Peptide Materials in Dye Sensitized Solar Cells
Abstract
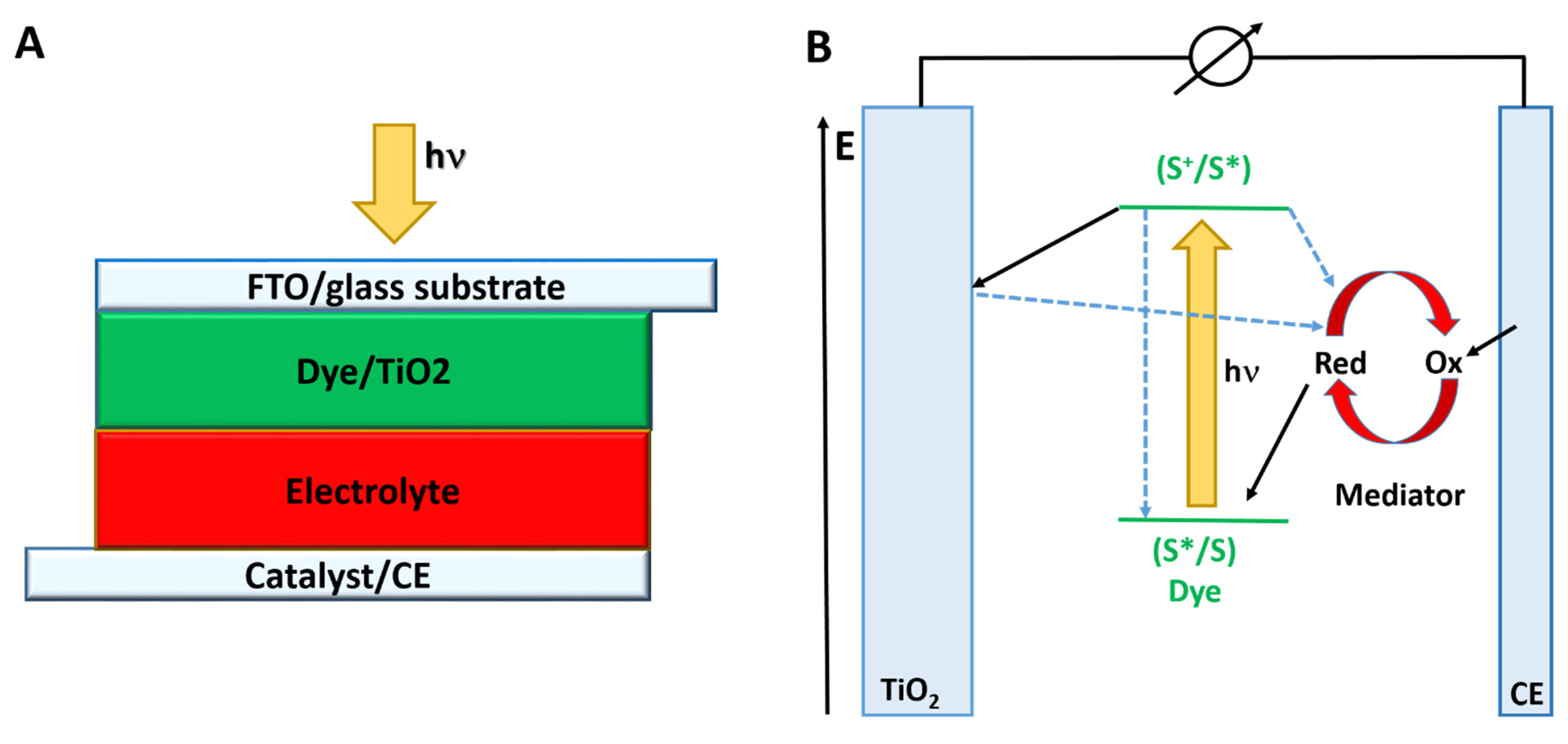
:1. Introduction
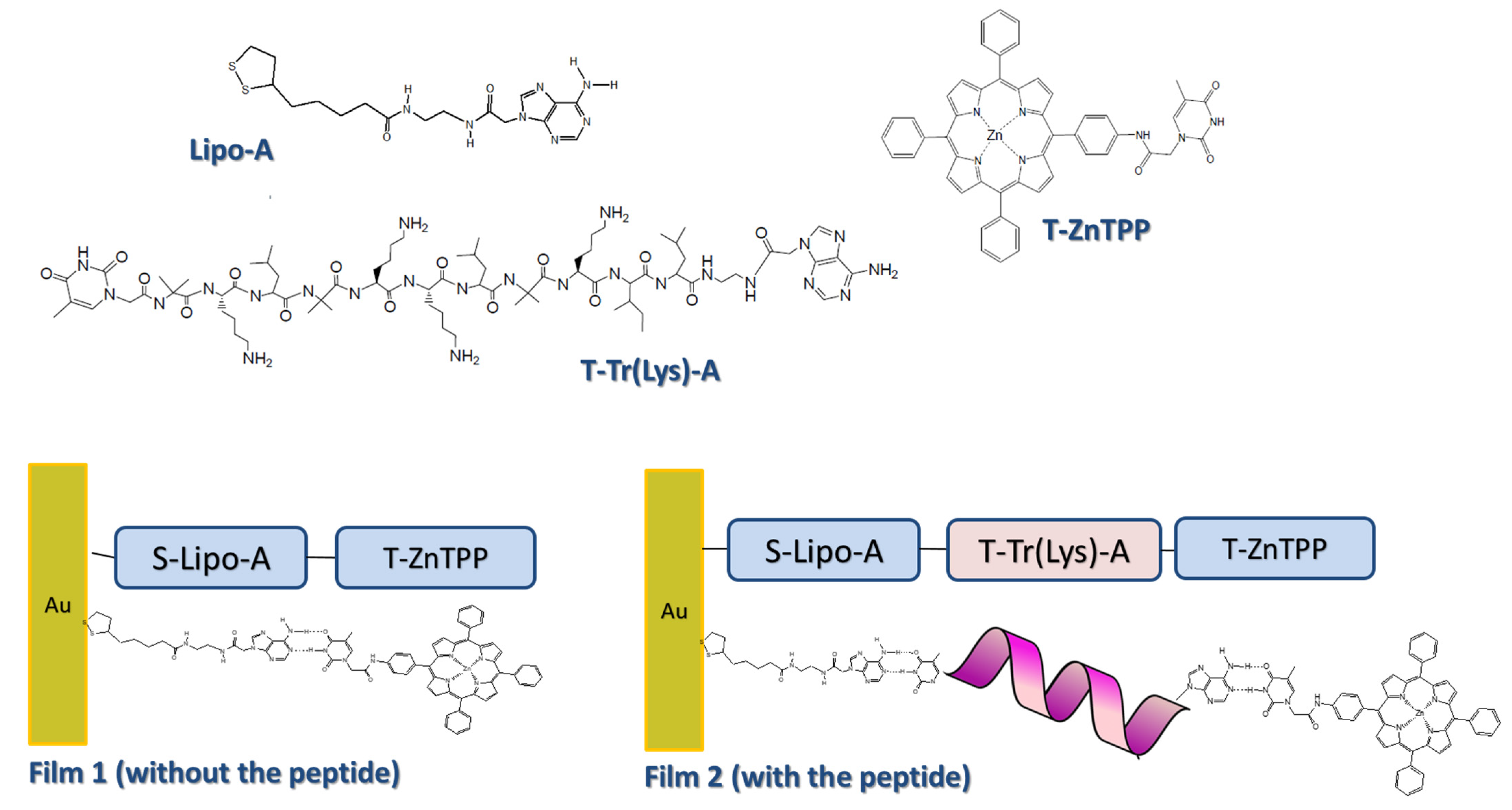
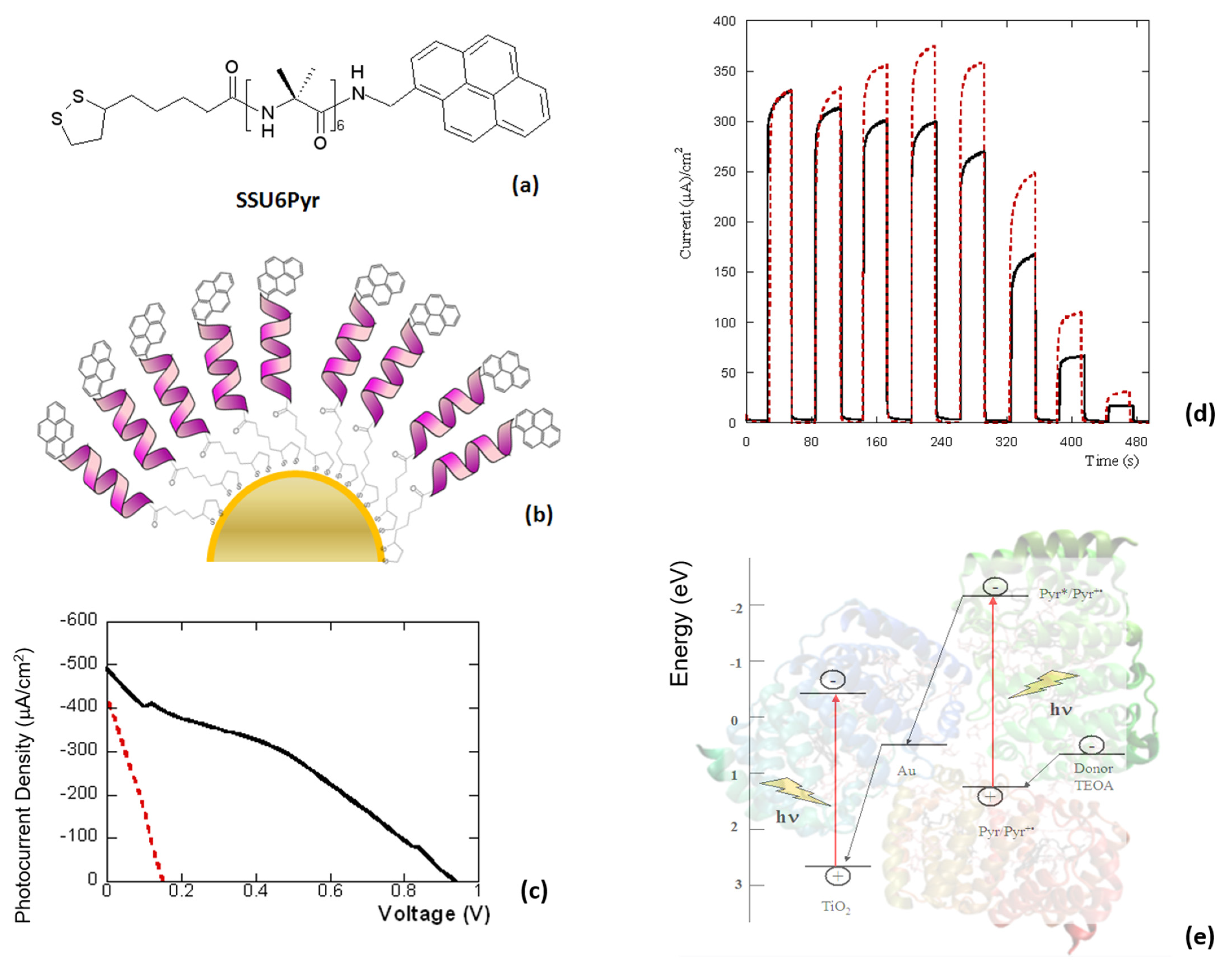
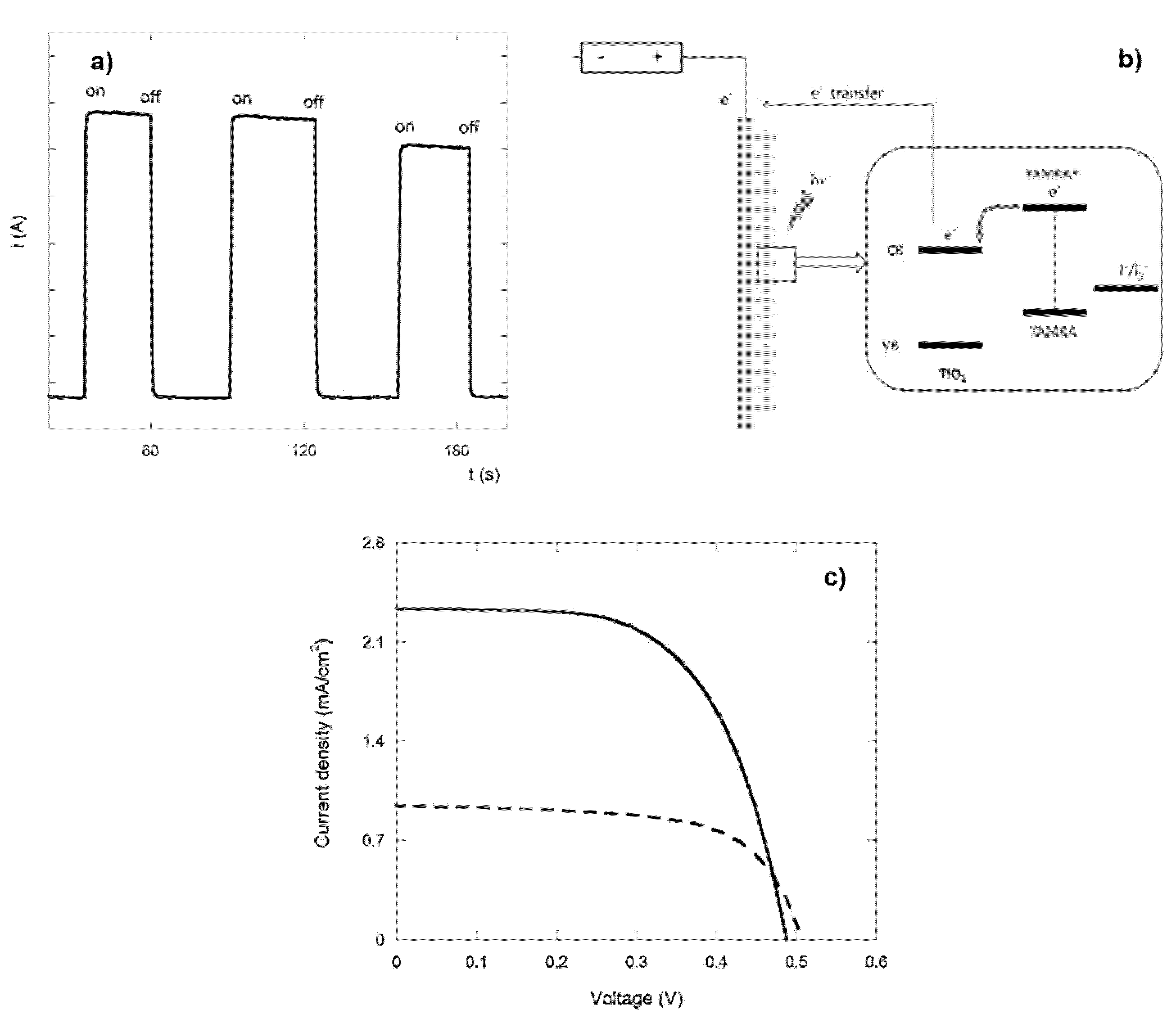
2. Peptides as Dyes
3. Peptides as Templating Materials
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beller, M.; Centi, G.; Sun, L. Chemistry Future: Priorities and Opportunities from the Sustainability Perspective. ChemSusChem 2017, 10, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Su, D.S. (Ed.) Special Issue: Energy Conversion and Storage. ChemSusChem 2012, 5, 441–599. [Google Scholar]
- Gong, J.; Li, C.; Wasielewski, M.R. Advances in solar energy conversion. Chem. Soc. Rev. 2019, 48, 1862–1864. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, G.W.; Lewis, N.S. Solar energy conversion. Phys. Today 2007, 60, 37–42. [Google Scholar] [CrossRef]
- Ginley, D.; Green, M.A.; Collins, R. Solar Energy Conversion toward 1 Terawatt. MRS Bull. 2011, 33, 355–364. [Google Scholar] [CrossRef] [Green Version]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Kalyanasundaram, K. Dye-Sensitized Solar Cells; EPFL Press: Lausanne, Switzerland, 2010. [Google Scholar]
- Nath, N.C.D.; Lee, J.J. Binary redox electrolytes used in dye-sensitized solar cells. J. Ind. Eng. Chem. 2019, 78, 53–65. [Google Scholar] [CrossRef]
- Ye, M.; Wen, X.; Wang, M.; Iocozzia, J.; Zhang, N.; Lin, C.; Lin, Z. Recent advances in dye-sensitized solar cells: From photoanodes, sensitizers and electrolytes to counter electrodes. Mater. Today 2015, 18, 155–162. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Khanna, S.; Sundaram, S.; Reddy, K.S.; Mallick, T.K. Performance analysis of perovskite and dye-sensitized solar cells under varying operating conditions and comparison with monocrystalline silicon cell. Appl. Therm. Eng. 2017, 127, 559–565. [Google Scholar] [CrossRef]
- Dominici, L.; Vesce, L.; Colonna, D.; Michelotti, F.; Brown, T.M.; Reale, A.; Di Carlo, A. Angular and prism coupling refractive enhancement in dye solar cells. Appl. Phys. Lett. 2010, 96, 13302. [Google Scholar] [CrossRef]
- Parisi, M.L.; Maranghi, S.; Vesce, L.; Sinicropi, A.; Di Carlo, A.; Basosi, R. Prospective life cycle assessment of third-generation photovoltaics at the pre-industrial scale: A long-term scenario approach. Renew. Sustain. Energy Rev. 2020, 121, 109703. [Google Scholar] [CrossRef]
- Borella, L.; Vesce, L.; Mariani, P.; Barichello, J.; Di Carlo, A.; Trivellin, N.; Sforza, E. Spectral changes by dye sensitized solar modules influence the pigment composition and productivity of arthrospira maxima and increase the overall energy efficiency. Adv. Sustain. Syst. 2022, 6, 2100346. [Google Scholar] [CrossRef]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; Curchod, B.F.E.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, M.K.; Grätzel, M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Stojanovic, M.; Ren, Y.; Cao, Y.; Eickemeyer, F.T.; Socie, E.; Vlachopoulos, N.; Moser, J.-E.; Zakeeruddin, S.M.; Hagfeldt, A.; et al. A molecular photosensitizer achieves a Voc of 1.24 V enabling highly efficient and stable dye-sensitized solar cells with copper(II/I)-based electrolyte. Nat. Commun. 2021, 12, 1777. [Google Scholar] [CrossRef] [PubMed]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.-I.; Hanaya, M. Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 2015, 51, 15894–15897. [Google Scholar] [CrossRef]
- Ji, J.-M.; Zhou, H.; Eom, Y.K.; Kim, C.H.; Kim, H.K. 14.2% Efficiency Dye-Sensitized Solar Cells by Co-sensitizing Novel Thieno[3,2-b]indole-Based Organic Dyes with a Promising Porphyrin Sensitizer. Adv. Energy Mater. 2020, 10, 2000124. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, V.; Sharma, S.S. Dye-Sensitized Solar Cells: Fundamentals and Current Status. Nanoscale Res. Lett. 2018, 28, 381–427. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Arakawa, H. Handbook of Photovoltaic Science and Engineering; Luque, A., Hegedus, S., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2003; pp. 663–700. [Google Scholar]
- Green, M.A.; Dunlop, E.D.; Hohl-Ebinger, J.; Yoshita, M.; Kopidakis, N.; Hao, X. Solar cell efficiency tables (version 59). Prog. Photovolt. Res. Appl. 2022, 30, 3–12. [Google Scholar] [CrossRef]
- Wan, J.; Tao, L.; Wang, B.; Zhang, J.; Wang, H.; Lund, P.D. A facile method to produce TiO2 nanorods for high-efficiency dye solar cells. J. Power Sources 2019, 438, 227012. [Google Scholar] [CrossRef]
- Zardetto, V.; De Angelis, G.; Vesce, L.; Caratto, V.; Mazzuca, C.; Gasiorowski, J.; Reale, A.; Di Carlo, A.; Brown, T.M. Formulations and processing of nanocrystalline TiO2 films for the different requirements of plastic, metal and glass dye solar cell applications. Nanotechnology 2013, 24, 255401. [Google Scholar] [CrossRef]
- Vesce, L.; Riccitelli, R.; Mincuzzi, G.; Orabona, A.; Soscia, G.; Brown, T.M.; Di Carlo, A.; Reale, A. Fabrication of spacer and catalytic layers in monolithic dye-sensitized solar cells. IEEE J. Photovolt. 2013, 3, 1004–1011. [Google Scholar] [CrossRef]
- Mincuzzi, G.; Vesce, L.; Liberatore, M.; Reale, A.; Di Carlo, A.; Brown, T.M. Laser-sintered TiO2 films for dye solar cell fabrication: An electrical, morphological, and electron lifetime investigation. IEEE Trans. Electron Devices 2011, 58, 3179–3188. [Google Scholar] [CrossRef]
- Vesce, L.; Riccitelli, R. Processing and characterization of a TiO2 paste based on small particle size powders for dye-sensitized solar cell semi-transparent photo-electrodes. Prog. Photovolt. Res. Appl. 2012, 20, 960–966. [Google Scholar] [CrossRef]
- Ozawa, H.; Sugiura, T.; Kuroda, T.; Nozawa, K.; Arakawa, H. Highly efficient dye-sensitized solar cells based on a ruthenium sensitizer bearing a hexylthiophene modified terpyridine ligand. J. Mater. Chem. A 2016, 4, 1762–1770. [Google Scholar] [CrossRef]
- Yella, A.; Mai, C.-L.; Zakeeruddin, S.-M.; Chang, S.-N.; Hsieh, C.-H.; Yeh, C.-Y.; Grätzel, M. Molecular Engineering of Push–Pull Porphyrin Dyes for Highly Efficient Dye-Sensitized Solar Cells: The Role of Benzene Spacers. Angew. Chem. Int. Ed. 2014, 53, 2973–2977. [Google Scholar] [CrossRef] [PubMed]
- Eom, Y.K.; Kang, S.H.; Choi, I.T.; Yoo, Y.; Kim, J.; Kim, H.K. Significant light absorption enhancement by a single heterocyclic unit change in the π-bridge moiety from thieno[3,2-b]benzothiophene to thieno[3,2-b]indole for high performance dye-sensitized and tandem solar cells. J. Mater. Chem. A 2017, 5, 2297–2308. [Google Scholar] [CrossRef]
- Zeng, K.; Chen, Y.; Zhu, W.-H.; Tian, H.; Xie, Y. Efficient Solar Cells Based on Concerted Companion Dyes Containing Two Complementary Components: An Alternative Approach for Cosensitization. J. Am. Chem. Soc. 2020, 142, 5154–5161. [Google Scholar] [CrossRef]
- Dessì, A.; Calamante, M.; Sinicropi, A.; Parisi, M.L.; Vesce, L.; Mariani, P.; Taheri, B.; Ciocca, M.; Di Carlo, A.; Zani, L.; et al. Thiazolo[5,4-d]thiazole-based organic sensitizers with improved spectral properties for application in greenhouse-integrated dye-sensitized solar cells. Sustain. Energy Fuels 2020, 4, 2309–2321. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, Y.; Zakeeruddin, S.M.; Hagfeldt, A.; Grätzel, M. Direct Contact of Selective Charge Extraction Layers Enables High-Efficiency Molecular Photovoltaics. Joule 2018, 2, 1108–1117. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-L.; Chang, T.-W.; Teng, H.; Wu, C.-G.; Chen, C.-Y.; Yang, Y.-M.; Lee, Y.-L. Highly efficient gel-state dye-sensitized solar cells prepared using poly(acrylonitrile-co-vinyl acetate) based polymer electrolytes. Phys. Chem. Chem. Phys. 2013, 15, 3640–3645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, Y.; Bahng, H.W.; Cao, Y.; Yi, C.; Saygili, Y.; Luo, J.; Liu, Y.; Kavan, L.; Moser, J.-E.; et al. Comprehensive control of voltage loss enables 11.7% efficient solid-state dye-sensitized solar cells. Energy Environ. Sci. 2018, 11, 1779–1787. [Google Scholar] [CrossRef] [Green Version]
- Munoz-García, A.B.; Benesperi, I.; Boschloo, G.; Concepcion, J.J.; Delcamp, J.H.; Gibson, E.A.; Meyer, G.J.; Pavone, M.; Pettersson, H.; Hagfeldt, A.; et al. Dye-sensitized solar cells strike back. Chem. Soc. Rev. 2021, 50, 12450–12550. [Google Scholar] [CrossRef]
- Vesce, L.; Guidobaldi, A.; Mariani, P.; Di Carlo, A.; Parisi, M.L.; Maranghi, S.; Basosi, R. World Scientific Reference of Hybrid Materials. World Scientific Publishing, Co., Pte Ltd.: Singapore, 2019; p. 423. [Google Scholar]
- Vesce, L.; Mariani, P.; Calamante, M.; Dessì, A.; Mordini, A.; Zani, L.; Di Carlo, A. Process engineering of semitransparent DSSC modules and panel incorporating an organic sensitizer. Solar RRL 2022, in press. [Google Scholar] [CrossRef]
- Niu, G.; Guo, X.; Wang, L. Review of recent progress in chemical stability of perovskite solar cells. J. Mater. Chem. A 2015, 3, 8970–8980. [Google Scholar] [CrossRef]
- Ansaria, M.I.H.; Qurashib, A.; Nazeeruddinc, M.K. Frontiers, opportunities, and challenges in perovskite solar cells: A critical review. J. Photochem. Photobiol. C Photochem. Rev. 2018, 35, 1–24. [Google Scholar] [CrossRef]
- Asghar, M.I.; Zhang, J.; Wang, H.; Lund, P.D. Device stability of perovskite solar cells—A review. Renew. Sustain. Energy Rev. 2017, 77, 131–146. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Lee, J.W.; Jung, H.S.; Shin, H.; Park, N.G. High-Efficiency Perovskite Solar Cells. Chem. Rev. 2020, 120, 7867–7918. [Google Scholar] [CrossRef] [PubMed]
- Vesce, L.; Stefanelli, M.; Herterich, J.P.; Castriotta, L.A.; Kohlstädt, M.; Würfel, U.; Di Carlo, A. Ambient air blade-coating fabrication of stable triple-cation perovskite solar modules by green solvent quenching. Solar RRL 2021, 5, 2100073. [Google Scholar] [CrossRef]
- Freitag, M.; Boschloo, G. The revival of dye-sensitized solar cells. Curr. Opin. Electrochem. 2017, 2, 111–119. [Google Scholar] [CrossRef]
- Mariotti, N.; Bonomo, M.; Fagiolari, L.; Barbero, N.; Gerbaldi, C.; Bella, F.; Barolo, C. Recent advances in eco-friendly and cost-effective materials towards sustainable dye-sensitized solar cells. Green Chem. 2020, 22, 7168–7218. [Google Scholar] [CrossRef]
- Parisi, M.L.; Dessì, A.; Zani, L.; Maranghi, S.; Mohammadpourasl, S.; Calamante, M.; Mordini, A.; Basosi, R.; Reginato, G.; Sinicropi, A. Combined LCA and Green Metrics Approach for the Sustainability Assessment of an Organic Dye Synthesis on Lab Scale. Front. Chem. 2020, 8, 214. [Google Scholar] [CrossRef] [PubMed]
- Bella, F.; Galliano, S.; Falco, M.; Viscardi, G.; Barolo, C.; Grätzel, M.; Gerbaldi, C. Approaching truly sustainable solar cells by the use of water and cellulose derivatives. Green Chem. 2017, 19, 1043–1051. [Google Scholar] [CrossRef]
- Lee, C.P.; Li, C.T.; Ho, K.C. Use of organic materials in dye-sensitized solar cells. Mater. Today 2017, 20, 267–283. [Google Scholar] [CrossRef]
- Bella, F.; Gerbaldi, C. Natural Polymers for Dye-Sensitized Solar Cells: Electrolytes and Electrodes. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Schoden, F.; Dotter, M.; Knefelkamp, D.; Blachowicz, T.; Schwenzfeier Hellkamp, E. Review of State of the Art Recycling Methods in the Context of Dye Sensitized Solar Cells. Energies 2021, 14, 3741. [Google Scholar] [CrossRef]
- Pandey, S.; Karakoti, M.; Surana, K.; Dhapola, P.S.; Bhushan, B.S.; Ganguly, S.; Singh, P.K.; Abbas, A.; Srivastava, A.; Sahoo, N.G. Graphene nanosheets derived from plastic waste for the application of DSSCs and supercapacitors. Sci. Rep. 2021, 11, 3916. [Google Scholar] [CrossRef] [PubMed]
- Soudi, N.; Nanayakkara, S.; Jahed, N.M.S.; Naahidi, S. Rise of nature-inspired solar photovoltaic energy convertors. Sol. Energy 2020, 208, 31–45. [Google Scholar] [CrossRef]
- Page, C.C.; Moser, C.C.; Chen, X.; Dutton, P.L. Natural Engineering Principles of Electron Tunneling in Biological Oxidation-Reduction. Nature 1999, 402, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Wasielewski, M.R. Photoinduced electron transfer in supramolecular systems for artificial photosynthesis. Chem. Rev. 1992, 92, 435–461. [Google Scholar] [CrossRef]
- Beratan, D.N.; Onuchic, J.N.; Winkler, J.R.; Gray, H.B. Electron-tunneling pathways in proteins. Science 1992, 258, 1740–1741. [Google Scholar] [CrossRef]
- Sek, S.; Misicka, A.; Swiatek, K.; Maicka, E. Asymmetry of electron transmission through monolayers of helical polyalanine adsorbed on gold surfaces. J. Phys. Chem. B 2005, 109, 18433–18438. [Google Scholar] [CrossRef] [PubMed]
- Galoppini, E.; Fox, M.A. Effect of the electric field generated by the helix dipole on photoinduced intramolecular Electron Transfer in dichromophoric α-helical peptides. J. Am. Chem. Soc. 1996, 118, 2299–2300. [Google Scholar] [CrossRef]
- Fox, M.A.; Galoppini, E. Electric field effect on Electron Transfer rates in dichromophoric peptides: The effect of helix unfolding. J. Am. Chem. Soc. 1997, 119, 5277–5285. [Google Scholar] [CrossRef]
- Watanabe, J.; Morita, T.; Kimura, S. Effects of dipole moment, linkers and chromophores at side chains on long-range Electron Transfer through helical peptides. J. Phys. Chem. B 2005, 109, 14416–14425. [Google Scholar] [CrossRef] [PubMed]
- Gatto, E.; Porchetta, A.; Stella, L.; Guryanov, I.; Formaggio, F.; Toniolo, C.; Kaptein, B.; Broxterman, Q.B.; Venanzi, M. Conformational Effects on the Electron-Transfer Efficiency in Peptide Foldamers Based on α,α-Disubstituted Glycyl Residues. Chem. Biodivers. 2008, 5, 1263–1278. [Google Scholar] [CrossRef]
- Gatto, E.; Stella, L.; Baldini, C.; Venanzi, M.; Toniolo, C.; Formaggio, F. Photocurrent generation in peptide-based self-assembled monolayers on gold electrodes. Superlatt. Microstruct. 2009, 46, 34–39. [Google Scholar] [CrossRef]
- Gatto, E.; Porchetta, A.; Scarselli, M.; De Crescenzi, M.; Formaggio, F.; Toniolo, C.; Venanzi, M. Playing with Peptides: How to Build a Supramolecular Peptide Nanostructure by Exploiting Helix···Helix Macrodipole Interaction. Langmuir 2012, 28, 2817–2826. [Google Scholar] [CrossRef] [PubMed]
- Venanzi, M.; Gatto, E.; Caruso, M.; Porchetta, A.; Formaggio, F.; Toniolo, C. Photoinduced Electron Transfer through Peptide-Based Self-Assembled Monolayers Chemisorbed on Gold Electrodes: Directing the Flow-in and Flow-out of Electrons through Peptide Helices. J. Phys. Chem. A 2014, 118, 6674–6684. [Google Scholar] [CrossRef]
- Shin, Y.K.; Newton, M.D.; Isied, S.S. Distance dependence of Electron Transfer across peptides with different secondary structures: The role of peptide energetic and electronic coupling. J. Am. Chem. Soc. 2003, 125, 3722–3732. [Google Scholar] [CrossRef] [PubMed]
- Creager, S.; Yu, C.J.; Bamdad, C.; O’Connor, S.; MacLean, T.; Lam, E.; Chong, Y.; Olsen, G.T.; Luo, J.; Gozin, M.; et al. Electron Transfer at Electrodes through Conjugated “Molecular Wire” Bridges. J. Am. Chem. Soc. 1999, 121, 1059–1064. [Google Scholar] [CrossRef]
- Fan, F.-R.F.; Yao, Y.; Cai, L.; Cheng, L.; Tour, J.M.; Bard, A.J. Structure-Dependent Charge Transport and Storage in Self-Assembled Monolayers of Compounds of Interest in Molecular Electronics: Effects of Tip Material, Headgroup, and Surface Concentration. J. Am. Chem. Soc. 2004, 126, 4035–4042. [Google Scholar] [CrossRef] [PubMed]
- Davis, W.B.; Svec, W.A.; Ratner, M.A.; Wasielewski, M.R. Molecular-wire behaviour in p-phenylenevinylene oligomers. Nature 1998, 396, 60–63. [Google Scholar] [CrossRef]
- Sikes, H.D.; Smalley, J.F.; Dudek, S.P.; Cook, A.R.; Newton, M.D. Rapid electron tunneling through oligophenylenevinylene bridges. Science 2001, 291, 1519–1523. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.K.; Hall, D.O.; Vlachopoulos, N.; Grätzel, M.; Evans, M.C.V.; Seibert, M. Photoelectrochemical responses of photosystem II particles immobilized on dye-derivatized TiO2 films. J. Photochem. Photobiol. B 1990, 5, 379–389. [Google Scholar] [CrossRef]
- Ciesielski, P.N.; Scott, A.M.; Faulkner, C.J.; Berron, B.J.; Cliffel, D.E.; Jennings, G.K. Functionalized Nanoporous Gold Leaf Electrode Films for the Immobilization of Photosystem I. ACS Nano 2008, 2, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Yehezkeli, O.; Tel-Vered, R.; Wasserman, J.; Trifonov, A.; Michaeli, D.; Nechushtai, R.; Willner, I. Integrated photosystem II-based photo-bioelectrochemical cells. Nat. Commun. 2012, 3, 742–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onoda, A.; Kakikura, Y.; Uematsu, T.; Kuwabata, S.; Hayashi, T. Photocurrent Generation of Hierarchical Zinc-substituted Hemoprotein Assemblies Immobilized on a Gold Electrode. Angew. Chem. Int. Ed. 2012, 51, 2628–2631. [Google Scholar] [CrossRef] [PubMed]
- Mershin, A.; Matsumoto, K.; Kaiser, L.; Yu, D.; Vaughn, M.; Nazeeruddin, M.K.; Bruce, B.D.; Graetzel, M.; Zhang, S. Self-assembled photosystem-I biophotovoltaics on nanostructured TiO2 and ZnO. Sci. Rep. 2012, 2, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, X.; Kalathil, S.; Reisner, E. Semi-biological approaches to solar-to-chemical conversion. Chem. Soc. Rev. 2020, 49, 4926–4952. [Google Scholar] [CrossRef] [PubMed]
- Thavasi, V.; Lazarova, T.; Filipek, S.; Kolinski, M.; Querol, E.; Kumar, A.; Ramakrishna, S.; Padrós, E.; Renugopalakrishnan, V. Study on the Feasibility of Bacteriorhodopsin as Bio-Photosensitizer in Excitonic Solar Cell: A First Report. J. Nanosci. Nanotechnol. 2008, 8, 1679–1687. [Google Scholar] [CrossRef] [Green Version]
- Yasutomi, S.; Morita, T.; Imanishi, Y.; Kimura, S. A molecular photodiode system that can switch photocurrent direction. Science 2004, 304, 1944–1947. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, K.; Morita, T.; Kimura, S. Efficient photocurrent generation by self-assembled monolayers composed of 310-helical peptides carrying linearly spaced Naphthyl groups at the side chains. J. Am. Chem. Soc. 2004, 126, 12780–12781. [Google Scholar] [CrossRef]
- Morita, T.; Kimura, S.; Kobayashi, S.; Imanishi, Y. Photocurrent generation under a large dipole moment formed by self-assembled monolayers of helical peptides having an N-Ethylcarbazolyl group. J. Am. Chem. Soc. 2000, 122, 2850–2859. [Google Scholar] [CrossRef]
- Yasutomi, S.; Morita, T.; Kimura, S. pH-controlled switching of photocurrent detection by self-assembled monolayer of helical peptides. J. Am. Chem. Soc. 2005, 127, 14564–14565. [Google Scholar] [CrossRef] [PubMed]
- Gatto, E.; Venanzi, M.; Palleschi, A.; Stella, L.; Pispisa, B.; Lorenzelli, L.; Toniolo, C.; Formaggio, F.; Marletta, G. Self-assembled peptide monolayers on interdigitated gold microelectrodes. Mater. Sci. Eng. C 2007, 27, 1309–1312. [Google Scholar] [CrossRef]
- Gatto, E.; Stella, L.; Formaggio, F.; Toniolo, C.; Lorenzelli, L.; Venanzi, M. Electroconductive and photocurrent generation properties of self-assembled monolayers formed by functionalized, conformationally constrained peptides on gold electrodes. J. Pept. Sci. 2008, 14, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Gatto, E.; Caruso, M.; Porchetta, A.; Toniolo, C.; Formaggio, F.; Crisma, M.; Venanzi, M. Photocurrent generation through peptide-based self-assembled monolayers on a gold surface: Antenna and junction effects. J. Pept. Sci. 2011, 17, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Gatto, E.; Venanzi, M. Self-assembled monolayers formed by helical peptide building blocks: A new tool for bioinspired nanotechnology. Polym. J. 2013, 45, 468–480. [Google Scholar] [CrossRef] [Green Version]
- Gatto, E.; Venanzi, M. Peptronics: Peptide Materials for Electron Transfer. In Peptide Materials from Nanostructures to Applications; Alemàn, C., Bianco, A., Venanzi, M., Eds.; Wiley-VCH: Weinheim, Germany, 2013; pp. 105–147. [Google Scholar]
- Gatto, E.; Venanzi, M. The Impervious Route to Peptide-Based Dye-Sensitized Solar Cells. Isr. J. Chem. 2015, 55, 671–681. [Google Scholar] [CrossRef]
- Gatto, E.; Kubitzky, S.; Schriever, M.; Cesaroni, S.; Mazzuca, C.; Venanzi, M.; De Zotti, M. Building Supramolecular DNA-Inspired Nanowires on Gold Surface: From 2D to 3D. Angew. Chem. 2019, 58, 7308–7312. [Google Scholar] [CrossRef]
- Kubitzky, S.; Venanzi, M.; Biondi, B.; Lettieri, R.; De Zotti, M.; Gatto, E. A pH-Induced Reversible Conformational Switch Able to Control the Photocurrent Efficiency in a Peptide Supramolecular System. Chem. Eur. J. 2021, 27, 2810–2817. [Google Scholar] [CrossRef] [PubMed]
- Umehara, S.; Pourmand, N.; Webb, C.D.; Davis, R.W.; Yasuda, K.; Karhanek, M. Current rectification with poly-L-lysine-coated quartz nanopipettes. Nano Lett. 2006, 6, 2486–2492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatto, E.; Quatela, A.; Caruso, M.; Tagliaferro, R.; De Zotti, M.; Formaggio, F.; Toniolo, C.; Di Carlo, A.; Venanzi, M. Mimicking Nature: A Novel Peptide-based Bio-inspired Approach for Solar Energy Conversion. ChemPhysChem 2014, 15, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Caruso, M.; Gatto, E.; Palleschi, A.; Morales, P.; Scarselli, M.; Casaluci, S.; Quatela, A.; Di Carlo, A.; Venanzi, M. A bioinspired dye sensitized solar cell based on a rhodamine-functionalized peptide immobilized on nanocrystalline TiO2. J. Photochem. Photobiol. A Chem. 2017, 347, 227–234. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Thelakkat, M.; Willert-Porada, M. Different mesoporous titania films for solid-state dye sensitised solar cells. Thin Solid Film. 2006, 511, 187–194. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, M.H.; Kim, H.J.; Lim, G.; Choi, Y.S.; Park, N.-G.; Kim, K.; Lee, W.I. Formation of Highly Efficient Dye-Sensitized Solar Cells by Hierarchical Pore Generation with Nanoporous TiO2 Spheres. Adv. Mater. 2009, 21, 3668–3673. [Google Scholar] [CrossRef]
- Schlichthörl, G.; Huang, S.; Sprague, J.; Frank, A. Band Edge Movement and Recombination Kinetics in Dye-Sensitized Nanocrystalline TiO2 Solar Cells: A Study by Intensity Modulated Photovoltage Spectroscopy. J. Phys. Chem. B 1997, 101, 8141–8155. [Google Scholar] [CrossRef]
- Grätzel, M. Recent Advances in Sensitized Mesoscopic Solar Cells. Acc. Chem. Res. 2009, 42, 1788–1798. [Google Scholar] [CrossRef] [PubMed]
- Han, T.H.; Moon, H.S.; Hwang, J.O.; Seok, S.I.; Im, S.H.; Kim, S.O. Peptide-templating dye-sensitized solar cells. Nanotechnology 2010, 21, 185601. [Google Scholar] [CrossRef]
- Acar, H.; Garifullin, R.; Aygun, L.E.; Okyayab, A.K.; Guler, M.O. Amyloid-like peptide nanofiber templated titania nanostructures as dye sensitized solar cell anodic materials. J. Mater. Chem. A 2013, 1, 10979–10984. [Google Scholar] [CrossRef] [Green Version]
- Reches, M.; Gazit, E. Casting metal nanowires within discrete self-assembled peptide nanotubes. Science 2003, 300, 625–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]

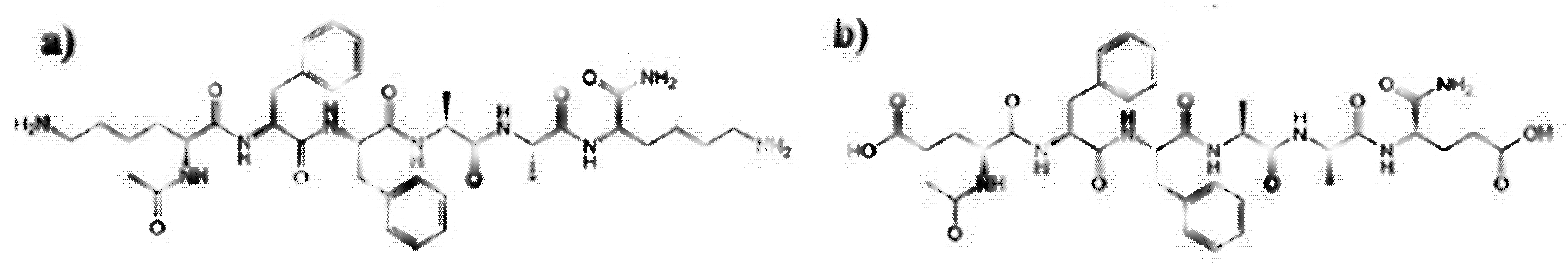

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatto, E.; Lettieri, R.; Vesce, L.; Venanzi, M. Peptide Materials in Dye Sensitized Solar Cells. Energies 2022, 15, 5632. https://doi.org/10.3390/en15155632
Gatto E, Lettieri R, Vesce L, Venanzi M. Peptide Materials in Dye Sensitized Solar Cells. Energies. 2022; 15(15):5632. https://doi.org/10.3390/en15155632
Chicago/Turabian StyleGatto, Emanuela, Raffaella Lettieri, Luigi Vesce, and Mariano Venanzi. 2022. "Peptide Materials in Dye Sensitized Solar Cells" Energies 15, no. 15: 5632. https://doi.org/10.3390/en15155632






