Morphology and Particle Size of a Synthesized NMC 811 Cathode Precursor with Mixed Hydroxide Precipitate and Nickel Sulfate as Nickel Sources and Comparison of Their Electrochemical Performances in an NMC 811 Lithium-Ion Battery
Abstract
:1. Introduction
2. Materials and Methods
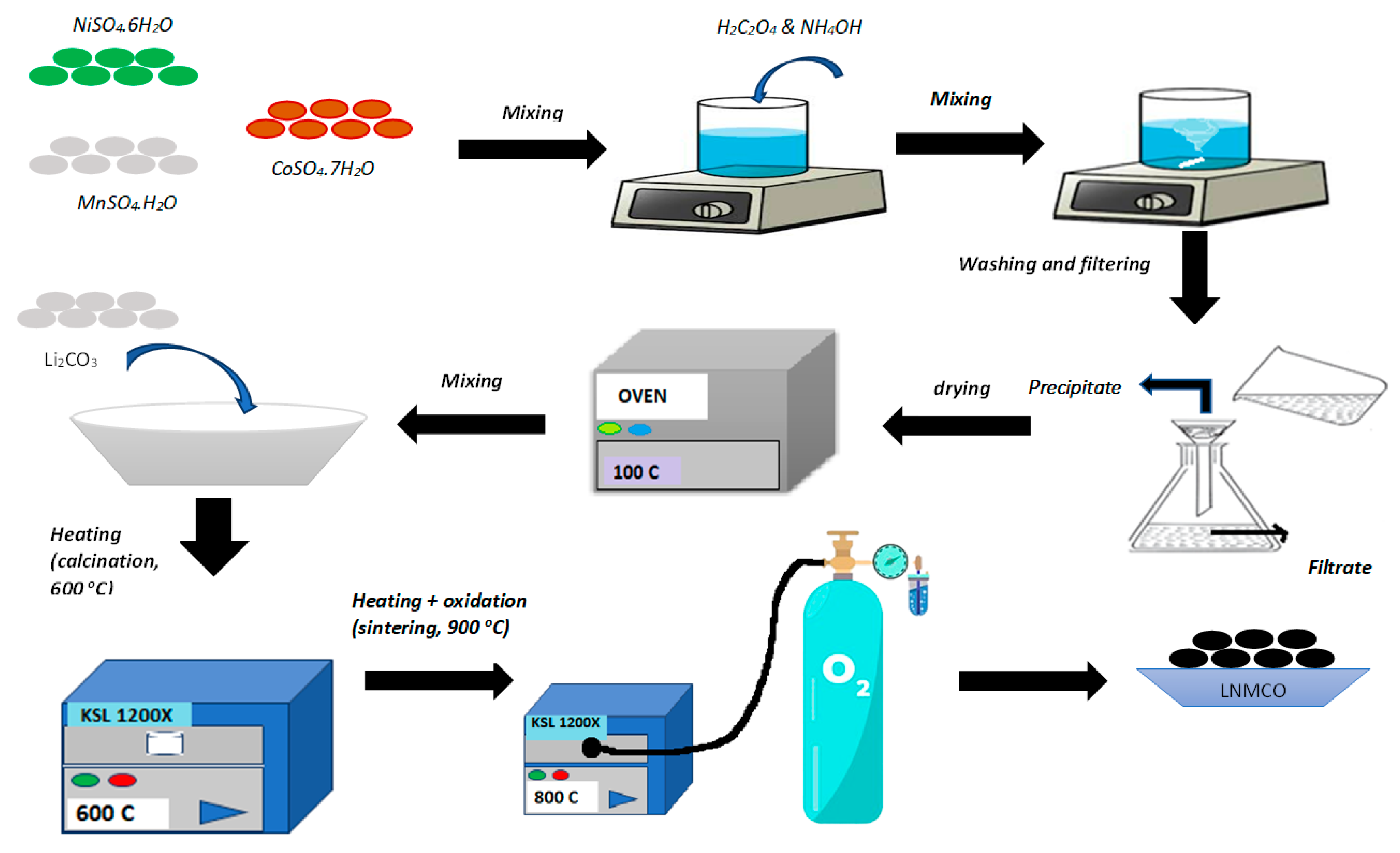
2.1. Cathode Precursor Synthesis and Battery Fabrication
2.2. Material Characterization
2.3. Electrochemical Performance Test of the Batteries
3. Results and Discussion
3.1. FTIR Analysis
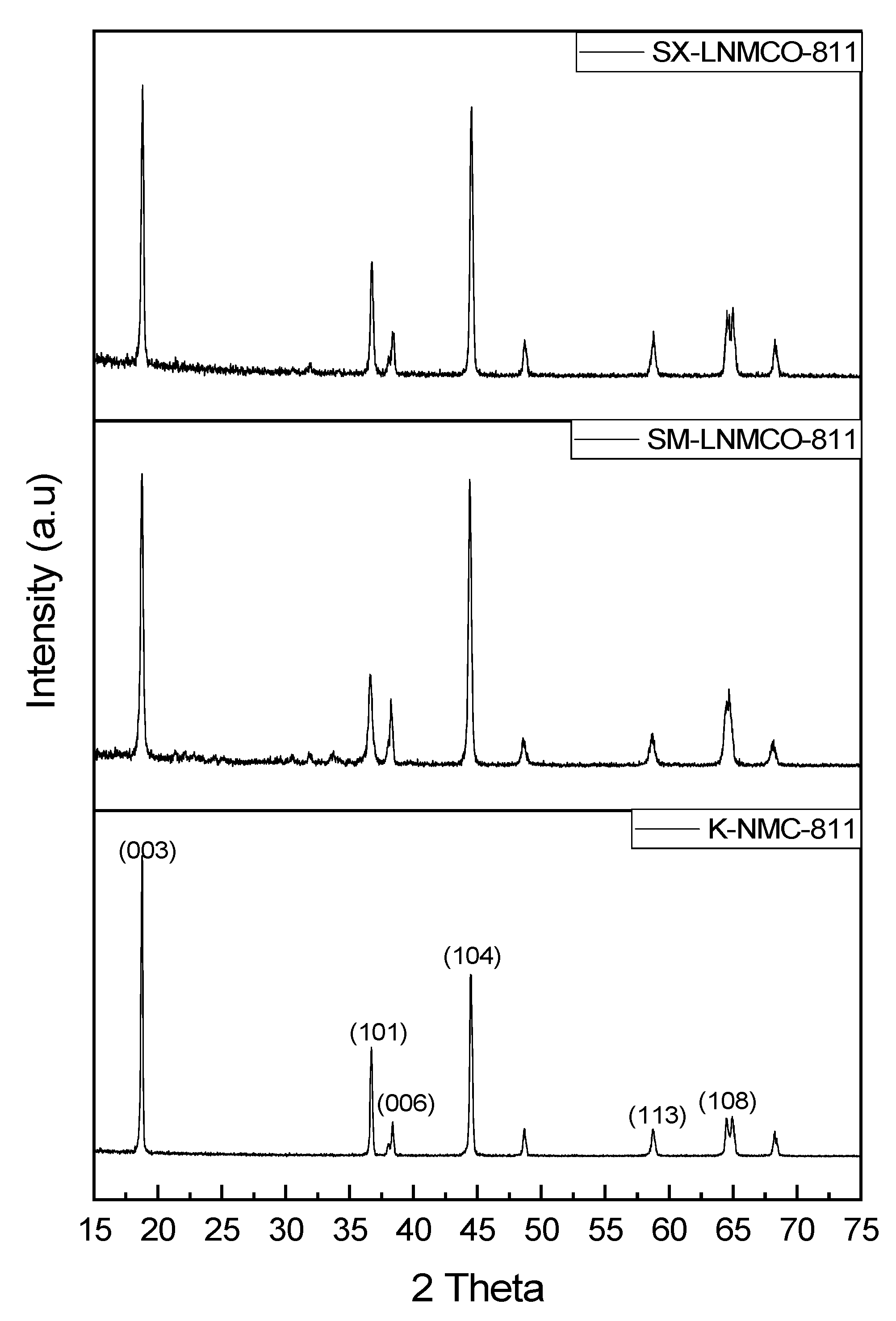
3.2. XRD Analysis
3.3. Elemental Analysis of the Precursor
3.4. Analysis of Morphology by SEM
3.5. PSA Analysis
3.6. Charge–Discharge Analyses of the Batteries
3.7. EIS Measurements Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martins, L.S.; Guimarães, L.F.; Botelho Junior, A.B.; Tenório, J.A.S.; Espinosa, D.C.R. Electric Car Battery: An overview on Global Demand, Recycling and Future Approaches towards Sustainability. J. Environ. Manag. 2021, 295, 113091. [Google Scholar] [CrossRef]
- Mossali, E.; Picone, N.; Gentilini, L.; Rodrìguez, O.; Pérez, J.M.; Colledani, M. Lithium-ion Batteries Towards Circular Economy: A Literature Review of Opportunities and Issues of Recycling Treatments. J. Environ. Manag. 2020, 264, 110500. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, X.Q.; Ding, F.; Huang, J.Q.; Xu, R.; Chen, X.; Yan, C.; Su, F.Y.; Chen, C.M.; Liu, X.; et al. Advanced Electrode Materials in Lithium Batteries: Retrospect and Prospect. Energy Mater. Adv. 2021, 2021, 1205324. [Google Scholar] [CrossRef]
- Wang, D.; Liu, W.; Zhang, X.; Huang, Y.; Xu, M.; Xiao, W. Review of Modified Nickel-Cobalt Lithium Aluminate Cathode Materials for Lithium-Ion Batteries. Int. J. Photoenergy 2019, 2019, 2730849. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Sasaki, Y.; Kageyama, M.; Kobayakawa, K.; Sato, Y. Structure, Morphology and Electrochemical Properties of LiNi0.5Mn0.5−xCoxO2 prepared by solid state reaction. J. Power Sources 2005, 148, 85–89. [Google Scholar] [CrossRef]
- Miao, Y.; Hynan, P.; Von Jouanne, A.; Yokochi, A. Current Li-ion Battery Technologies in Electric Vehicles and Opportunities for Advancements. Energies 2019, 12, 1074. [Google Scholar] [CrossRef] [Green Version]
- Bensalah, N.; Dawood, H.D. Review on Synthesis, Characterizations, and Electrochemical Properties of Cathode Materials for Lithium-Ion Batteries. J. Mater. Sci. Eng. 2016, 396. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Sun, Y.; Yue, Y.; Hu, X.; Xia, M. Carbon Modified Li-rich Cathode Materials Li1.26Fe0.22Mn0.52O2 Synthesized via Molten Salt Method with Excellent Rate Ability for Li-ion Batteries. Electrochim. Acta 2014, 30, 66–75. [Google Scholar] [CrossRef]
- Peralta, D.; Salomon, J.; Colin, J.F.; Boulineau, A.; Fabre, F.; Bourbon, C. Submicronic LiNi1/3Mn1/3Co1/3O2 Synthesized by Co-precipitation for Lithium-Ion Batteries—Tailoring a Classic Process for Enhanced Energy and Power Density. J. Power Sources 2018, 396, 527–532. [Google Scholar] [CrossRef]
- Nisa, S.S.; Rahmawati, M.; Yudha, C.S.; Nilasary, H.; Nursukatmo, H.; Oktaviano, H.S.; Muzayanha, S.U.; Purwanto, A. A Fast Approach to Obtain Layered Transition-Metal Cathode Material for Rechargeable Batteries. Batteries 2022, 8, 4. [Google Scholar] [CrossRef]
- Nayaka, G.P.; Zhang, Y.; Dong, P.; Wang, D.; Zhou, Z.; Duan, J.; Li, X.; Lin, Y.; Meng, Q.; Pai, K.V.; et al. An environmental Friendly Attempt to Recycle the Spent Li-ion Battery Cathode through Organic Acid Leaching. J. Environ. Chem. Eng. 2019, 7, 102854. [Google Scholar] [CrossRef]
- Dhayal Raj, A.; Suresh Kumar, P.; Mangalaraj, D.; Ponpandian, N.; Albert Irudayaraj, A.; Yang, Q. Gas sensing behavior of high surface area Co3O4 Micro/Nano structures synthesized by simple sonication process. Sens. Lett. 2012, 10, 826. [Google Scholar]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indonesian. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar]
- Xuan, W.; Otsuki, A.; Chagnes, A. Investigation of the Leaching Mechanism of NMC 811 (LiNi0.8Mn0.1Co0.1O2) by Hydrochloric Acid for Recycling Lithium-Ion Battery Cathodes. RSC Adv. 2019, 9, 66. [Google Scholar] [CrossRef] [Green Version]
- Lin, F.; Nordlund, D.; Li, Y.; Quan, M.K.; Cheng, L.; Weng, T.; Liu, Y.; Xin, H.L.; Doeff, M.M. Metal Segregation in Hierarchically Structured Cathode Materials for High-Energy Lithium Batteries. Nat. Energy 2016, 1, 15004. [Google Scholar] [CrossRef]
- Bi, Y.; Yang, W.; Du, R.; Zhou, J.; Liu, M.; Liu, Y.; Wang, D. Correlation of Oxygen Non-stoichiometry to the Instabilities and Electrochemical Performance of LiNi0.8Co0.1Mn0.1O2 Utilized in Lithium-Ion Battery. J. Power Sources 2015, 283, 211–218. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.; Xie, Q.; You, Y.; Chi, M.; Manthiram, A. Long-Term Cyclability of NCM-811 at High Voltages in Lithium-Ion Batteries: An In-Depth Diagnostic Study. Chem. Mater. 2020, 32, 18. [Google Scholar] [CrossRef]
- Lipson, A.L.; Ross, B.J.; Durham, J.L.; Liu, D.; Leresche, M.; Fister, T.T.; Liu, L.; Kim, K. Stabilizing NMC 811 Li-Ion Battery Cathode through a Rapid Coprecipitation Process. ACS Appl. Energy Mater. 2021, 4, 1972–1977. [Google Scholar] [CrossRef]
- Ramasamy, H.V.; Sinha, S.; Park, J.; Gong, M.; Aravindan, V.; Heo, J.; Lee, Y.S. Enhancement of electrochemical activity of Ni-rich LiNi0.8Mn0.1Co0.1O2 by Precisely Controlled Al2O3 Nanocoatings via Atomic Layer Deposition. J. Electrochem. Sci. Technol. 2019, 10, 196–205. [Google Scholar] [CrossRef]
- Sim, S.J.; Lee, S.H.; Jin, B.S.; Kim, H.S. Use of Carbon Coating on LiNi0.8Co0.1Mn0.1O2 Cathode Material for Enhanced Performances of Lithium-Ion Batteries. Sci. Rep. 2020, 10, 11114. [Google Scholar] [CrossRef]
- Xu, X.; Xiang, L.; Wang, L.; Jian, J.; Du, C.; He, X.; Huo, H.; Cheng, X.; Yin, G. Progressive Concentration Gradient Nickel-Rich Oxide Cathode Material for High-Energy and Long-Life Lithium-Ion Batteries. J. Mater. Chem. A 2019, 7, 7728–7735. [Google Scholar] [CrossRef]
- Zhang, M.; Shen, J.; Li, J.; Zhang, D.; Yan, Y.; Huang, Y.; Li, Z. Effect of Micron Sized Particle on the Electrochemical Properties of Nickel-rich LiNi0.8Co0.1Mn0.1O2 Cathode Materials. Ceram. Int. 2020, 46, 4643–4651. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Shin, H.C.; Kim, J.M.; Choi, J.Y.; Yoon, W.S. Modeling and Applications of Electrochemical Impedance Spectroscopy (EIS) for Lithium-Ion Batteries. J. Electrochem. Sci. Technol. 2020, 11, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Steinle, D.; Nguyen, H.; Kim, J.; Mayer, A.; Shi, J.; Paillard, E.; Iojoiu, C.; Passerini, S.; Bresser, D. High-energy Lithium Batteries Based on Single-ion Li [Ni0.8Co0.1Mn0.1]O2 Cathodes. Nano Energy 2020, 77, 105129. [Google Scholar] [CrossRef]
- Xi, Y.; Liu, Y.; Zhang, D.; Jin, S.; Zhang, R.; Jin, M. Comparative Study of the Electrochemical Performance of LiNi0.5Co0.2Mn0.3O2 and LiNi0.8Co0.1Mn0.1O2 Cathode Materials for Lithium Ion Batteries. Solid State Ion. 2018, 327, 27–31. [Google Scholar] [CrossRef]





| Element | Content (%) |
|---|---|
| Ni | 47.22 |
| S | 11.94 |
| Mg | 1.41 |
| Mn | 1.80 |
| Co | 1.12 |
| Zn | 0.72 |
| Cl | 0.26 |
| Sb | 0.20 |
| Al | 0.11 |
| Pb | 0.56 |
| K | 0.06 |
| Cr | 0.03 |
| Fe | 0.02 |
| P | 0.02 |
| O & H | balance |
| Element | Content | Unit |
|---|---|---|
| Ni | 21.53 | % |
| Co | 132.65 | ppm |
| Na | 5379.1 | ppm |
| Mg | 226.52 | ppm |
| Ca | 123.93 | ppm |
| Mn | 18.23 | ppm |
| Fe | 205.19 | ppm |
| Parameter | Voltage | Electrical Charge |
|---|---|---|
| Constant current charge | 4.25 | 1/20 C |
| Constant voltage charge | 4.25 | 1/30 C |
| Constant current discharge | 2.7 | 1/20 C |
| Precursor Sample | FoM (Figure(s) of Merit) to Li0.05Ni0.75Co0.1Mn0.1O2 |
|---|---|
| SM-LNMCO-811 | 0.81 |
| SX-LNMCO-811 | 0.88 |
| K-NMC-811 | 0.90 |
| Sample | Ni (%) | Mn (%) | Co (%) |
|---|---|---|---|
| SM-LNMCO-811 | 71.01 | 6.85 | 10.30 |
| SX-LNMCO-811 | 71.34 | 9.75 | 11.7 |
| K-NMC-811 | 75.25 | 4.42 | 11.5 |
| Sample | Mol Ratio of Ni:Mn:Co | ||
|---|---|---|---|
| Ni | Mn | Co | |
| SM-LNMCO-811 | 0.8 | 0.08 | 0.12 |
| SX-LNMCO-811 | 0.76 | 0.11 | 0.13 |
| K-NMC-811 | 0.82 | 0.05 | 0.13 |
| Sample Code | D10 (µm) | D50 (µm) | D90 (µm) | Average (µm) |
|---|---|---|---|---|
| SM-LNMCO-811 | 10.05 | 245.4 | 643.4 | 285.2 |
| SX-LNMCO-811 | 5.345 | 12.94 | 38.36 | 17.16 |
| K-NMC-811 | 2.159 | 15.74 | 38.85 | 18.28 |
| Cathode Precursor Variation | Specific Capacity Charge (mAh/g) | Specific Capacity Discharge (mAh/g) | Initial Efficiency (%) |
|---|---|---|---|
| SM-LNMCO-811 | 164.75 | 60.97 | 37 |
| SX-LNMCO-811 | 189.71 | 178.93 | 94.32 |
| K-NMC-811 | 158.28 | 149.05 | 94.17 |
| Sample | Rct | Ionic Conductivity (S/cm) |
|---|---|---|
| SX-LNMCO-811 | 826.066 | 1.20 × 10−7 |
| K-NMC-811 | 952.59 | 1.04 × 10−7 |
| Sample | DLi+ (cm2/s) |
|---|---|
| SX-LNMCO-811 | 4.22 × 10−9 |
| K-NMC-811 | 1.57 × 10−9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijareni, A.S.; Widiyandari, H.; Purwanto, A.; Arif, A.F.; Mubarok, M.Z. Morphology and Particle Size of a Synthesized NMC 811 Cathode Precursor with Mixed Hydroxide Precipitate and Nickel Sulfate as Nickel Sources and Comparison of Their Electrochemical Performances in an NMC 811 Lithium-Ion Battery. Energies 2022, 15, 5794. https://doi.org/10.3390/en15165794
Wijareni AS, Widiyandari H, Purwanto A, Arif AF, Mubarok MZ. Morphology and Particle Size of a Synthesized NMC 811 Cathode Precursor with Mixed Hydroxide Precipitate and Nickel Sulfate as Nickel Sources and Comparison of Their Electrochemical Performances in an NMC 811 Lithium-Ion Battery. Energies. 2022; 15(16):5794. https://doi.org/10.3390/en15165794
Chicago/Turabian StyleWijareni, Anisa Surya, Hendri Widiyandari, Agus Purwanto, Aditya Farhan Arif, and Mohammad Zaki Mubarok. 2022. "Morphology and Particle Size of a Synthesized NMC 811 Cathode Precursor with Mixed Hydroxide Precipitate and Nickel Sulfate as Nickel Sources and Comparison of Their Electrochemical Performances in an NMC 811 Lithium-Ion Battery" Energies 15, no. 16: 5794. https://doi.org/10.3390/en15165794






