Changes in Stabile Organic Carbon in Differently Managed Fluvisol Treated by Two Types of Anaerobic Digestate
Abstract
1. Introduction
2. Materials and Methods
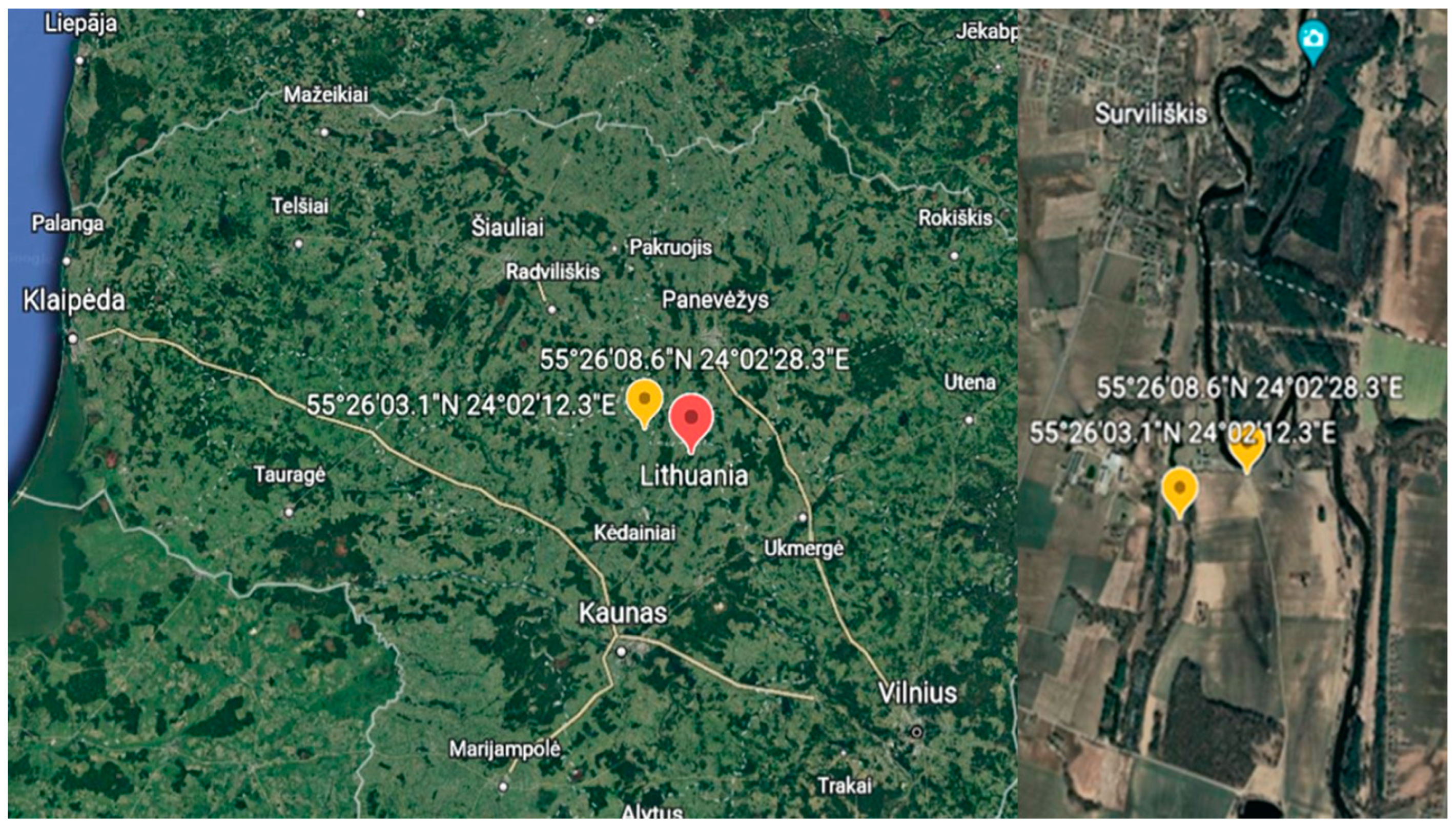
2.1. Study Area
2.2. Digestate Sampling and Analyses
2.3. Soil Sampling and Analyses
2.4. Statistical Analysis
3. Results and Discussion
3.1. The Effect of Digestate-Treated Soils on the Stratification Ratio of Soil Organic Carbon
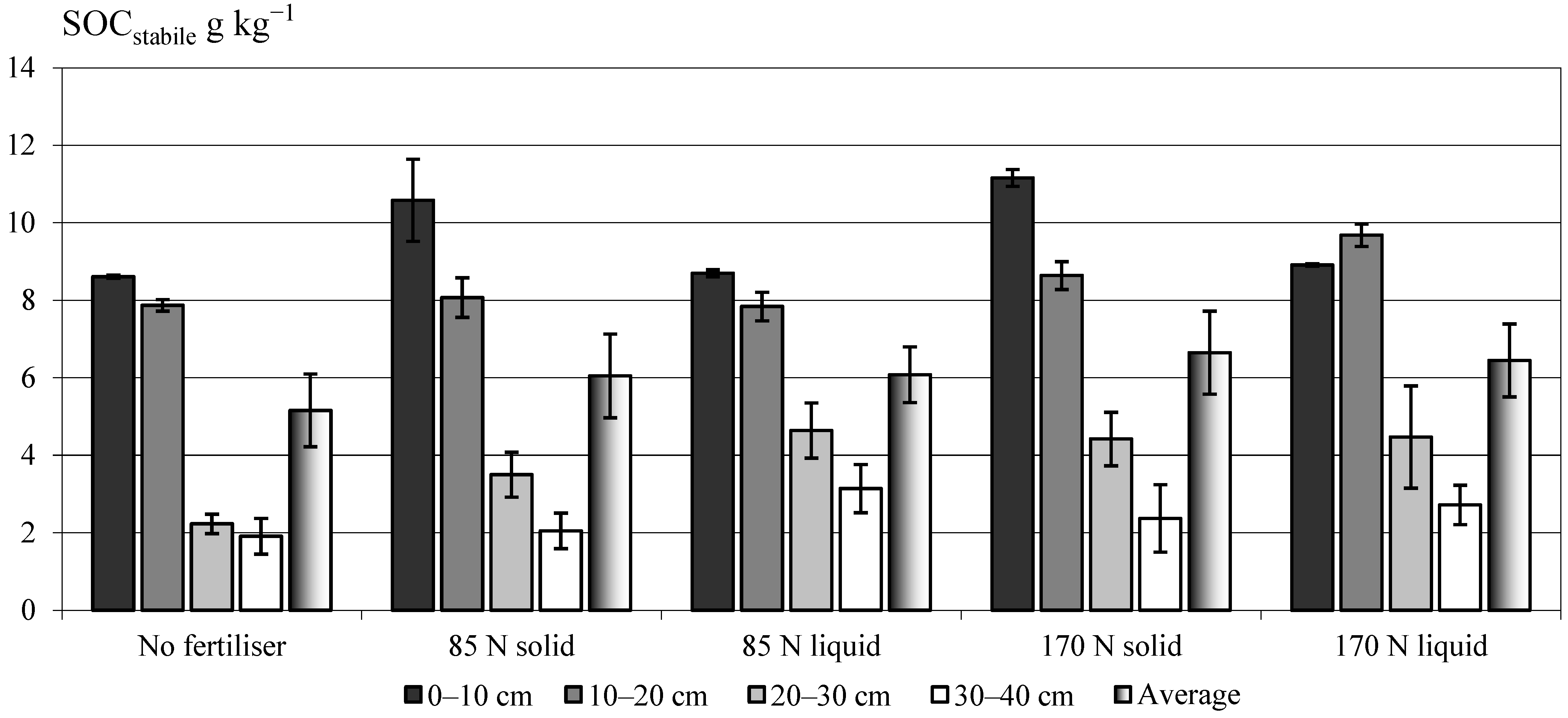
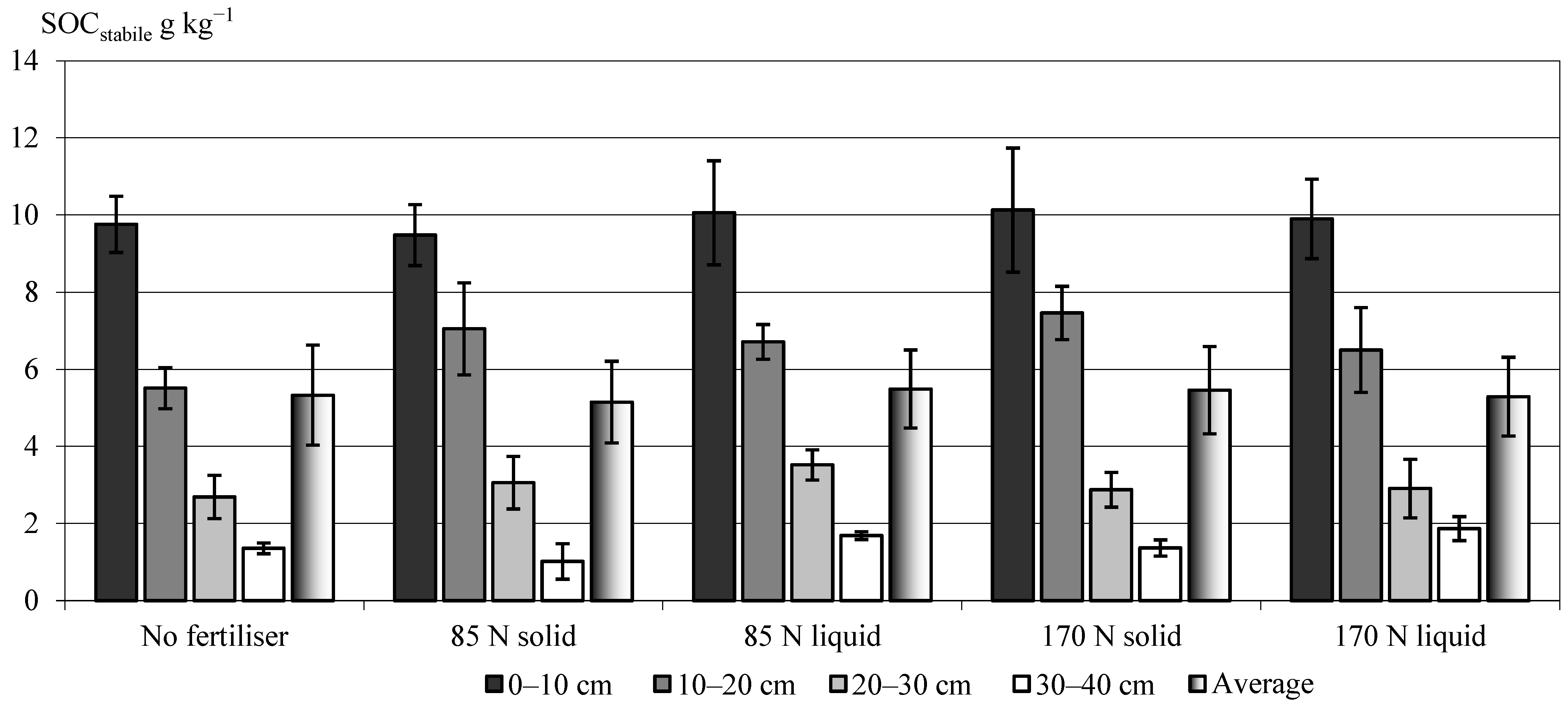
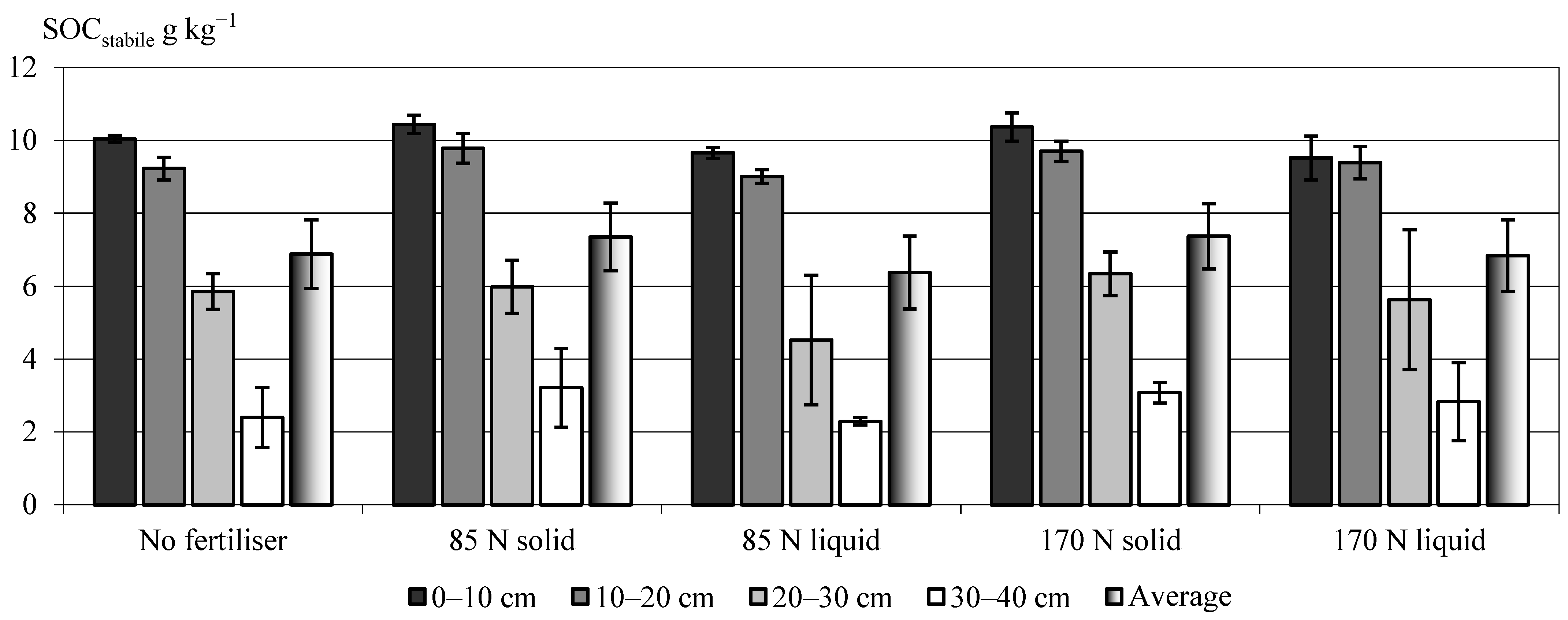
3.2. Effect of Two Fractions of Digestate on the Stabile Soil Organic Carbon (SOCstabile) Pool
3.3. The Effect of Digestate-Treated Soils on the Stratification Ratio of Stabile Soil Organic Carbon Pool
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| SOCstabile | stabile soil organic carbon |
| MHS | mobile humic substances |
| SD | solid digestate |
| LD | liquid digestate |
| OC | organic carbon |
| TKN | Kjeldahl nitrogen |
References
- Bong, C.P.C.; Lim, L.Y.; Lee, C.T.; Klemeš, J.J.; Ho, C.S.; Ho, W.S. The characterisation and treatment of food waste for improvement of biogas production during anaerobic digestion—A review. J. Clean Prod. 2018, 172, 1545–1558. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.F.; Fahl, F. Biogas: Developments and perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Klinglmair, M.; Thomsen, M. Using food waste in organic fertilizer: Modelling biogenic carbon sequestration with associated nutrient and micropollutant loads. Sustainability 2020, 12, 7399. [Google Scholar] [CrossRef]
- World Biogas Association. The Contribution of Anaerobic Digestion and Biogas towards achieving the UN Sustainable Development Goals. WBA SGD Biogas Report. 2018. Available online: https://www.worldbiogasassociation.org/wp-content/uploads/2018/12/WBA_SDG_Biogas_Report.pdf (accessed on 29 July 2022).
- Caruso, M.C.; Braghieri, A.; Capece, A.; Napolitano, F.; Romano, P.; Galgano, F.; Altieri, G.; Genovese, F. Recent updates on the use of agro-food waste for biogas production. Appl. Sci. 2019, 9, 1217. [Google Scholar] [CrossRef]
- Nsair, A.; Onen Cinar, S.; Alassali, A.; Abu Qdais, H.; Kuchta, K. Operational parameters of biogas plants: A review and evaluation study. Energies 2020, 13, 3761. [Google Scholar] [CrossRef]
- Laiq Ur Rehman, M.; Iqbal, A.; Chang, C.C.; Li, W.; Ju, M. Anaerobic digestion. Water Environ. Res. 2019, 91, 1253–1271. [Google Scholar] [CrossRef]
- O’Shea, R.; Lin, R.; Wall, D.M.; Browne, J.D.; Murphy, J.D. Using biogas to reduce natural gas consumption and greenhouse gas emissions at a large distillery. Appl. Energy 2020, 279, 115812. [Google Scholar] [CrossRef]
- Gray, N.; O’Shea, R.; Smyth, B.; Lens, P.N.; Murphy, J.D. What is the energy balance of electrofuels produced through power-to-fuel integration with biogas facilities? Renew. Sustain. Energy Rev. 2021, 155, 111886. [Google Scholar] [CrossRef]
- European Union. Directive 2006/12/EC of the European Parliament and of the Council of 5 April 2006 on Waste. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32006L0012 (accessed on 29 July 2022).
- Alrefai, R.; Benyounis, K.Y.; Stokes, J. Integration approach of anaerobic digestion and fermentation process towards producing biogas and bioethanol with zero waste: Technical. J. Fundam. Renew. Energy Appl. 2017, 7, 243. [Google Scholar] [CrossRef]
- Stürmer, B.; Pfundtner, E.; Kirchmeyr, F.; Uschnig, S. Legal requirements for digestate as fertilizer in Austria and the European Union compared to actual technical parameters. J. Environ. Manag. 2020, 253, 109756. [Google Scholar] [CrossRef]
- Meyer, A.K.P.; Ehimen, E.A.; Holm-Nielsen, J.B. Future European biogas: Animal manure, straw and grass potentials for a sustainable European biogas production. Biomass Bioenergy 2018, 111, 154–164. [Google Scholar] [CrossRef]
- Abubaker, J.; Risberg, K.; Pell, M. Biogas residues as fertilisers—Effects on wheat growth and soil microbial activities. Appl. Energy 2012, 99, 126–134. [Google Scholar] [CrossRef]
- Pawlita-Posmyk, M.; Wzorek, M. Biogas production from the perspective of sustainable development. Econ. Environ. Stud. (EES) 2018, 18, 1043–1057. [Google Scholar] [CrossRef]
- Koszel, M.; Lorencowicz, E. Agricultural use of biogas digestate as a replacement fertilizers. Agric. Agric. Sci. Procedia 2015, 7, 119–124. [Google Scholar] [CrossRef]
- Ehmann, A.; Thumm, U.; Lewandowski, I. Fertilizing potential of separated biogas digestates in annual and perennial biomass production systems. Front. Sustain. Food Syst. 2018, 2, 12. [Google Scholar] [CrossRef]
- Doyeni, M.O.; Stulpinaite, U.; Baksinskaite, A.; Suproniene, S.; Tilvikiene, V. The Effectiveness of Digestate Use for Fertilization in an Agricultural Cropping System. Plants 2021, 10, 1734. [Google Scholar] [CrossRef]
- Pastorelli, R.; Valboa, G.; Lagomarsino, A.; Fabiani, A.; Simoncini, S.; Zaghi, M.; Vignozzi, N. Recycling biogas digestate from energy crops: Effects on soil properties and crop productivity. Appl. Sci. 2021, 11, 750. [Google Scholar] [CrossRef]
- Teglia, C.; Tremier, A.; Martel, J.L. Characterization of solid digestates: Part 1, review of existing indicators to assess solid digestates agricultural use. Waste Biomass Valorization 2011, 2, 43–58. [Google Scholar] [CrossRef]
- Egene, C.E.; Sigurnjak, I.; Regelink, I.C.; Schoumans, O.F.; Adani, F.; Michels, E.; Sleutel, S.; Tack, F.M.G.; Meers, E. Solid fraction of separated digestate as soil improver: Implications for soil fertility and carbon sequestration. J. Soils Sediments 2021, 21, 678–688. [Google Scholar] [CrossRef]
- Trivedi, P.; Singh, B.P.; Singh, B.K. Soil Carbon: Introduction, Importance, Status, Threat, and Mitigation. In Soil Carbon Storage Modulators, Mechanisms and Modeling; Singh, B.K., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–6. ISBN 978-0-12-812766-7. [Google Scholar]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Yang, J.; Li, A.; Yang, Y.; Li, G.; Zhang, F. Soil organic carbon stability under natural and anthropogenic-induced perturbations. Earth-Sci. Rev. 2020, 205, 103199. [Google Scholar] [CrossRef]
- Błonska, E.; Lasota, J.; Vasconcelos da Silva, G.R.; Vanguelova, E.; Ashwood, F.; Tibbett, M.; Watts, K.; Lukac, M. Soil organic matter stabilization and carbon-cycling enzyme activity are affected by land management. Ann. For. Res. 2020, 63, 71–86. [Google Scholar] [CrossRef]
- Dynarski, K.A.; Bossio, D.A.; Scow, K.M. Dynamic stability of soil carbon: Reassessing the “permanence” of soil carbon sequestration. Front. Environ. Sci. 2020, 8, 514701. [Google Scholar] [CrossRef]
- Witing, F.; Prays, N.; O’Keeffe, S.; Gründling, R.; Gebel, M.; Kurzer, H.J.; Daniel-Gromke, J.; Franko, U. Biogas production and changes in soil carbon input-A regional analysis. Geoderma 2018, 320, 105–114. [Google Scholar] [CrossRef]
- Holatko, J.; Hammerschmiedt, T.; Kintl, A.; Danish, S.; Skarpa, P.; Latal, O.; Baltazar, T.; Fahad, S.; Akca, H.; Taban, S.; et al. Effect of carbon-enriched digestate on the microbial soil activity. PLoS ONE 2021, 16, 0252262. [Google Scholar] [CrossRef]
- Levin, K.S.; Auerswald, K.; Reents, H.J.; Hülsbergen, K.J. Effects of Organic Energy Crop Rotations and Fertilisation with the Liquid Digestate Phase on Organic Carbon in the Topsoil. Agronomy 2021, 11, 1393. [Google Scholar] [CrossRef]
- Bellamy, P.H.; Loveland, P.J.; Bradley, R.I.; Lark, R.M.; Kirk, G.J. Carbon losses from all soils across England and Wales 1978–2003. Nature 2005, 437, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Soil erosion and carbon dynamics. Soil. Till. Res. 2005, 81, 137–142. [Google Scholar] [CrossRef]
- Liaudanskiene, I.; Zukaitis, T.; Velykis, A.; Satkus, A.; Parasotas, I. The impact of tillage practices on the distribution of humified organic carbon in a clay loam. Zemdirbyste 2021, 108, 11–18. [Google Scholar] [CrossRef]
- Yang, Y.; Mohammat, A.; Feng, J.; Zhou, R.; Fang, J. Storage, patterns and environmental controls of soil organic carbon in China. Biogeochemistry 2007, 84, 131–141. [Google Scholar] [CrossRef]
- De Moraes, S.; Lal, J.C. Stratification ratio of soil organic matter pools as an indicator of carbon sequestration in a tillage chronosequence on a Brazilian Oxisol. Soil Tillage Res. 2009, 103, 46–56. [Google Scholar] [CrossRef]
- Zhao, X.; Xue, J.F.; Zhang, X.Q.; Kong, F.L.; Chen, F.; Lal, R.; Zhang, H.L. Stratification and storage of soil organic carbon and nitrogen as affected by tillage practices in the North China Plain. PLoS ONE 2015, 10, e0128873. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, Z.; Zhao, Y. Stratification ratio of soil organic carbon as an indicator of carbon sequestration and soil quality in ecological restoration. Restor. Ecol. 2017, 26, 555–562. [Google Scholar] [CrossRef]
- European Union. Nitrates Directive (91/676/EEC). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:01991L0676-20081211 (accessed on 29 July 2022).
- Slepetiene, A.; Kochiieru, M.; Jurgutis, L.; Mankeviciene, A.; Skersiene, A.; Belova, O. The Effect of Anaerobic Digestate on the Soil Organic Carbon and Humified Carbon Fractions in Different Land-Use Systems in Lithuania. Land 2022, 11, 133. [Google Scholar] [CrossRef]
- Nikitin, B.A. A method for soil humus determination. Agric. Chem. 1999, 3, 156–158. [Google Scholar]
- Ponomareva, V.V.; Plotnikova, T.A. Humus and Soil Formation; Nauka: Leningrad, Russia, 1980. [Google Scholar]
- Bhogal, A.; Nicholson, F.A.; Rollett, A.; Taylor, M.; Litterick, A.; Whittingham, M.J.; Williams, J.R. Improvements in the quality of agricultural soils following organic material additions depend on both the quantity and quality of the materials applied. Front. Sustain. Food Syst. 2018, 2, 9. [Google Scholar] [CrossRef]
- Tobiášová, E. Potential of the soil for stabilisation of organic carbon in soil aggregates. Agriculture 2015, 61, 50–60. [Google Scholar] [CrossRef][Green Version]
- Crespo, C.; Wyngaard, N.; Rozas, H.S.; Studdert, G.; Barraco, M.; Gudelj, V.; Barbagelata, P.; Barbieri, P. Effect of the intensification of cropping sequences on soil organic carbon and its stratification ratio in contrasting environments. Catena 2021, 200, 105145. [Google Scholar] [CrossRef]
- Tambone, F.; Scaglia, B.; D’Imporzano, G.; Schievano, A.; Orzi, V.; Salati, S.; Adani, F. Assessing amendment and fertilizing properties of digestates from anaerobic digestion through a comparative study with digested sludge and compost. Chemosphere 2010, 81, 577–583. [Google Scholar] [CrossRef]
- Franzluebbers, A.J. Soil organic matter stratification ratio as an indicator of soil quality. Soil Tillage Res. 2002, 66, 95–106. [Google Scholar] [CrossRef]
- Qiaorui, S.; Ali, A.; Biaobiao, W.; Wang, P.; Bois, G.; Jianping, Y.; Kubar, A.A. Numerical study on gas–liquid two phase flow characteristic of multistage electrical submersible pump by using a novel multiple-size group (MUSIG) modePhys. Fluids 2022, 34, 063311. [Google Scholar] [CrossRef]

| Land Use, Year | Depth, cm | Fertilization | ||||
|---|---|---|---|---|---|---|
| No Fertilizer | 85 N Solid | 85 N Liquid | 170 N Solid | 170 N Liquid | ||
| SOC (g kg−1) | ||||||
| Crop rotation field, 2019 | 0–10 | 11.35 ± 0.11 | 13.66 ± 1.28 | 11.39 ± 0.14 | 14.22 ± 0.25 | 11.99 ± 0.10 |
| 10–20 | 10.54 ± 0.20 | 10.93 ± 0.75 | 10.75 ± 0.37 | 11.74 ± 0.32 | 12.60 ± 0.26 | |
| 20–30 | 3.66 ± 0.90 | 4.38 ± 0.88 | 6.27 ± 1.00 | 5.64 ± 0.81 | 5.52 ± 1.69 | |
| 30–40 | 2.28 ± 0.53 | 2.31 ± 0.49 | 3.68 ± 0.77 | 2.80 ± 1.07 | 3.28 ± 0.56 | |
| Pr > F | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0002 | |
| Grassland, 2019 | 0–10 | 18.26 ± 3.08 | 15.13 ± 0.88 | 15.71 ± 1.61 | 15.54 ± 1.61 | 16.11 ± 1.51 |
| 10–20 | 9.30 ± 0.58 | 11.00 ± 1.03 | 11.87 ± 0.82 | 12.03 ± 0.66 | 10.78 ± 1.48 | |
| 20–30 | 3.98 ± 0.68 | 4.50 ± 0.89 | 5.40 ± 0.64 | 4.29 ± 0.67 | 4.32 ± 0.99 | |
| 30–40 | 1.79 ± 0.14 | 1.56 ± 0.51 | 2.23 ± 0.14 | 1.79 ± 0.21 | 2.36 ± 0.41 | |
| Pr > F | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0001 | |
| Crop rotation field, 2020 | 0–10 | 13.18 ± 0.08 | 13.38 ± 0.19 | 12.56 ± 0.16 | 13.34 ± 0.52 | 12.67 ± 0.59 |
| 10–20 | 12.23 ± 0.15 | 12.70 ± 0.49 | 11.78 ± 0.22 | 12.78 ± 0.20 | 12.27 ± 0.61 | |
| 20–30 | 7.52 ± 0.81 | 7.63 ± 0.94 | 5.82 ± 2.41 | 8.14 ± 0.76 | 7.16 ± 2.50 | |
| 30–40 | 2.94 ± 0.98 | 3.97 ± 1.42 | 2.69 ± 0.06 | 3.92 ± 0.27 | 3.44 ± 1.34 | |
| Pr > F | <0.0001 | 0.0002 | 0.0011 | <0.0001 | 0.0063 | |
| Grassland, 2020 | 0–10 | 14.82 ± 1.27 | 13.41 ± 1.26 | 14.90 ± 0.87 | 15.09 ± 1.26 | 15.71 ± 1.01 |
| 10–20 | 10.57 ± 0.94 | 8.29 ± 0.46 | 9.39 ± 1.27 | 10.45 ± 1.45 | 10.03 ± 0.87 | |
| 20–30 | 4.74 ± 0.69 | 3.15 ± 0.39 | 4.62 ± 1.55 | 3.07 ± 0.34 | 4.40 ± 0.84 | |
| 30–40 | 1.90 ± 0.19 | 1.83 ± 0.31 | 1.90 ± 0.42 | 1.94 ± 0.56 | 2.28 ± 0.27 | |
| Pr > F | <0.0001 | <0.0001 | 0.0002 | <0.0001 | <0.0001 | |
| Stratification ratio, SOC | ||||||
| Crop rotation field, 2019 | (0–10:10–20) | 1.08 ± 0.03 | 1.25 ± 0.05 | 1.06 ± 0.02 | 1.21 ± 0.02 | 0.95 ± 0.03 |
| (0–10:20–30) | 3.45 ± 0.71 | 3.26 ± 0.40 | 1.92 ± 0.34 | 2.63 ± 0.36 | 2.93 ± 1.24 | |
| (0–10:30–40) | 5.60 ± 1.37 | 6.20 ± 0.66 | 3.45 ± 0.85 | 6.52 ± 1.88 | 3.88 ± 0.67 | |
| Pr > F | 0.0323 | 0.0007 | 0.0479 | 0.0351 | 0.1051 | |
| Grassland, 2019 | (0–10:10–20) | 1.65 ± 0.06 | 1.40 ± 0.14 | 1.32 ± 0.05 | 1.29 ± 0.07 | 1.51 ± 0.07 |
| (0–10:20–30) | 4.08 ± 0.74 | 3.65 ± 0.74 | 2.92 ± 0.14 | 3.76 ± 0.53 | 4.05 ± 0.72 | |
| (0–10:30–40) | 8.64 ± 0.75 | 11.72 ± 3.11 | 7.00 ± 0.29 | 9.03 ± 1.81 | 7.05 ± 0.68 | |
| Pr > F | 0.0005 | 0.0171 | 0.0001 | 0.0064 | 0.0014 | |
| Crop rotation field, 2020 | (0–10:10–20) | 1.08 ± 0.02 | 1.06 ± 0.03 | 1.07 ± 0.02 | 1.04 ± 0.03 | 1.03 ± 0.03 |
| (0–10:20–30) | 1.79 ± 0.19 | 1.81 ± 0.22 | 2.90 ± 0.91 | 1.66 ± 0.11 | 2.48 ± 1.06 | |
| (0–10:30–40) | 5.51 ± 1.62 | 4.40 ± 1.55 | 4.68 ± 0.16 | 3.44 ± 0.29 | 4.60 ± 1.20 | |
| Pr > F | 0.0328 | 0.0875 | 0.0088 | 0.0002 | 0.0873 | |
| Grassland, 2020 | (0–10:10–20) | 1.40 ± 0.05 | 1.64 ± 0.23 | 1.65 ± 0.26 | 1.54 ± 0.37 | 1.61 ± 0.25 |
| (0–10:20–30) | 3.21 ± 0.32 | 4.43 ± 0.76 | 3.80 ± 0.86 | 5.04 ± 0.73 | 4.04 ± 1.23 | |
| (0–10:30–40) | 7.93 ± 1.01 | 7.91 ± 1.78 | 8.61 ± 1.94 | 9.30 ± 2.74 | 7.23 ± 1.44 | |
| Pr > F | 0.0007 | 0.0213 | 0.0185 | 0.0438 | 0.0313 | |
| Land Use, Year | Depth, cm | Fertilization | ||||
|---|---|---|---|---|---|---|
| No Fertilizer | 85 N Solid | 85 N Liquid | 170 N Solid | 170 N Liquid | ||
| Stratification Ratio, SOCstabile | ||||||
| Crop rotation field, 2019 | (0–10:10–20) | 1.10 ± 0.02 | 1.31 ± 0.06 | 1.11 ± 0.04 | 1.29 ± 0.04 | 0.92 ± 0.03 |
| (0–10:20–30) | 3.96 ± 0.43 | 3.10 ± 0.32 | 1.98 ± 0.35 | 2.64 ± 0.39 | 2.60 ± 1.04 | |
| (0–10:30–40) | 5.18 ± 1.41 | 5.45 ± 0.62 | 3.05 ± 0.72 | 5.92 ± 1.66 | 3.50 ± 0.59 | |
| Pr > F | 0.0362 | 0.011 | 0.0656 | 0.0394 | 0.0941 | |
| Grassland, 2019 | (0–10:10–20) | 1.78 ± 0.04 | 1.42 ± 0.24 | 1.49 ± 0.11 | 1.35 ± 0.10 | 1.56 ± 0.11 |
| (0–10:20–30) | 3.91 ± 0.79 | 3.48 ± 0.84 | 2.84 ± 0.08 | 3.60 ± 0.46 | 3.87 ± 0.90 | |
| (0–10:30–40) | 7.36 ± 1.01 | 13.38 ± 4.55 | 5.90 ± 0.54 | 7.97 ± 2.20 | 5.42 ± 0.41 | |
| Pr > F | 0.0051 | 0.0406 | 0.0002 | 0.0295 | 0.0088 | |
| Crop rotation field, 2020 | (0–10:10–20) | 1.09 ± 0.04 | 1.07 ± 0.04 | 1.07 ± 0.02 | 1.07 ± 0.01 | 1.01 ± 0.02 |
| (0–10:20–30) | 1.74 ± 0.14 | 1.80 ± 0.23 | 2.77 ± 0.83 | 1.65 ± 0.11 | 2.30 ± 0.92 | |
| (0–10:30–40) | 5.26 ± 1.66 | 4.15 ± 1.44 | 4.21 ± 0.08 | 3.42 ± 0.34 | 4.14 ± 1.04 | |
| Pr > F | 0.0451 | 0.0916 | 0.0106 | 0.0005 | 0.0841 | |
| Grassland, 2020 | (0–10:10–20) | 1.35 ± 0.03 | 1.56 ± 0.21 | 1.57 ± 0.27 | 1.60 ± 0.31 | 1.53 ± 0.23 |
| (0–10:20–30) | 2.90 ± 0.39 | 3.89 ± 0.79 | 3.32 ± 0.75 | 5.19 ± 0.83 | 3.78 ± 1.20 | |
| (0–10:30–40) | 6.30 ± 0.86 | 6.07 ± 1.58 | 6.46 ± 1.27 | 7.57 ± 1.88 | 5.43 ± 1.05 | |
| Pr > F | 0.0018 | 0.0561 | 0.0193 | 0.0342 | 0.0649 | |
| Land Use, Year | Axis | Equations | R2 | Significant Level | |
|---|---|---|---|---|---|
| X | Y | ||||
| Crop rotation field, 2019 | SOCstabile | SOC | Y = 0.74X + 0.26 | 0.97 | <0.0001 |
| Grassland, 2019 | SOCstabile | SOC | Y = 0.66X + 0.43 | 0.79 | <0.0001 |
| Crop rotation field, 2020 | SOCstabile | SOC | Y = 0.61X + 0.41 | 0.59 | 0.0008 |
| Grassland, 2020 | SOCstabile | SOC | Y = 1.02X + 0.01 | 0.89 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slepetiene, A.; Kochiieru, M.; Skersiene, A.; Mankeviciene, A.; Belova, O. Changes in Stabile Organic Carbon in Differently Managed Fluvisol Treated by Two Types of Anaerobic Digestate. Energies 2022, 15, 5876. https://doi.org/10.3390/en15165876
Slepetiene A, Kochiieru M, Skersiene A, Mankeviciene A, Belova O. Changes in Stabile Organic Carbon in Differently Managed Fluvisol Treated by Two Types of Anaerobic Digestate. Energies. 2022; 15(16):5876. https://doi.org/10.3390/en15165876
Chicago/Turabian StyleSlepetiene, Alvyra, Mykola Kochiieru, Aida Skersiene, Audrone Mankeviciene, and Olgirda Belova. 2022. "Changes in Stabile Organic Carbon in Differently Managed Fluvisol Treated by Two Types of Anaerobic Digestate" Energies 15, no. 16: 5876. https://doi.org/10.3390/en15165876
APA StyleSlepetiene, A., Kochiieru, M., Skersiene, A., Mankeviciene, A., & Belova, O. (2022). Changes in Stabile Organic Carbon in Differently Managed Fluvisol Treated by Two Types of Anaerobic Digestate. Energies, 15(16), 5876. https://doi.org/10.3390/en15165876








