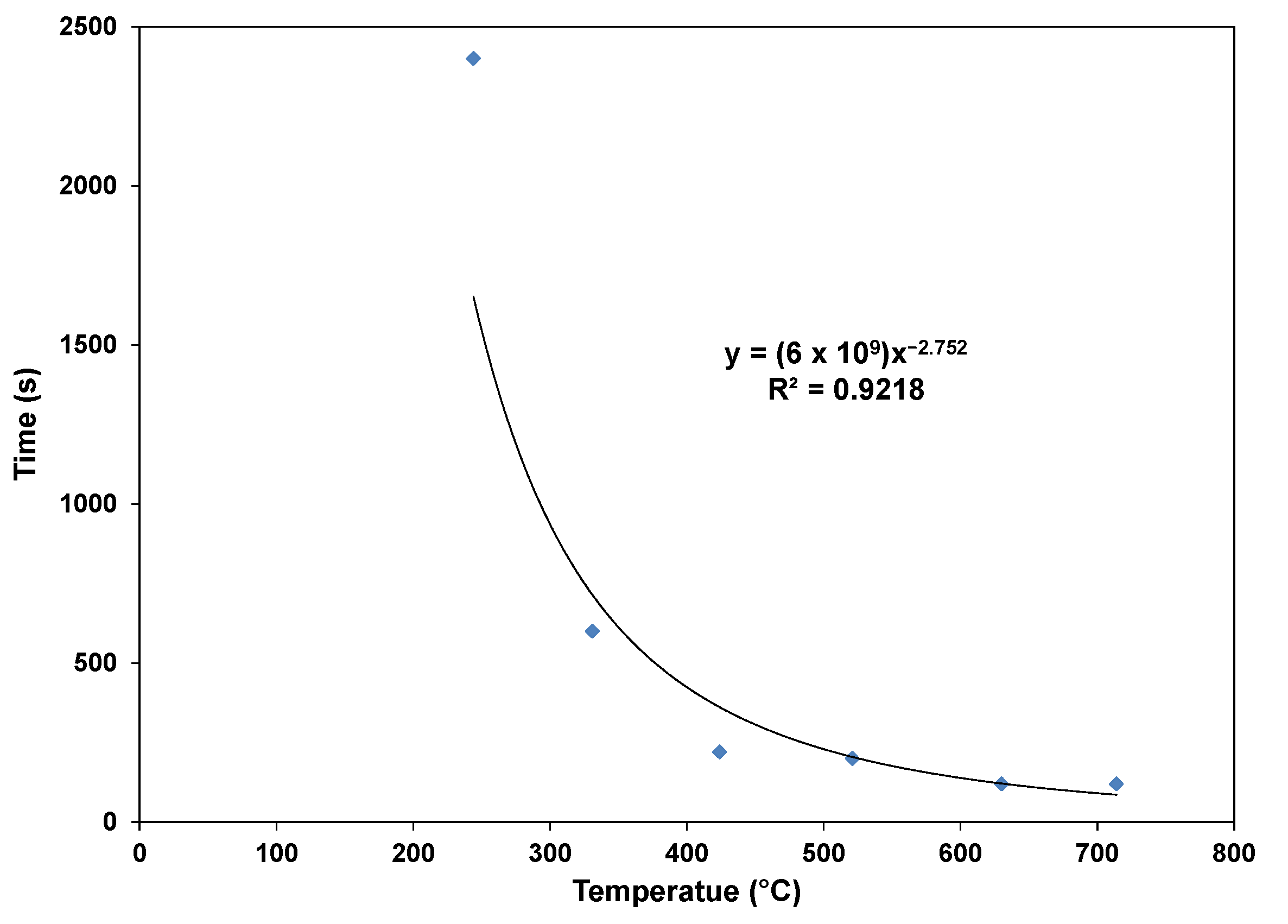
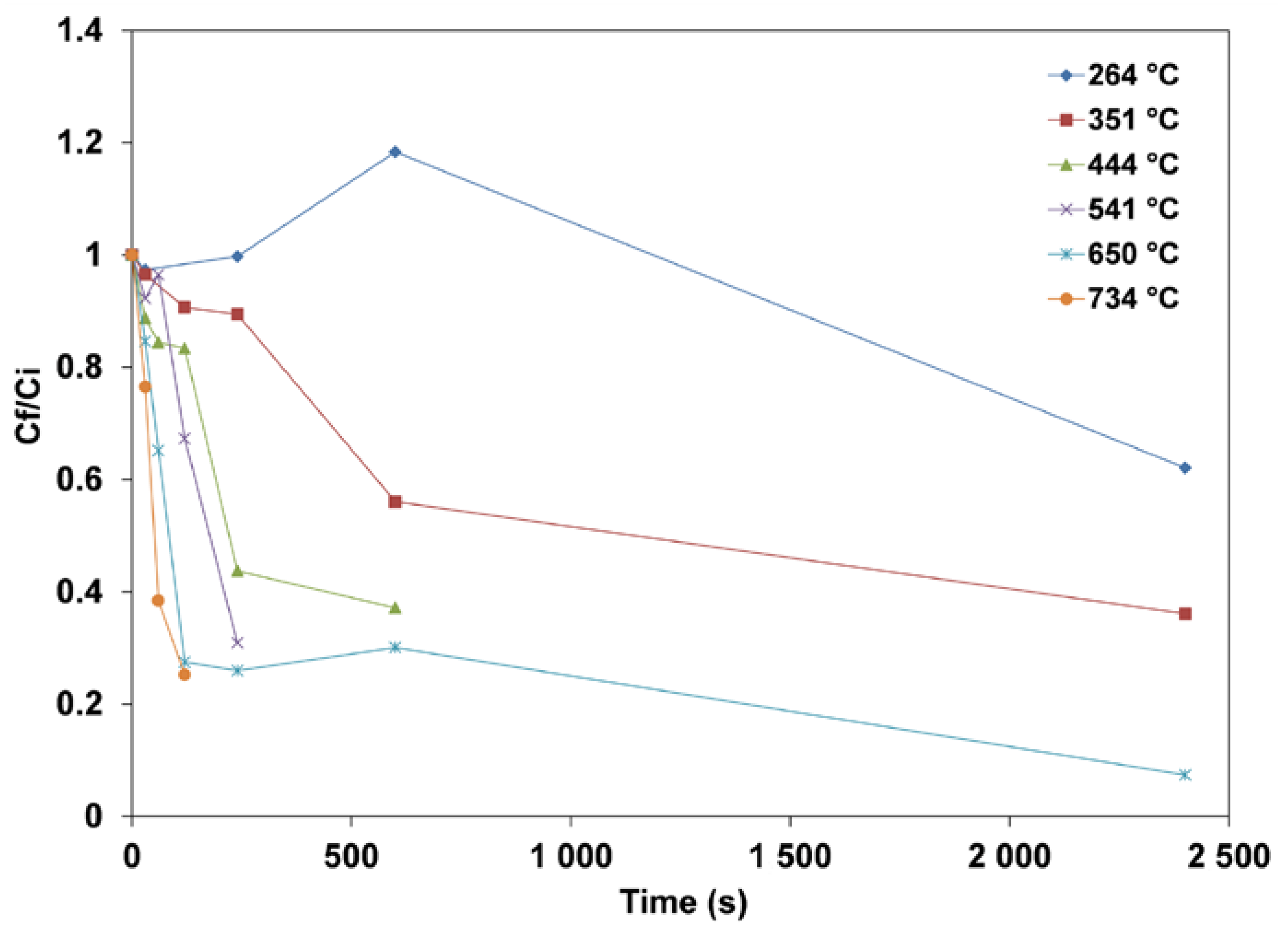
3.1. Mass Loss of Wood Pellets
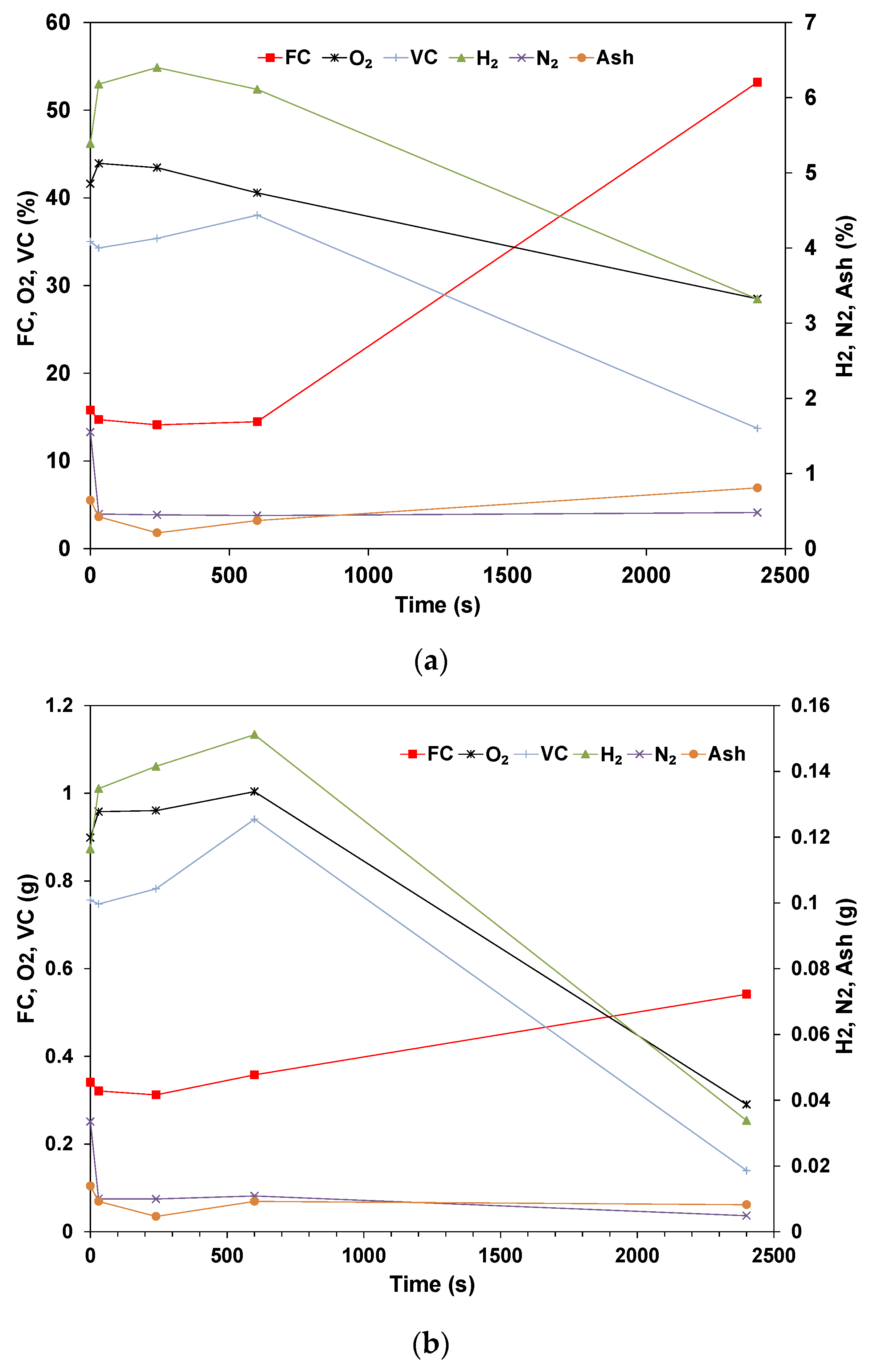
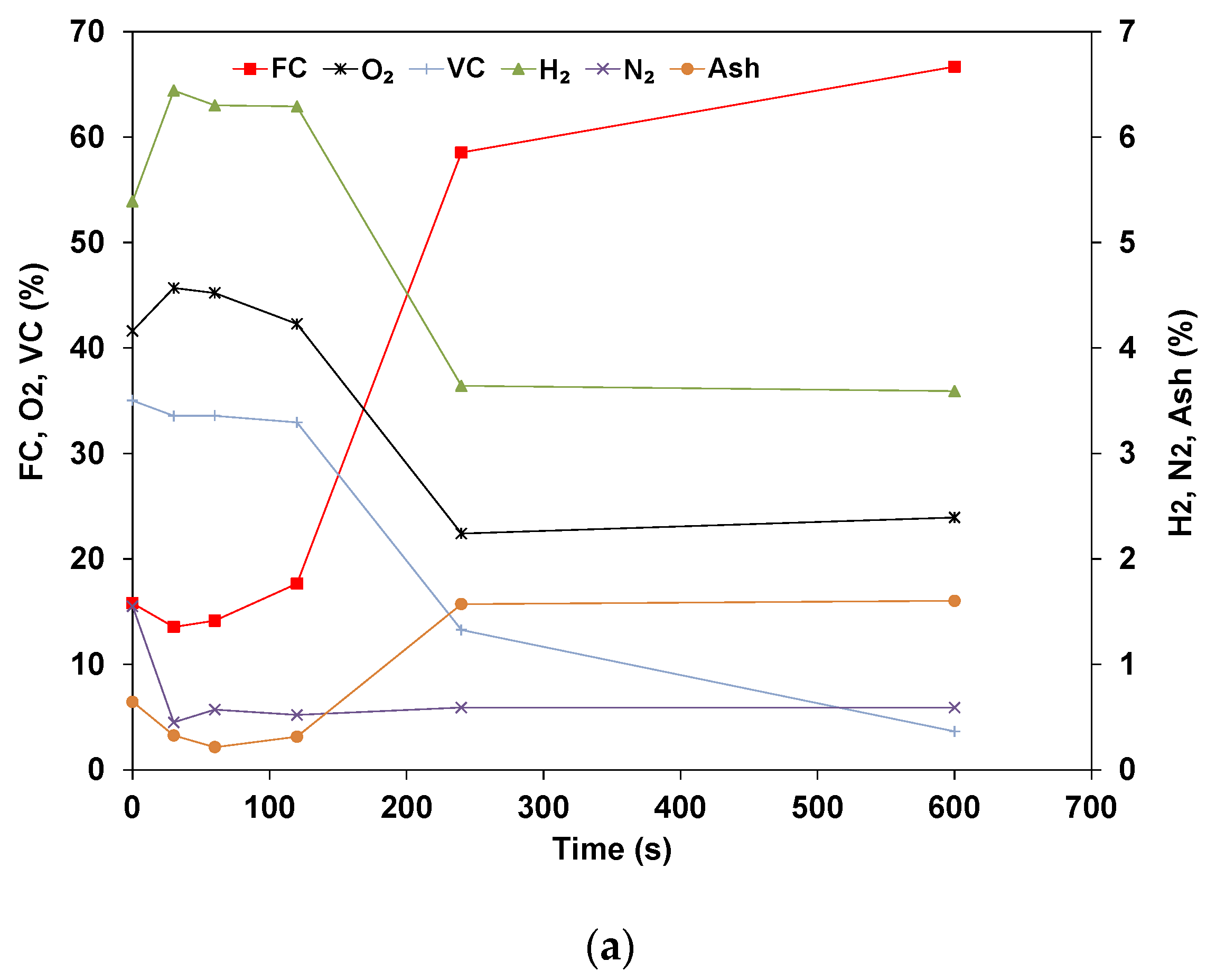
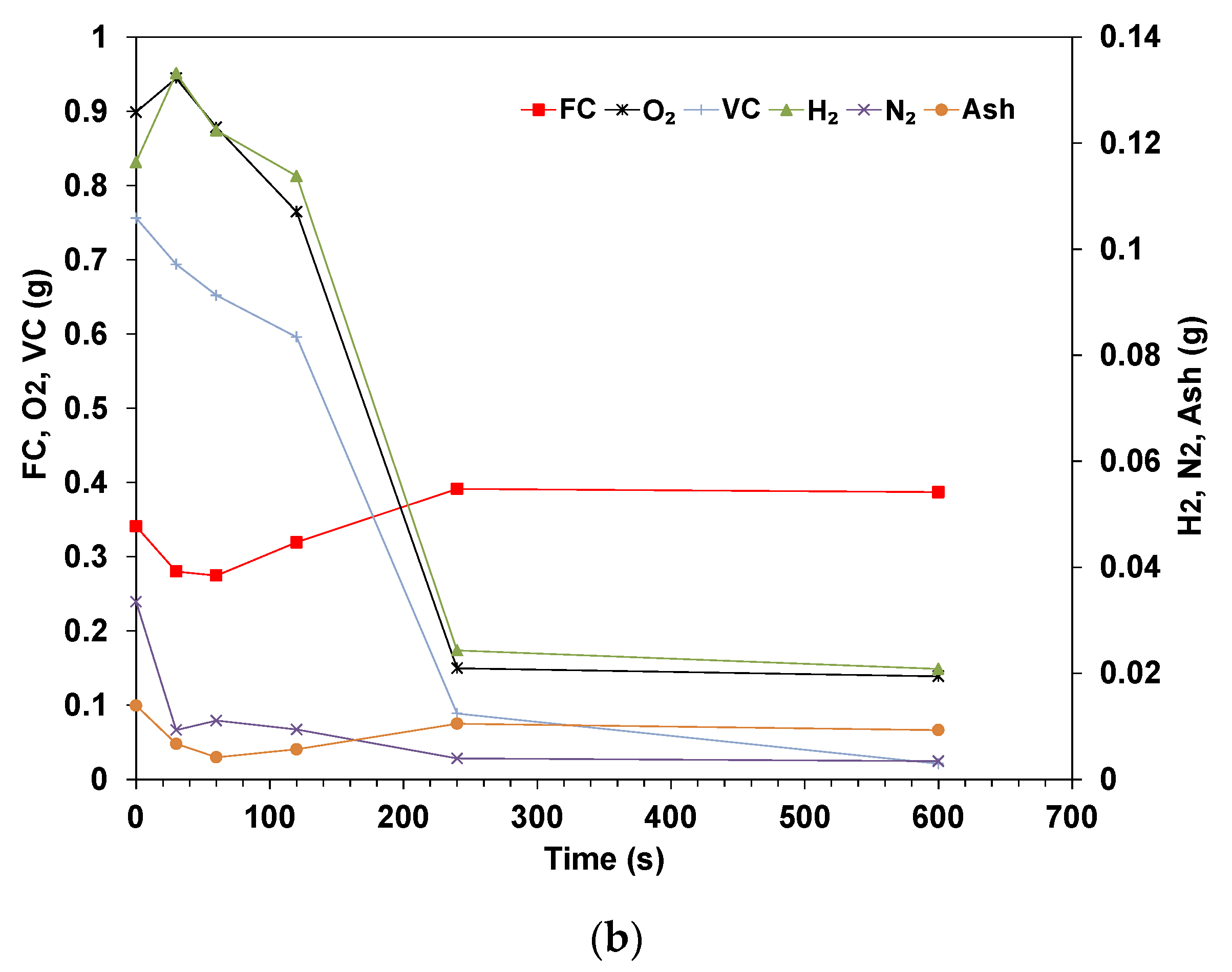
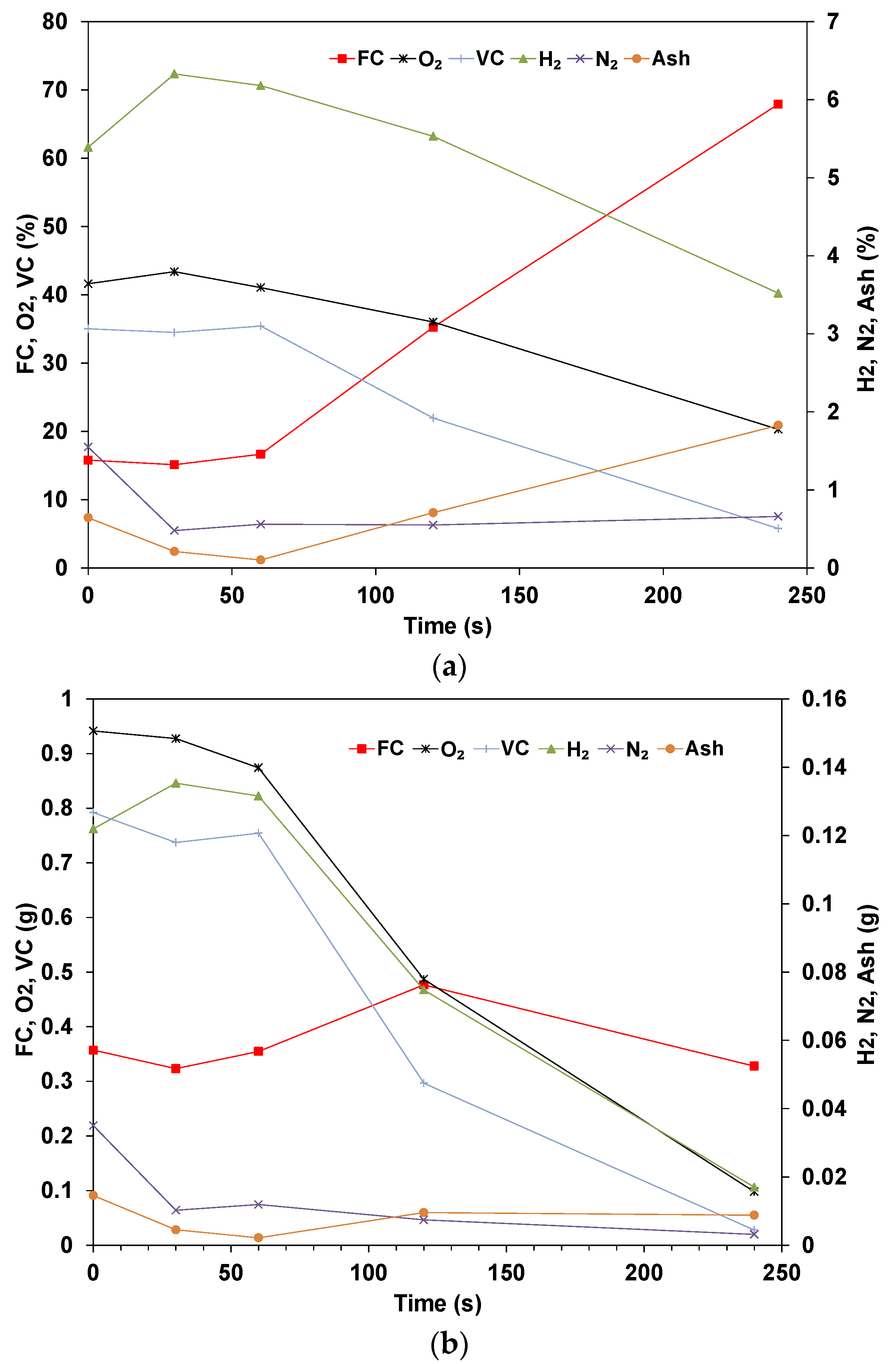
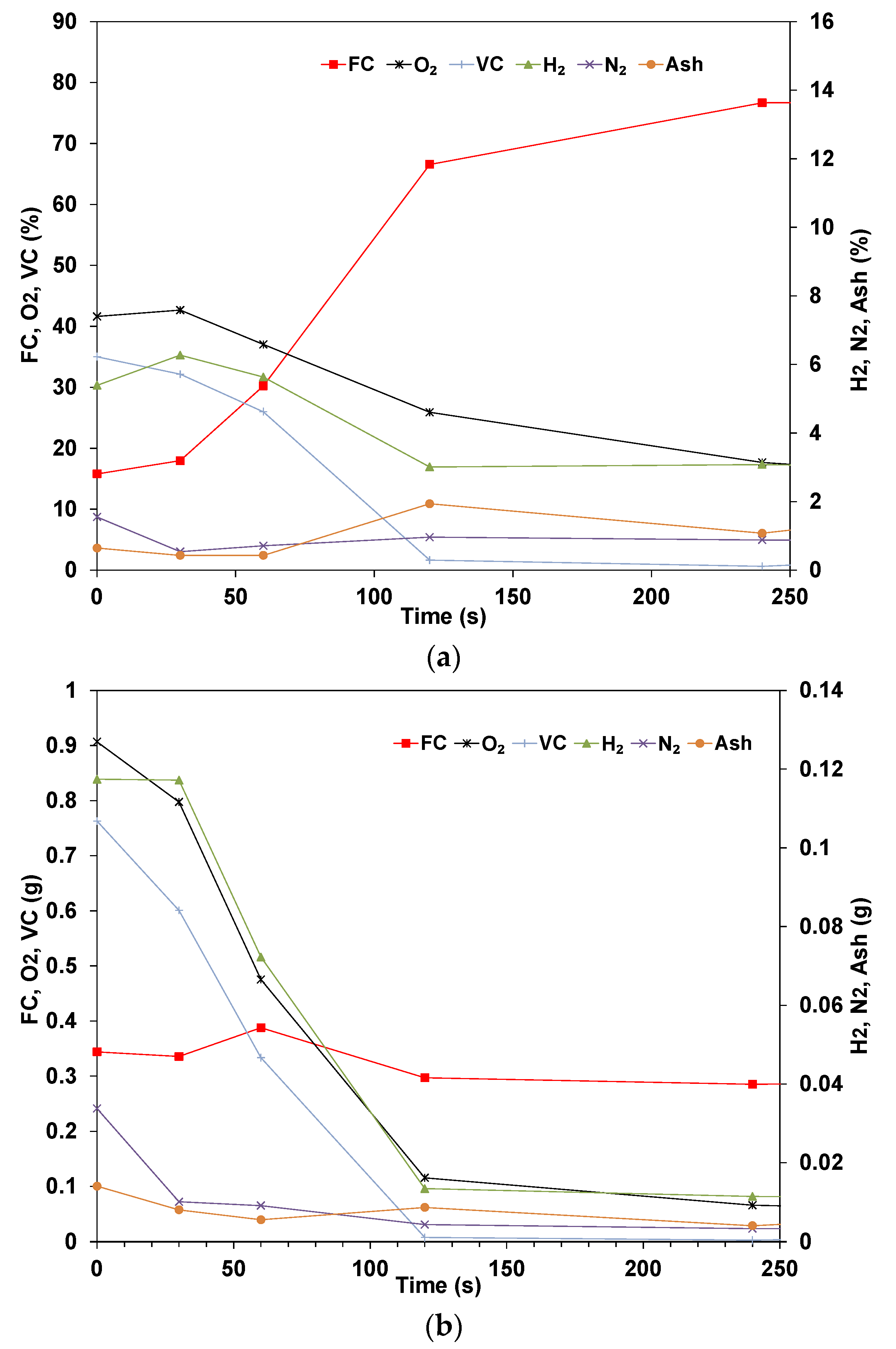
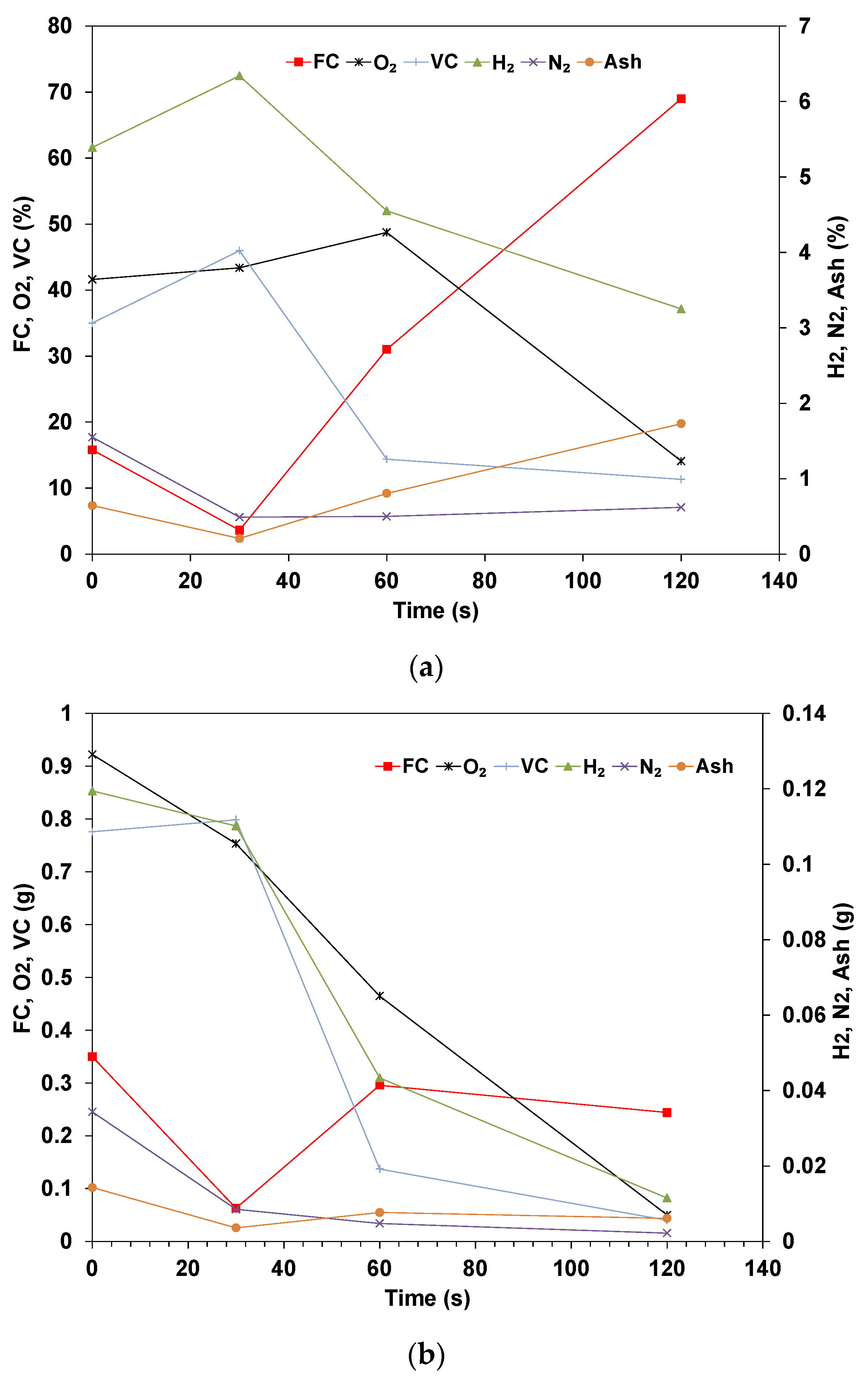
3.2. Elemental Analysis of Wood Pellets
4. Conclusions
- The mass losses increase for any specific temperature, and the higher the temperature, the faster the pellets release the volatile matter. Devolatilization occurs at a very slow rate at low temperatures. The maximum mass loss obtained at the highest temperatures (650 and 734 °C) for a 1-h test leveled off at about 97%, with the remaining substances, including fixed carbon and ashes, being about 3%. The mass loss of dry pellets increases with the temperature, and at a higher temperature, the dry pellets devolatilize faster than wet pellets.
- The elemental results show that the volatile matter is released in the early stages while the ratio of fixed carbon and ash steadily increases. The rate of conversion for the volatile is similar for all compounds, though Nitrogen shows a higher rate of conversion. The mass of fixed carbon increases due to the Carbon diffusion from the volatile fraction. The decomposition of these compounds is dependent on the temperature and residence time applied.
- The total Carbon decreases with time as it is being oxidized, and the species concentration in the sample is expected to increase in concentration if their rate of volatilization is lower than others. The results for dry pellets show that the mass of H and O always decreases, which indicates that in the drying phase, some of the O and H are transferred from the water to the biomass’s structure. In addition, the decomposition of biomass is related to hemicellulose, cellulose, and lignin, which start at different temperatures and stages.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Nomenclature | |
| T | Temperature (K) |
| m | Mass (g) |
| t | Time (s) |
| Subscripts | |
| f | Final |
| l | Loss |
| i | Initial |
References
- Euh, S.H.; Kafle, S.; Choi, Y.S.; Oh, J.H.; Kim, D.H. A study on the effect of tar fouled on thermal efficiency of a wood pellet boiler: A performance analysis and simulation using Computation Fluid Dynamics. Energy 2016, 103, 305–312. [Google Scholar] [CrossRef]
- Gonzaga Fraga, L.; Carlos, F.; Teixeira, J.; Eduardo, C. Ferreira, M. The potential of renewable energy in Timor-Leste: An assessment for biomass. Energies 2019, 12, 1441. [Google Scholar] [CrossRef] [Green Version]
- Halim, S.A.; Swithenbank, J. Characterisation of Malaysian wood pellets and rubberwood using slow pyrolysis and microwave technology. J. Anal. Appl. Pyrolysis 2016, 122, 64–75. [Google Scholar] [CrossRef]
- Kraiem, N.; Lajili, M.; Limousy, L.; Said, R.; Jeguirim, M. Energy recovery from Tunisian agri-food wastes: Evaluation of combustion performance and emissions characteristics of green pellets prepared from tomato residues and grape marc. Energy 2016, 107, 409–418. [Google Scholar] [CrossRef]
- Harun, N.Y.; Afzal, M.T. Effect of particle size on mechanical properties of pellets made from biomass blends. Procedia Eng. 2016, 148, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Fraga, L.; Teixeira, J.C.F.; Ferreira, M.E.C. Technical review on biomass resource for wood pellets in Timor-Leste. In Proceedings of the 3rd International Conference, Production of Scientific Knowledge in Timor-Leste, Dili, Timor-Leste, 12–14 September 2018; pp. 1–13. [Google Scholar]
- Rabaçal, M.; Fernandes, U.; Costa, M. Combustion and emission characteristics of a domestic boiler fired with pellets of pine, industrial wood wastes and peach stones. Renew. Energy 2013, 51, 220–226. [Google Scholar] [CrossRef]
- Bäfver, L.S.; Leckner, B.; Tullin, C.; Berntsen, M. Particle emissions from pellets stoves and modern and old-type wood stoves. Biomass Bioenergy 2011, 35, 3648–3655. [Google Scholar] [CrossRef]
- Olsson, M. Residential Biomass Combustion—Emissions of Organic Compounds to Air from Wood Pellets and Other New Alternatives. Doctoral Thesis, Chalmers University of Technology, Gothenburg, Sweden, 2006. [Google Scholar]
- Stahl, M.K.R.; Henrich, E.; Gehrmann, H.J.; Vodegel, S. Definition of a standard biomass. Contract 2004, 1–14. Available online: http://www.renew-fuel.com/del_sp2_wp1_2-1-1_05-01-10-fzk7ff0.pdf?dl=del_sp2_wp1_2-1-1_05-01-10-fzk.pdf&kat=14 (accessed on 17 May 2018).
- Saarela, K.E.; Harju, L.; Rajander, J.; Lill, J.O.; Heselius, S.J.; Lindroos, A.; Mattsson, K. Elemental analyses of pine bark and wood in an environmental study. Sci. Total Environ. 2005, 343, 231–241. [Google Scholar] [CrossRef]
- Järvinen, T.; Agar, D. Experimentally determined storage and handling properties of fuel pellets made from torrefied whole-tree pine chips, logging residues and beech stem wood. Fuel 2014, 129, 330–339. [Google Scholar] [CrossRef]
- Melkior, T.; Barthomeuf, C.; Bardet, M. Inputs of solid-state NMR to evaluate and compare thermal reactivity of pine and beech woods under torrefaction conditions and modified atmosphere. Fuel 2017, 187, 250–260. [Google Scholar] [CrossRef]
- Paulauskas, R.; Džiugys, A.; Striūgas, N. Experimental investigation of wood pellet swelling and shrinking during pyrolysis. Fuel 2015, 142, 145–151. [Google Scholar] [CrossRef]
- Fagerström, J.; Steinvall, E.; Boström, D.; Boman, C. Alkali transformation during single pellet combustion of soft wood and wheat straw. Fuel Process. Technol. 2016, 143, 204–212. [Google Scholar] [CrossRef]
- Mehrabian, R.; Scharler, R.; Obernberger, I. Effects of pyrolysis conditions on the heating rate in biomass particles and applicability of TGA kinetic parameters in particle thermal conversion modelling. Fuel 2012, 93, 567–575. [Google Scholar] [CrossRef]
- Miccio, F.; Russo, S.; Silvestri, N. Assessment of the devolatilization behavior of fuel pellets in fluidized bed. Fuel Process. Technol. 2013, 115, 122–129. [Google Scholar] [CrossRef]
- González, J.F.; Ledesma, B.; Alkassir, A.; González, J. Study of the influence of the composition of several biomass pellets on the drying process. Biomass Bioenergy 2011, 35, 4399–4406. [Google Scholar] [CrossRef]
- Wang, G.; Silva, R.B.; Azevedo, J.L.T.; Martins-Dias, S.; Costa, M. Evaluation of the combustion behaviour and ash characteristics of biomass waste derived fuels, pine and coal in a drop tube furnace. Fuel 2014, 117, 809–824. [Google Scholar] [CrossRef]
- Chapela, S.; Porteiro, J.; Costa, M. Effect of the Turbulence-Chemistry Interaction in Packed-Bed Biomass Combustion. Energy Fuels 2017, 31, 9967–9982. [Google Scholar] [CrossRef]
- European Pellet Council. ENplus Quality Certification Scheme Part 3: Pellet Quality Requirements. Available online: https://www.enplus-pellets.eu/en-in/resources-en-in/technical-documentation-en-in.html#handbook (accessed on 17 May 2018).
- Hansen, M.T.; Jein, A.R.; Hayes, S.; Bateman, P. English Handbook for Wood Pellet Combustion. National Energy Foundation: Milton Keynes, UK, 2009. [Google Scholar]
- Neves, D.; Thunman, H.; Matos, A.; Tarelho, L.; Gómez-Barea, A. Characterization and prediction of biomass pyrolysis products. Prog. Energy Combust. Sci. 2011, 37, 611–630. [Google Scholar] [CrossRef]
- Borman, L.G.; Ragland, W.K. Combustion Engineering; McGraw-Hill: New York, NY, USA, 1998. [Google Scholar]
- Yeo, J.Y.; Chin, B.L.F.; Tan, J.K.; Loh, Y.S. Comparative studies on the pyrolysis of cellulose, hemicellulose, and lignin based on combined kinetics. J. Energy Inst. 2019, 92, 27–37. [Google Scholar] [CrossRef]
- Madzaki, H.; Karimghani, W.A.W.A.; NurzalikhaRebitanim; AzilBahariAlias. Carbon Dioxide Adsorption on Sawdust Biochar. Procedia Eng. 2016, 148, 718–725. [Google Scholar] [CrossRef] [Green Version]



| Sample | Proximate Analysis, (%) | Ultimate Analysis, (%) | LHV (MJ/kg) | References | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moisture | VM | Ash | Fixed Carbon | C | H | N | S | O | CO2 (kg/MJ) | |||
| Pine pellets | 6.90 | 77.80 | 0.60 | 14.70 | 50.80 | 5.39 | 1.55 | 0.037 | 42.22 | - | 17.10 | Recent study |
| Whole-tree pine chips | 7.89 | 83.2 | 0.5 | 15.8 | 49.7 | 6.2 | 0.17 | - | 43.6 | - | 17.13 | [12] |
| Pine pellets | 6.65 | 76.62 | 2.50 | 14.23 | 46.8 | 5.99 | 0.675 | 0.021 | - | 0.0979 | 17.53 | [18] |
| Pine branches | 12.5 | 63.7 | 2.6 | 21.2 | 46.6 | 6.3 | 0.9 | <0.02 | 31.1 | - | 17 | [19] |
| Pine pellets | 7.3 | 80.5 | 1.3 | 10.9 | 46.0 | 6.2 | 0.5 | <0.01 | 47.3 | - | 17.1 | [20] |
| Proximate Analysis (wt.%, as Received) | Ultimate Analysis (wt.%, Dry Ash-Free) | ||
|---|---|---|---|
| Moisture | 6.90 | Carbon | 50.80 |
| Volatile matter | 77.80 | Hydrogen | 5.39 |
| Ash | 0.60 | Nitrogen | 1.55 |
| Fixed carbon | 14.70 | Sulphur | 0.037 |
| Lower Heating Value (MJ/kg) | 17.10 | Oxygen | 42.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraga, L.G.; Silva, J.; Teixeira, J.C.; Ferreira, M.E.C.; Teixeira, S.F.; Vilarinho, C.; Gonçalves, M.M. Study of Mass Loss and Elemental Analysis of Pine Wood Pellets in a Small-Scale Reactor. Energies 2022, 15, 5253. https://doi.org/10.3390/en15145253
Fraga LG, Silva J, Teixeira JC, Ferreira MEC, Teixeira SF, Vilarinho C, Gonçalves MM. Study of Mass Loss and Elemental Analysis of Pine Wood Pellets in a Small-Scale Reactor. Energies. 2022; 15(14):5253. https://doi.org/10.3390/en15145253
Chicago/Turabian StyleFraga, Lelis Gonzaga, João Silva, José Carlos Teixeira, Manuel E. C. Ferreira, Senhorinha F. Teixeira, Cândida Vilarinho, and Maria Margarida Gonçalves. 2022. "Study of Mass Loss and Elemental Analysis of Pine Wood Pellets in a Small-Scale Reactor" Energies 15, no. 14: 5253. https://doi.org/10.3390/en15145253
APA StyleFraga, L. G., Silva, J., Teixeira, J. C., Ferreira, M. E. C., Teixeira, S. F., Vilarinho, C., & Gonçalves, M. M. (2022). Study of Mass Loss and Elemental Analysis of Pine Wood Pellets in a Small-Scale Reactor. Energies, 15(14), 5253. https://doi.org/10.3390/en15145253










