Thermal, Microstructural and Electrochemical Hydriding Performance of a Mg65Ni20Cu5Y10 Metallic Glass Catalyzed by CNT and Processed by High-Pressure Torsion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Thermal Characterization
2.3. Structural Characterization
2.4. Transmission Electron Microscopy
2.5. Electrochemical Hydriding
3. Results and Discussion
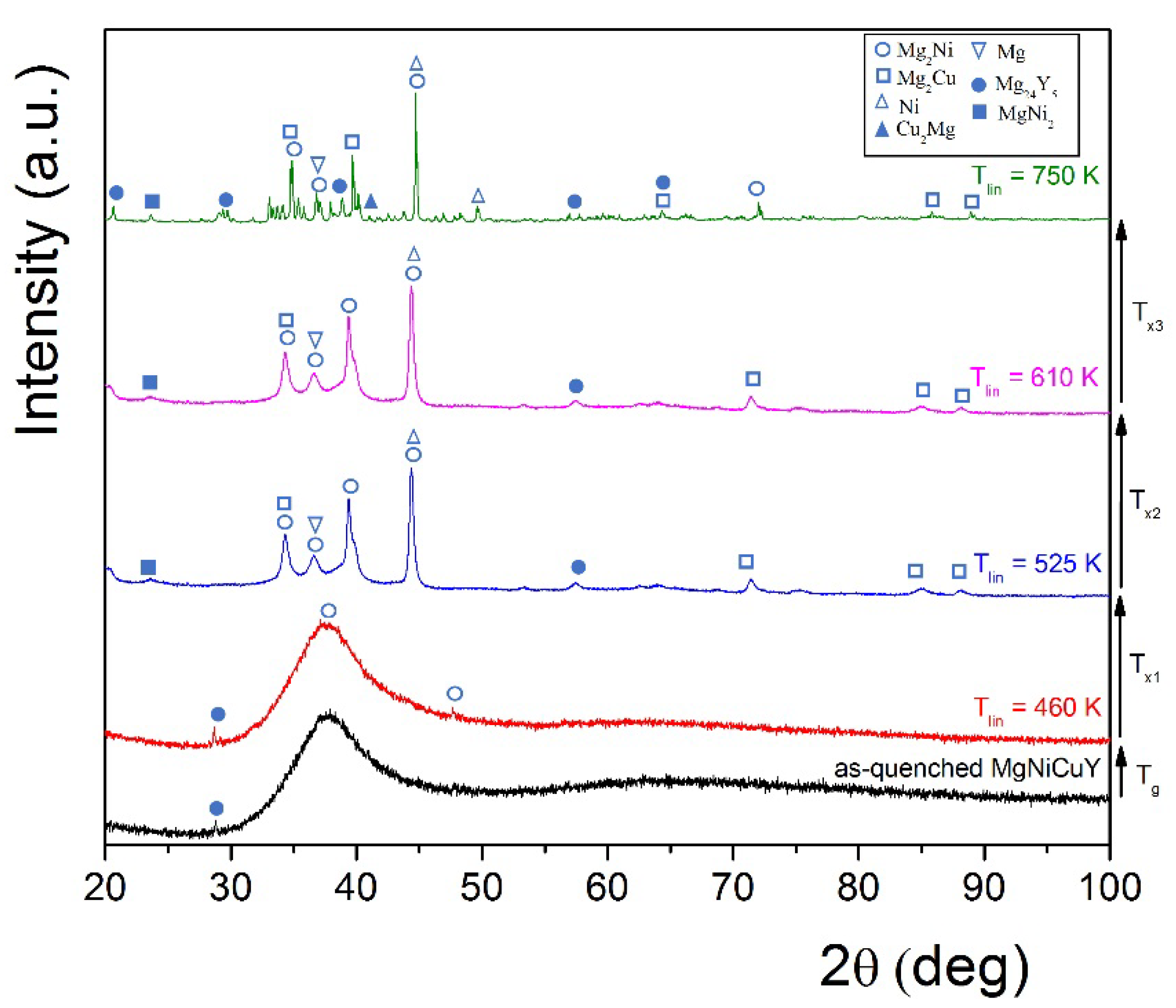
3.1. Characterization of the As-Spun Ribbon
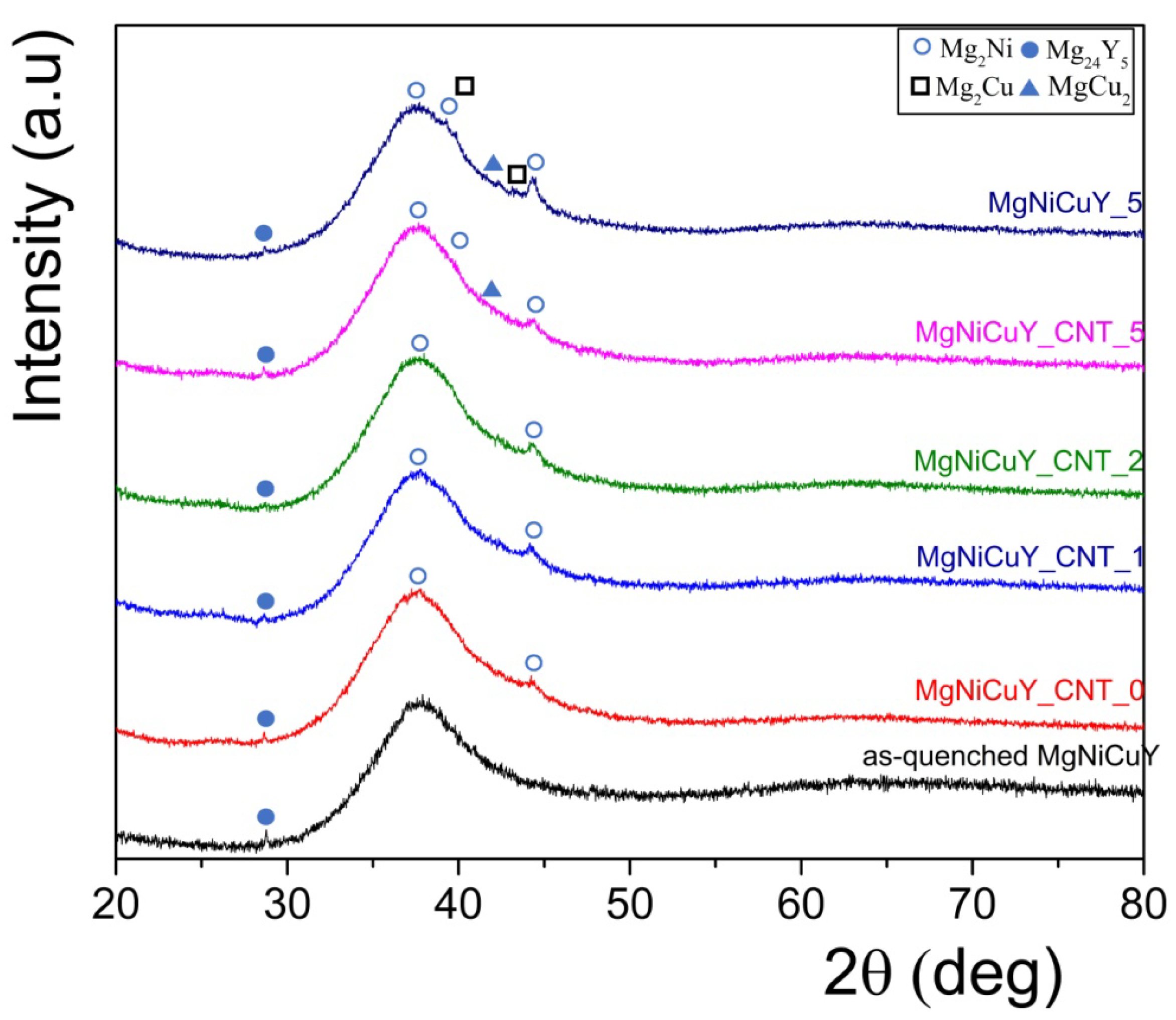
3.2. Characterization of HPT-Treated and CNT-Catalyzed Mg65Ni20Cu5Y10
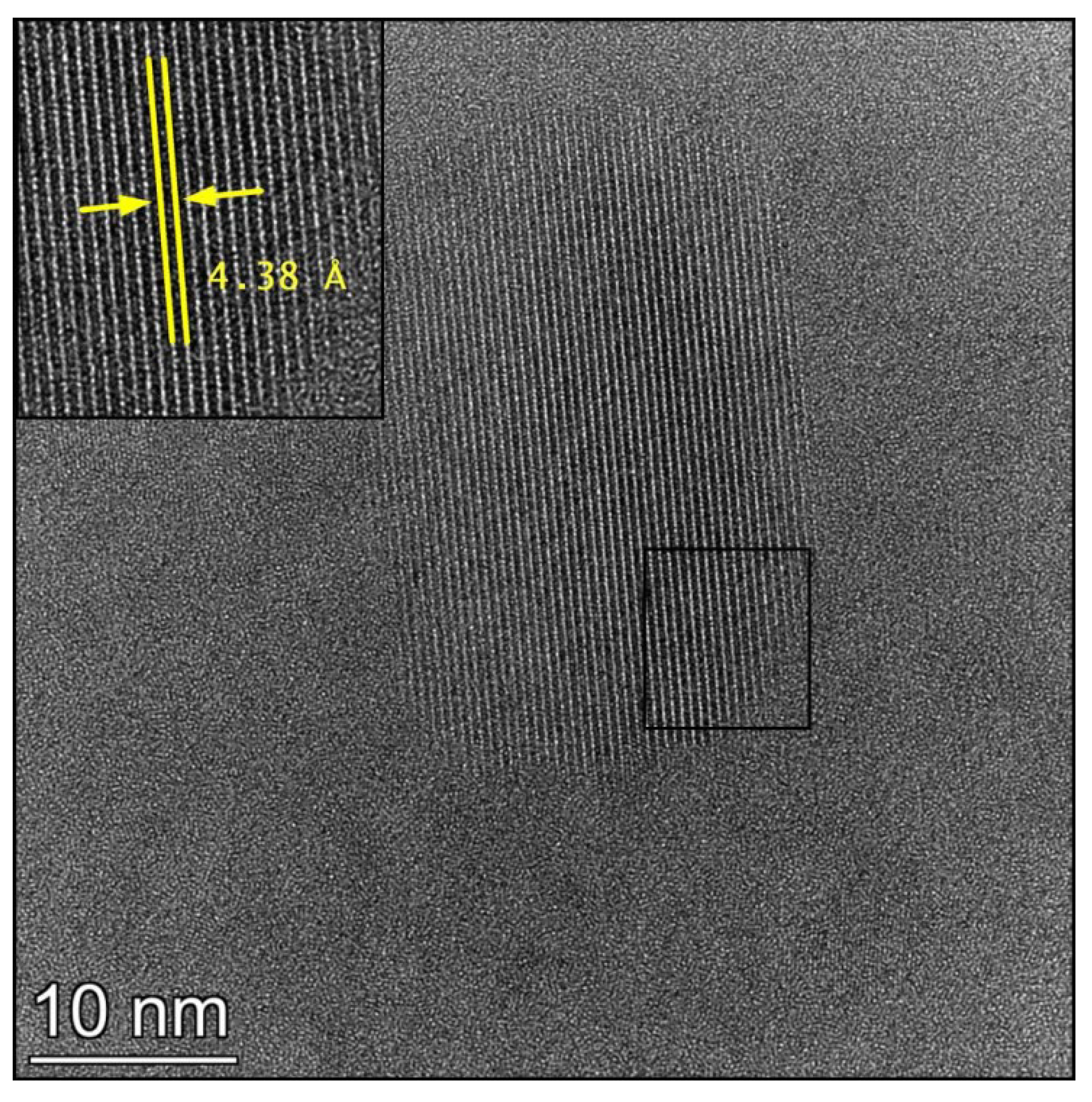
3.3. TEM Study on the MgNiCuY_CNT_5 HPT Disk
3.4. Electrochemical Experiments
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ren, J.; Musyoka, N.M.; Langmi, H.W.; Mathe, M.; Liao, S. Current Research Trends and Perspectives on Materials-Based Hydrogen Storage Solutions: A Critical Review. Int. J. Hydrogen Energy 2017, 42, 289–311. [Google Scholar] [CrossRef]
- Borgschulte, A. The Hydrogen Grand Challenge. Front. Energy Res. 2016, 4, 11. [Google Scholar] [CrossRef] [Green Version]
- Schlapbach, L.; Züttel, A. Hydrogen-Storage Materials for Mobile Applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Varin, R.A.; Czujko, T.; Wronski, Z.S. Nanomaterials for Solid State Hydrogen Storage; Springer Science: New York, NY, USA, 2009. [Google Scholar]
- Aguey-Zinsou, K.-F.; Ares-Fernández, J.-R. Hydrogen in Magnesium: New Perspectives toward Functional Stores. Energy Environ. Sci. 2010, 3, 526–543. [Google Scholar] [CrossRef]
- Pasquini, L. The Effects of Nanostructure on the Hydrogen Sorption Properties of Magnesium-Based Metallic Compounds: A Review. Crystals 2018, 8, 106. [Google Scholar] [CrossRef] [Green Version]
- Crivello, J.-C.; Dam, B.; Denys, R.V.; Dornheim, M.; Grant, D.M.; Huot, J.; Jensen, T.R.; de Jongh, P.; Latroche, M.; Milanese, C.; et al. Review of Magnesium Hydride-Based Materials: Development and Optimisation. Appl. Phys. A 2016, 122, 97. [Google Scholar] [CrossRef] [Green Version]
- Révész, Á.; Gajdics, M. Improved H-Storage Performance of Novel Mg-Based Nanocomposites Prepared by High-Energy Ball Milling: A Review. Energies 2021, 14, 6400. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Shao, H.; Li, W.; Lin, H. Catalysis and Downsizing in Mg-Based Hydrogen Storage Materials. Catalysts 2018, 8, 89. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.-J.; Lu, Y.-S.; Zhang, L.-T.; Liu, H.-Z.; Edalati, K.; Révész, Á. Recent Advances in Metastable Alloys for Hydrogen Storage: A Review. Rare Met. 2022, 41, 1797–1817. [Google Scholar] [CrossRef]
- Hara, M.; Morozumi, S.; Watanabe, K. Effect of a Magnesium Depletion on the Mg–Ni–Y Alloy Hydrogen Absorption Properties. J. Alloys Compd. 2006, 414, 207–214. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, D.; Li, B.; Ren, H.; Guo, S.; Wang, X. Electrochemical Hydrogen Storage Characteristics of Nanocrystalline Mg20Ni10-xCox (X = 0–4) Alloys Prepared by Melt-Spinning. J. Alloys Compd. 2010, 491, 589–594. [Google Scholar] [CrossRef]
- Si, T.Z.; Liu, Y.F.; Zhang, Q.A. Hydrogen Storage Properties of the Supersaturated Mg12YNi Solid Solution. J. Alloys Compd. 2010, 507, 489–493. [Google Scholar] [CrossRef]
- Kalinichenka, S.; Röntzsch, L.; Baehtz, C.; Kieback, B. Hydrogen Desorption Kinetics of Melt-Spun and Hydrogenated Mg90Ni10 and Mg80Ni10Y10 Using in Situ Synchrotron, X-Ray Diffraction and Thermogravimetry. J. Alloys Compd. 2010, 496, 608–613. [Google Scholar] [CrossRef]
- Spassov, T.; Köster, U. Thermal Stability and Hydriding Properties of Nanocrystalline Melt-Spun Mg63Ni30Y7 Alloy. J. Alloys Compd. 1998, 279, 279–286. [Google Scholar] [CrossRef]
- Zhang, Q.A.; Jiang, C.J.; Liu, D.D. Comparative Investigations on the Hydrogenation Characteristics and Hydrogen Storage Kinetics of Melt-Spun Mg10NiR (R = La, Nd and Sm) Alloys. Int. J. Hydrogen Energy 2012, 37, 10709–10714. [Google Scholar] [CrossRef]
- Huang, L.J.; Wang, H.; Ouyang, L.Z.; Sun, D.L.; Lin, H.J.; Zhu, M. Achieving Fast Hydrogenation by Hydrogen-Induced Phase Separation in Mg-Based Amorphous Alloys. J. Alloys Compd. 2021, 887, 161476. [Google Scholar] [CrossRef]
- Lin, H.-J.; He, M.; Pan, S.-P.; Gu, L.; Li, H.-W.; Wang, H.; Ouyang, L.-Z.; Liu, J.-W.; Ge, T.-P.; Wang, D.-P.; et al. Towards Easily Tunable Hydrogen Storage via a Hydrogen-Induced Glass-to-Glass Transition in Mg-Based Metallic Glasses. Acta Mater. 2016, 120, 68–74. [Google Scholar] [CrossRef]
- Spassov, T.; Köster, U. Hydrogenation of Amorphous and Nanocrystalline Mg-Based Alloys. J. Alloys Compd. 1999, 287, 243–250. [Google Scholar] [CrossRef]
- Kalinichenka, S.; Röntzsch, L.; Kieback, B. Structural and Hydrogen Storage Properties of Melt-Spun Mg–Ni–Y Alloys. Int. J. Hydrogen Energy 2009, 34, 7749–7755. [Google Scholar] [CrossRef]
- Zhou, H.; Tan, J.; Dong, Q.; Ma, L.F.; Ding, D.Y.; Guo, S.F.; Li, Q.; Chen, Y.A.; Pan, F.S. Nonisothermal Crystallization Behavior of Micron-Sized Mg85Ni5Y10 Amorphous Wires. J. Non-Cryst. Solids 2022, 581, 121412. [Google Scholar] [CrossRef]
- Yang, T.; Wang, P.; Xia, C.; Li, Q.; Liang, C.; Zhang, Y. Characterization of Microstructure, Hydrogen Storage Kinetics and Thermodynamics of a Melt-Spun Mg86Y10Ni4 Alloy. Int. J. Hydrogen Energy 2019, 44, 6728–6737. [Google Scholar] [CrossRef]
- Pang, X.; Ran, L.; Chen, Y.; Luo, Y.; Pan, F. Enhancing Hydrogen Storage Performance via Optimizing Y and Ni Element in Magnesium Alloy. J. Magnes. Alloys 2022, 10, 821–835. [Google Scholar] [CrossRef]
- Révész, Á.; Kis-Tóth, Á.; Varga, L.K.; Lábár, J.L.; Spassov, T. High Glass Forming Ability Correlated with Microstructure and Hydrogen Storage Properties of a Mg–Cu–Ag–Y Glass. Int. J. Hydrogen Energy 2014, 39, 9230–9240. [Google Scholar] [CrossRef] [Green Version]
- Lass, E.A. Hydrogen Storage Measurements in Novel Mg-Based Nanostructured Alloys Produced via Rapid Solidification and Devitrification. Int. J. Hydrogen Energy 2011, 36, 10787–10796. [Google Scholar] [CrossRef]
- Zhang, Q.A.; Liu, D.D.; Wang, Q.Q.; Fang, F.; Sun, D.L.; Ouyang, L.Z.; Zhu, M. Superior Hydrogen Storage Kinetics of Mg12YNi Alloy with a Long-Period Stacking Ordered Phase. Scr. Mater. 2011, 65, 233–236. [Google Scholar] [CrossRef]
- Wu, Y.; Lototskyy, M.V.; Solberg, J.K.; Yartys, V.A. Effect of Microstructure on the Phase Composition and Hydrogen Absorption-Desorption Behaviour of Melt-Spun Mg-20Ni-8Mm Alloys. Int. J. Hydrogen Energy 2012, 37, 1495–1508. [Google Scholar] [CrossRef]
- Wu, Y.; Solberg, J.K.; Yartys, V.A. The Effect of Solidification Rate on Microstructural Evolution of a Melt-Spun Mg–20Ni–8Mm Hydrogen Storage Alloy. J. Alloys Compd. 2007, 446–447, 178–182. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.; Ren, H.; Guo, S.; Zhao, D.; Wang, X. Hydrogenation and Dehydrogenation Behaviours of Nanocrystalline Mg20Ni10-xCux (X = 0−4) Alloys Prepared by Melt Spinning. Int. J. Hydrogen Energy 2010, 35, 2040–2047. [Google Scholar] [CrossRef]
- Ding, X.; Chen, R.; Chen, X.; Pu, J.; Su, Y.; Guo, J. Study on the Eutectic Formation and Its Correlation with the Hydrogen Storage Properties of Mg98Ni2-xLax Alloys. Int. J. Hydrogen Energy 2021, 46, 17814–17826. [Google Scholar] [CrossRef]
- Ding, X.; Chen, R.; Zhang, J.; Chen, X.; Su, Y.; Guo, J. Achieving Superior Hydrogen Storage Properties via In-Situ Formed Nanostructures: A High-Capacity Mg–Ni Alloy with La Microalloying. Int. J. Hydrogen Energy 2022, 47, 6755–6766. [Google Scholar] [CrossRef]
- Denys, R.V.; Poletaev, A.A.; Maehlen, J.P.; Solberg, J.K.; Tarasov, B.P.; Yartys, V.A. Nanostructured Rapidly Solidified LaMg11Ni Alloy. II. In Situ Synchrotron X-Ray Diffraction Studies of Hydrogen Absorption–Desorption Behaviours. Int. J. Hydrogen Energy 2012, 37, 5710–5722. [Google Scholar] [CrossRef]
- Li, H.; Wan, C.; Li, X.; Ju, X. Structural, Hydrogen Storage, and Electrochemical Performance of LaMgNi4 Alloy and Theoretical Investigation of Its Hydrides. Int. J. Hydrogen Energy 2022, 47, 1723–1734. [Google Scholar] [CrossRef]
- Guo, F.; Zhang, T.; Shi, L.; Chen, Y.; Song, L. Mechanisms of Hydrides’ Nucleation and the Effect of Hydrogen Pressure Induced Driving Force on de-/Hydrogenation Kinetics of Mg-Based Nanocrystalline Alloys. Int. J. Hydrogen Energy 2022, 47, 1063–1075. [Google Scholar] [CrossRef]
- Han, B.; Yu, S.; Wang, H.; Lu, Y.; Lin, H.-J. Nanosize Effect on the Hydrogen Storage Properties of Mg-Based Amorphous Alloy. Scr. Mater. 2022, 216, 114736. [Google Scholar] [CrossRef]
- Song, F.; Yao, J.; Yong, H.; Wang, S.; Xu, X.; Chen, Y.; Zhang, L.; Hu, J. Investigation of Ball-Milling Process on Microstructure, Thermodynamics and Kinetics of Ce–Mg–Ni-Based Hydrogen Storage Alloy. Int. J. Hydrogen Energy 2022, in press. [Google Scholar] [CrossRef]
- Kang, H.; Yong, H.; Wang, J.; Xu, S.; Li, L.; Wang, S.; Hu, J.; Zhang, Y. Characterization on the Kinetics and Thermodynamics of Mg-Based Hydrogen Storage Alloy by the Multiple Alloying of Ce, Ni and Y Elements. Mater. Charact. 2021, 182, 111583. [Google Scholar] [CrossRef]
- Cao, W.; Ding, X.; Zhang, Y.; Zhang, J.; Chen, R.; Su, Y.; Guo, J.; Fu, H. Enhanced De-/Hydrogenation Kinetics of a Hyper-Eutectic Mg85Ni15-xAgx Alloy Facilitated by Ag Dissolving in Mg2Ni. J. Alloys Compd. 2022, 917, 165457. [Google Scholar] [CrossRef]
- Guo, F.; Zhang, T.; Shi, L.; Chen, Y.; Song, L. Ameliorated Microstructure and Hydrogen Absorption/Desorption Properties of Novel Mg–Ni–La Alloy Doped with MWCNTs and Co Nanoparticles. Int. J. Hydrogen Energy 2022, 47, 18044–18057. [Google Scholar] [CrossRef]
- Révész, Á.; Gajdics, M. High-Pressure Torsion of Non-Equilibrium Hydrogen Storage Materials: A Review. Energies 2021, 14, 819. [Google Scholar] [CrossRef]
- Révész, Á.; Kovács, Z. Severe Plastic Deformation of Amorphous Alloys. Mater. Trans. 2019, 60, 1283–1293. [Google Scholar] [CrossRef] [Green Version]
- Edalati, K.; Bachmaier, A.; Beloshenko, V.A.; Beygelzimer, Y.; Blank, V.D.; Botta, W.J.; Bryła, K.; Čížek, J.; Divinski, S.; Enikeev, N.A.; et al. Nanomaterials by Severe Plastic Deformation: Review of Historical Developments and Recent Advances. Mater. Res. Lett. 2022, 10, 163–256. [Google Scholar] [CrossRef]
- Strozi, R.B.; Ivanisenko, J.; Koudriachova, N.; Huot, J. Effect of HPT on the First Hydrogenation of LaNi5 Metal Hydride. Energies 2021, 14, 6710. [Google Scholar] [CrossRef]
- Chu, F.; Wu, K.; Meng, Y.; Edalati, K.; Lin, H.-J. Effect of High-Pressure Torsion on the Hydrogen Evolution Performances of a Melt-Spun Amorphous Fe73.5Si13.5B9Cu1Nb3 Alloy. Int. J. Hydrogen Energy 2021, 46, 25029–25038. [Google Scholar] [CrossRef]
- Gajdics, M.; Spassov, T.; Kis, V.K.; Schafler, E.; Révész, Á. Microstructural and Morphological Investigations on Mg-Nb2O5-CNT Nanocomposites Processed by High-Pressure Torsion for Hydrogen Storage Applications. Int. J. Hydrogen Energy 2020, 45, 7917–7928. [Google Scholar] [CrossRef]
- Gajdics, M.; Spassov, T.; Kovács Kis, V.; Béke, F.; Novák, Z.; Schafler, E.; Révész, Á. Microstructural Investigation of Nanocrystalline Hydrogen-Storing Mg-Titanate Nanotube Composites Processed by High-Pressure Torsion. Energies 2020, 13, 563. [Google Scholar] [CrossRef] [Green Version]
- Valiev, R.Z.; Islamgaliev, R.K.; Alexandrov, I.V. Bulk Nanostructured Materials from Severe Plastic Deformation. Prog. Mater. Sci. 2000, 45, 103–189. [Google Scholar] [CrossRef]
- Xu, C.; Lin, H.-J.; Edalati, K.; Li, W.; Li, L.; Zhu, Y. Superior Hydrogenation Properties in a Mg65Ce10Ni20Cu5 Nanoglass Processed by Melt-Spinning Followed by High-Pressure Torsion. Scr. Mater. 2018, 152, 137–140. [Google Scholar] [CrossRef]
- Révész, Á.; Kis-Tóth, Á.; Szommer, P.; Spassov, T. Hydrogen Storage, Microstructure and Mechanical Properties of Strained Mg65Ni20Cu5Y10 Metallic Glass. Mater. Sci. Forum 2013, 729, 74–79. [Google Scholar] [CrossRef]
- Révész, Á.; Kis-Tóth, Á.; Varga, L.K.; Schafler, E.; Bakonyi, I.; Spassov, T. Hydrogen Storage of Melt-Spun Amorphous Mg65Ni20Cu5Y10 Alloy Deformed by High-Pressure Torsion. Int. J. Hydrogen Energy 2012, 37, 5769–5776. [Google Scholar] [CrossRef]
- Zhilyaev, A.; Langdon, T. Using High-Pressure Torsion for Metal Processing: Fundamentals and Applications. Prog. Mater. Sci. 2008, 53, 893–979. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Jiang, J.Z.; Kato, H.; Ohsuna, T.; Saida, J.; Inoue, A.; Saksl, K.; Franz, H.; Ståhl, K. Origin of Nondetectable X-Ray Diffraction Peaks in Nanocomposite CuTiZr Alloys. Appl. Phys. Lett. 2003, 83, 3299–3301. [Google Scholar] [CrossRef] [Green Version]
- Hou, Z.; Zhang, W.; Wei, X.; Yuan, Z.; Ge, Q. Hydrogen Storage Behavior of Nanocrystalline and Amorphous Mg–Ni–Cu–La Alloys. RSC Adv. 2020, 10, 33103–33111. [Google Scholar] [CrossRef]






| Sample | ΔHHPT (J/g) | (η) |
|---|---|---|
| MgNiCuY_5 | 79 | 0.25 |
| MgNiCuY_CNT_5 | 74 | 0.29 |
| MgNiCuY_CNT_2 | 83 | 0.2 |
| MgNiCuY_CNT_1 | 86 | 0.18 |
| MgNiCuY_CNT_0 | 103 | 0.02 |
| As Quenched MgNiCuY | 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Révész, Á.; Gajdics, M.; Alifah, M.; Kovács Kis, V.; Schafler, E.; Varga, L.K.; Todorova, S.; Spassov, T.; Baricco, M. Thermal, Microstructural and Electrochemical Hydriding Performance of a Mg65Ni20Cu5Y10 Metallic Glass Catalyzed by CNT and Processed by High-Pressure Torsion. Energies 2022, 15, 5710. https://doi.org/10.3390/en15155710
Révész Á, Gajdics M, Alifah M, Kovács Kis V, Schafler E, Varga LK, Todorova S, Spassov T, Baricco M. Thermal, Microstructural and Electrochemical Hydriding Performance of a Mg65Ni20Cu5Y10 Metallic Glass Catalyzed by CNT and Processed by High-Pressure Torsion. Energies. 2022; 15(15):5710. https://doi.org/10.3390/en15155710
Chicago/Turabian StyleRévész, Ádám, Marcell Gajdics, Miratul Alifah, Viktória Kovács Kis, Erhard Schafler, Lajos Károly Varga, Stanislava Todorova, Tony Spassov, and Marcello Baricco. 2022. "Thermal, Microstructural and Electrochemical Hydriding Performance of a Mg65Ni20Cu5Y10 Metallic Glass Catalyzed by CNT and Processed by High-Pressure Torsion" Energies 15, no. 15: 5710. https://doi.org/10.3390/en15155710






