1. Introduction
The power lithium-ion battery has become a research hotspot in the electric vehicle industry because of its high energy density, low self-discharge, good safety performance, and long life [
1,
2]. In [
3], a new material for lithium batteries was proposed to improve the energy of lithium batteries. In article [
4], a new lithium battery structure was designed, which can effectively avoid the influence of temperature on battery parameters and improve battery energy utilization rates. On this basis, battery management technology also needs to be studied. A battery management system (BMS) can monitor and manage the working state of a power battery, making the battery run safely and reliably, improving the life of the battery, and extending the cruising range of the electric vehicle. The state of charge (
SOC), which is a physical quantity representing the amount of energy left in a battery, is an important basis for BMS to monitor the status of the battery and control decision making [
5]. Therefore,
SOC estimation has become a key part of the effective operation of BMSs.
At present, the
SOC estimation for power batteries mainly focuses on two aspects. Firstly, the equivalent model of batteries is studied. At present, the main battery models include the electrochemical model [
6], the equivalent circuit model [
7], the black box model [
8], and the battery thermal model [
9]. The electrochemical model has higher accuracy, but there are many model parameters, and most of the parameters are affected by the material, structure, and size of the battery. The black box model is simple and flexible, which is suitable for practical use. However, considering its high requirements for the quantity and quality of sample data, it needs more mature development. Although the battery thermal model has some research on battery heat generation, it cannot accurately reflect the heat generation and temperature rise pattern of the internal structure of large power lithium-ion batteries. The equivalent circuit model can well describe the electrochemical reaction with the circuit, which is suitable for a variety of batteries. However, its accuracy largely depends on the design of the specific equivalent circuit and the accuracy of parameter identification.
Secondly, the research aims at
SOC itself. The
SOC estimation methods mainly include the ampere-time integral method [
10], neural networks [
11], the particle filter (PF) algorithm [
12,
13,
14], and the Kalman filter (KF) and its improved algorithm [
15,
16,
17,
18,
19,
20]. The ampere-time integral method is easy to implement, but it does not have feedback correction, so there will be accumulated errors. The neural network method is suitable for the simulation of highly nonlinear systems, but establishing an accurate network needs a lot of data to train and the training process is usually time-consuming. The filtering method (i.e., PF and KF mentioned above) can continuously modify the estimated value in the iterative process, which has a good suppression effect on the system noise, but it relies largely upon the accuracy of the underlying battery models.
Temperature changes have a great impact on the performance of lithium-ion batteries. The capacity, terminal voltage, and internal resistance of the battery are all affected by temperature, which brings a lot of inconvenience to the use of power batteries. Poor estimations have been reported for
SOC at low temperatures [
21]. Therefore, the influence of temperature must be considered when estimating the
SOC of batteries.
In this paper, the battery capacity test and the hybrid pulse power characteristic (HPPC) test are carried out at different temperatures to obtain the characteristics of the battery. This paper analyzes the influence of different SOCs and temperatures on the open-circuit voltage, ohmic internal resistance, polarization resistance, and polarization capacitance of lithium-ion batteries. Based on this, an equivalent circuit model adapting to temperature and SOC changes is established, and the UKF algorithm is used to accurately estimate SOC.
The rest of this paper is organized as follows: In
Section 2, the equivalent circuit model of the battery is established, the ohmic resistance and polarization parameters of the battery are identified, and UDDS working conditions are used to verify the model at different temperatures. In
Section 3, an
SOC estimation algorithm based on UKF is proposed and verified. Finally,
Section 4 concludes this article.
2. Establishment of Battery Equivalent Model
2.1. Second-Order RC Equivalent Circuit Model
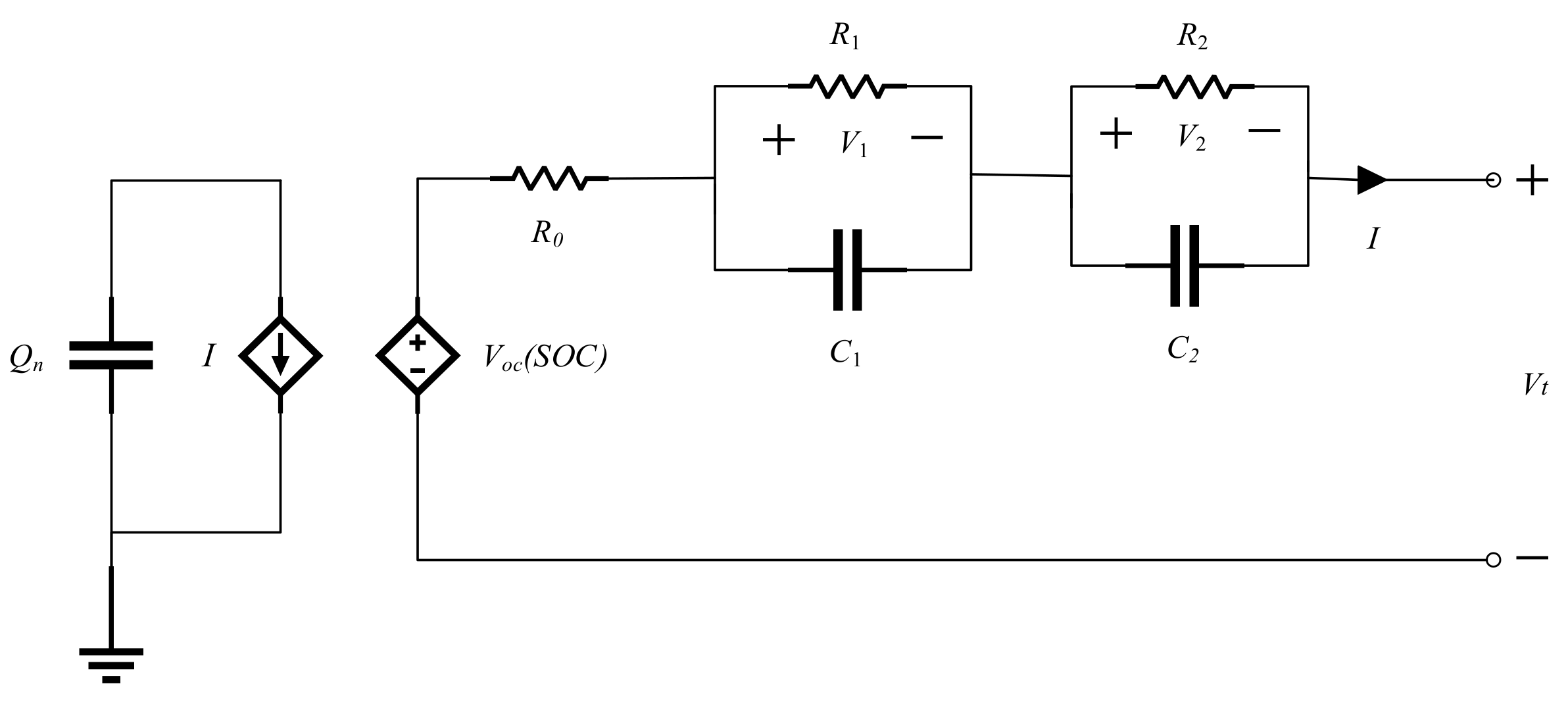
This paper adopts the second-order
RC equivalent circuit model [
22] to analyze the battery characteristics. As shown in
Figure 1,
is the battery’s nominal capacity and indicates the total energy stored in the battery.
,
represent terminal voltage and current (it is positive when discharging and negative when charging).
SOC represents the ratio of the available capacity to the nominal capacity of the battery [
23], as shown in Equation (1).
is the
SOC at time
.
(the voltage across
) changes with the
SOC variations from 0% to 100%. The controlled voltage source
reflects the nonlinear relationship between
SOC and open-circuit voltage (OCV).
is the ohmic resistance.
represents electrochemical polarization, describing the structure of the electric double layer at the electrode or solution interface.
indicates concentration polarization. The electrical characteristic of the equivalent circuit model can be described as follows:
The terminal voltage
and current
are taken as the output
and input
of the equivalent circuit model, respectively.
SOC,
and
are state variables. Discretizing Equations (1) and (2), the state-space equation of the second-order
RC equivalent circuit model is as follows:
In the Equation (3), , , , , , , , is the interval of sampling time, represents the time constant, , .
The second-order RC equivalent circuit model described by the Equation (3) will be used for the SOC estimation of batteries in the following section.
2.2. Parameter Identification and Fitting
The battery used in this paper is a ternary lithium-ion battery with a nominal capacity of 2800 mAh. The experimental equipment used in the test include a Neware charging and discharging machine, programmable temperatures, and a humidity chamber. According to the operating temperature range of the lithium-ion battery, when testing the battery, the experimental environment temperatures −10 °C, 0 °C, 10 °C, 20 °C, 25 °C, 30 °C, 40 °C, and 50 °C were chosen. Five batteries were placed under each temperature.
2.2.1. Open-Circuit Voltage
Since the open-circuit voltage (OCV) of charging is a little higher than that of discharging, the OCV of lithium-ion batteries at different temperatures and
SOCs is measured by averaging the two values, as shown in
Figure 2.
Figure 2 demonstrates that the OCV is affected by the ambient temperature. When the temperature
T > 10 °C, the OCV decreases slowly with the increase of temperature, and the variation range is small. When
T ≤ 10 °C, the open-circuit voltage decreases obviously with the increase of temperature, especially when the
SOC of the battery is close to 0. Therefore, the influence of temperature and
SOC on the OCV of a ternary lithium-ion battery cannot be ignored and should be fully considered when building the battery model.
The Curve Fitting Toolbox in MATLAB was used to establish the functional relationship of OCV related to
SOC and temperature
T.
The values of the fitting coefficients are shown in
Table 1.
2.2.2. Ohmic Internal Resistance
The ohmic internal resistance of equivalent circuit under different temperatures and
SOCs is identified accurately by a hybrid pulse power characteristic (HPPC) test.
Figure 3 shows the voltage change (point a → point b) under a pulse discharge in the HPPC test. In the figure, the voltage is recorded every 0.1 s. The battery is at rest before point a. Between point a and c, the battery is discharged at a constant current of 1C for 10 s. Between point c and e, the battery is rested again.
At the beginning (point a → point b) and end (point c → point d) of its discharge, the terminal voltage of lithium-ion battery will jump up and down. This phenomenon is caused by the ohmic internal resistance [
24]. The average of the voltage difference between two segments was taken to reduce the identification error. The calculation formula is shown in Equation (5). The change of ohmic internal resistance at different temperatures and
SOC is shown in
Figure 4.
Figure 4 shows that both temperature and
SOC changes can affect
R0, especially when the ambient temperature is low. When the temperature is fixed,
R0 increases slowly with the decrease of
SOC, the numerical fluctuation is more obvious at low temperatures. Under the same
SOC,
R0 is increasing with the decrease of temperature, the change rate gradually increases. It is calculated that the value of
R0 at −10 °C is about 3 times higher than that at 25 °C, while at 25 °C, it is only 1.37 times of that at 55 °C.
As shown in
Figure 5a, the effect of temperature on
R0 is much greater than that of
SOC. The change of
R0 with
SOC is not obvious at the same temperature, but the change of temperature will cause a significant fluctuation. The changing rate of
R0 at low temperatures is obviously greater than that at high temperatures.
As shown in
Figure 5b, the value of
R0 varies with the change of
SOC at a given temperature. Under high temperatures, the fluctuation of
R0 with
SOC is obviously smaller than that at low temperatures, so the slight change at high temperatures can almost be ignored. The relationship between
R0, temperature, and
SOC is fitted respectively for low temperatures and high temperatures.
At low temperature (
T < 20 °C),
R0 is chosen as the binary function of
SOC and temperature
T, such as the Equation (6). The fitting results and coefficient values are shown in
Figure 6 and
Table 2.
At room temperature and high temperature (
T ≥ 20 °C), the effect of
SOC on
R0 is negligible. The average value of
R0 at the same temperature was taken and
R0 as a function of
T was set, as shown in Equation (7). The fitting results and coefficient values are shown in
Figure 7 and
Table 3, respectively.
2.2.3. Polarization Capacitance and Polarization Resistance
The first-order
RC circuit response with resistance
R, capacitance
C and constant current
I is very important for identification. The equation is as follows:
In the Equation (8), , is the initial time.
- ①
The time constant
and
in the relaxation process was determined (point c → point e in
Figure 3):
Therefore, the terminal voltage equation of battery is:
In the Equation (11), , , , , are the unknown coefficients. can be fitted in the relaxation process point d→ point e segment and measured at the end of the relaxation process, i.e., point e. The optimum value of , , , and can be obtained by the fitting of custom equation in cftool. Thus, we determined the time constants , and the voltages , .
- ②
The parameters were determined: electrochemical polarization resistance
, electrochemical polarization capacitance
, concentration polarization resistance
, and concentration polarization capacitance
in the discharge process (point a → point b → point c in
Figure 3).
Obviously, = 0, = 0.
,
,
and
can be determined by the following equation.
Figure 8a–d show the
,
,
, and
of the equivalent circuit model at different temperatures and
SOC.
Electrochemical Polarization Resistance R1
Figure 9b demonstrates that
will fluctuate violently only when
T ≤ 20 °C and
SOC ≤ 30%. Therefore, this paper only analyzes the change of
at low temperatures, as shown in
Figure 10. At high temperatures, the model parameters can be directly substituted by the standard values without re-identification.
Figure 10b shows that when 0% ≤
SOC < 30%,
changes significantly with temperature. Especially when
SOC < 10%,
changes more violently than when 10% ≤
SOC < 30%. When 30% ≤
SOC < 100%,
is generally stable with a small fluctuation. According to the specific numerical fluctuation, the change of
can be divided into 30% ≤
SOC < 50% and 50% ≤
SOC ≤ 100%. Therefore, in the low-temperature environments,
can be fitted with segmented
SOC, as shown in Equation (14), and the value of the coefficients are shown in
Table 4.
Concentration Polarization Resistance R2
The processing of
is similar to
, but the
SOC segmentation is different. The expression is shown in Equation (15) and the value of the coefficients are shown in
Table 5.
Electrochemical Polarization Capacitance
This is similar to the processing of R1. Since the relationship between the average value of C1 and temperature in each segment of SOC is difficult to be expressed by a definite equation, we decided to use the interpolation function to deal with the value of at low temperature.
Concentration Polarization Capacitance
This is also similar to the method of
R1, but the
SOC segmentation is different. The expression is shown in Equation (16), the coefficients’ values are shown in
Table 6.
2.2.4. Available Battery Capacity
The available battery capacity in this paper is defined as the amount of discharge measured by discharging at the same rate to the cut-off voltage at different ambient temperatures.
The available battery capacity at different temperatures is shown in
Figure 11. The battery capacity varies from 2.24 Ah to 2.92 Ah in the range of −10 °C to 50 °C, with a variation range of 24.7%. Therefore, the influence of temperature on battery capacity should be considered in
SOC estimation.
Since the available battery capacity is affected by temperature and the charge and discharge rate, the
SOC calculation formula in Equation (1) has some errors in practical application [
25]. Considering the influence of temperature on the available battery capacity, this paper introduces a capacity compensation factor to modify the calculation of
SOC [
19,
20]. The
SOC calculation formula for the battery compensation factor is as follows:
Equation (17) shows that
SOC is a function of current
, temperature
, and time
. In the equation,
is the initial
SOC value [
21];
is the coulombic efficiency, it refers to the ratio of battery discharge capacity to charging capacity in the same cycle, which is approximately equal to 1.
is the nominal battery capacity (the available battery capacity at 25 °C) and
is the compensation factor of the available battery capacity at
T °C. Equation (18) is the calculation formula of
and
is the available battery capacity at
T °C.
According to
Figure 12, the relationship between temperature and battery capacity compensation factor are fitted [
20], as shown in Equation (19).
In the equation, a, b and c are the fitting coefficients, a = 1.211, b = 15.2, c = 46.26.
2.3. Verification of Optimized Equivalent Circuit Model
UDDS (Urban Dynamometer Driving Schedule) is used by the US Environmental Protection Agency to simulate the driving current conditions of light vehicles. In this paper, 3 °C, 23 °C, and 43 °C are set as the ambient temperature of low temperature, room temperature and high temperature respectively, and the Urban Dynamometer Driving Schedule (UDDS) working condition was run to verify the accuracy of the model [
23,
24]. The current waveform of the UDDS is shown in
Figure 13.
Figure 14 shows the terminal voltage error after using the optimized model to run the UDDS working condition at the selected temperature. The result shows that at 23 °C and 43 °C, the average terminal voltage error is 0.4%, the maximum error is about 1.1%. At 3 °C, the error fluctuates greatly, the average terminal voltage error is 0.7%, and the maximum error is about 3.8%. This indicates that the optimized model performs well, but at the low temperature, its accuracy is lower than that at room and high temperatures. There are two main reasons for the difference of model accuracy between high and low temperatures. First, there are some errors in the parameter identification at the low temperature [
23], which are less accurate than those at room and high temperatures. Second, since the temperature of the battery will rise when discharging, which is more obvious at low temperature, the internal parameters of the battery change more violently at low temperature than those at room and high temperatures.