Syntheses and Photovoltaic Properties of New Pyrazine-Based Organic Photosensitizers for Dye-Sensitized Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Regents
2.2. Measurements
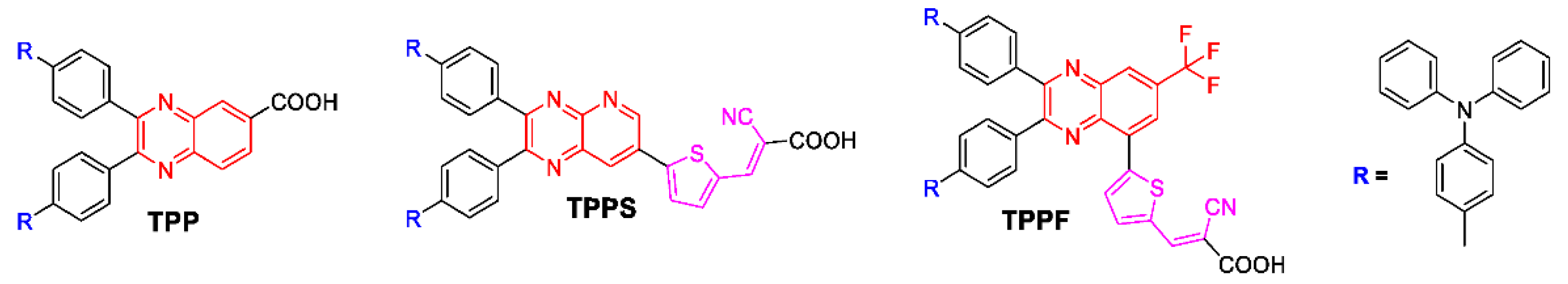
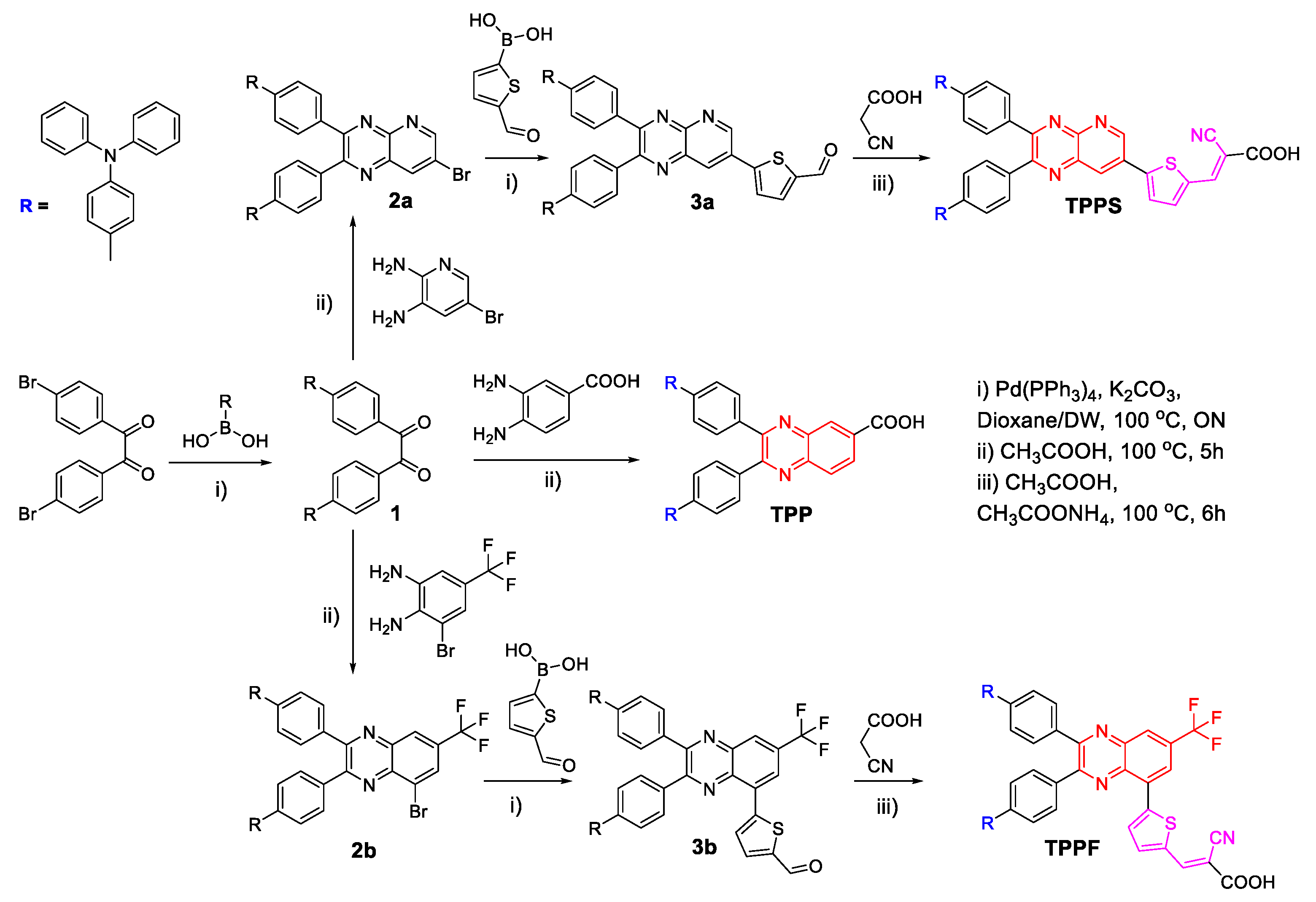
2.3. Synthesis of Photosensitizers
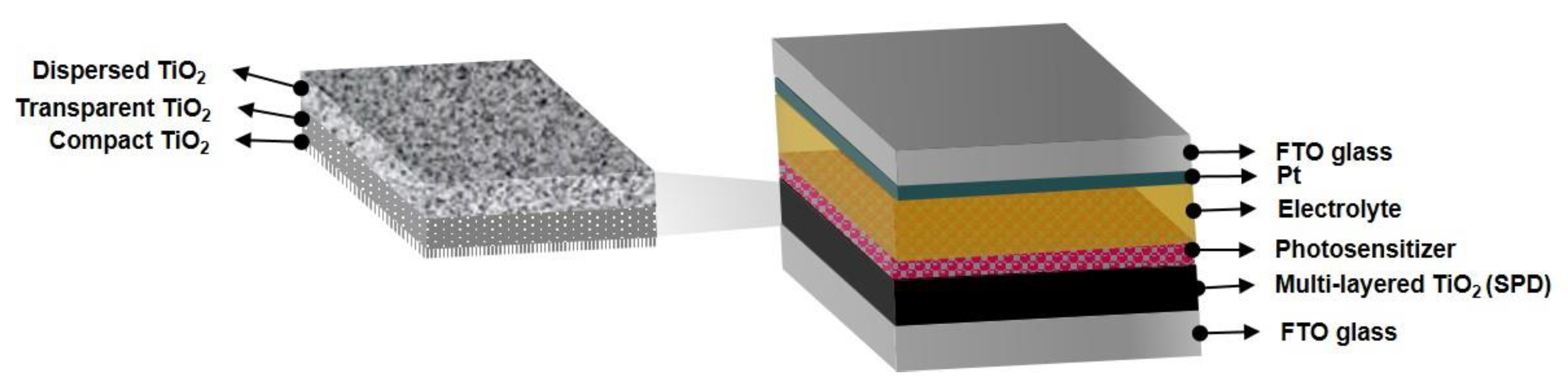
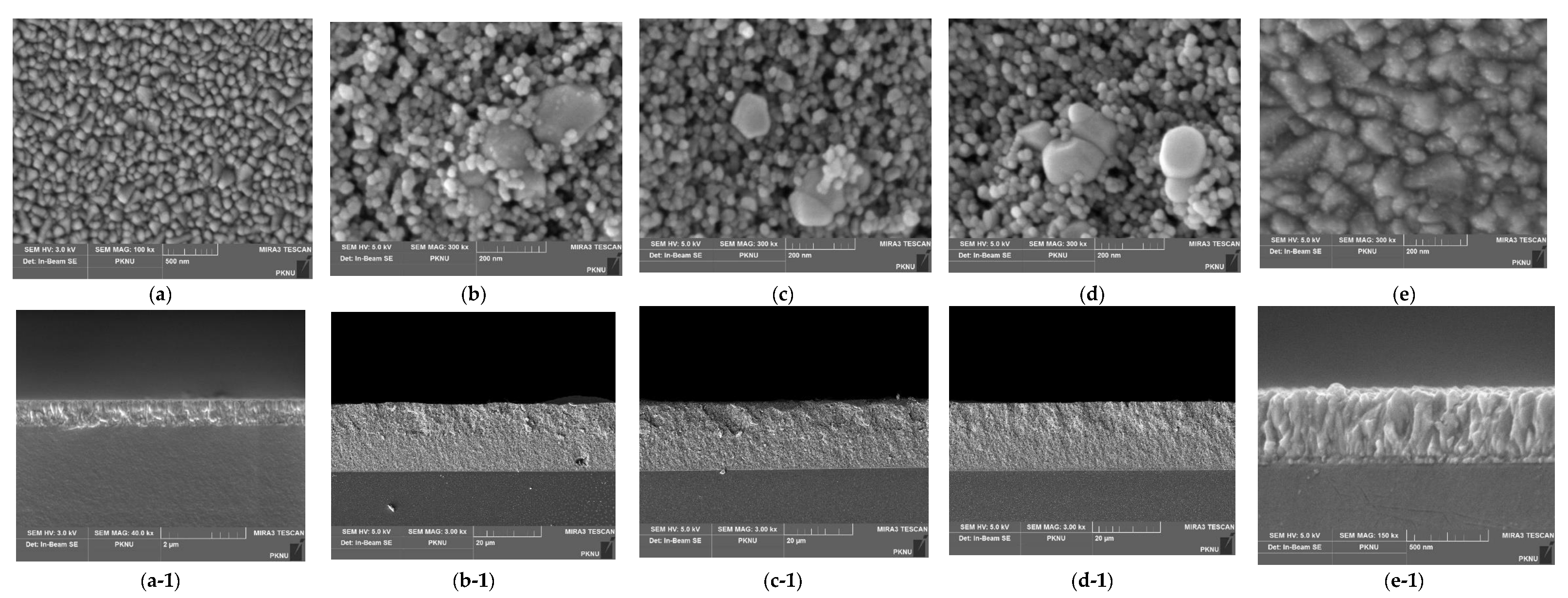
2.4. Fabrications of DSSC Devices
3. Results and Discussion
3.1. Synthesis of Photosensitizers
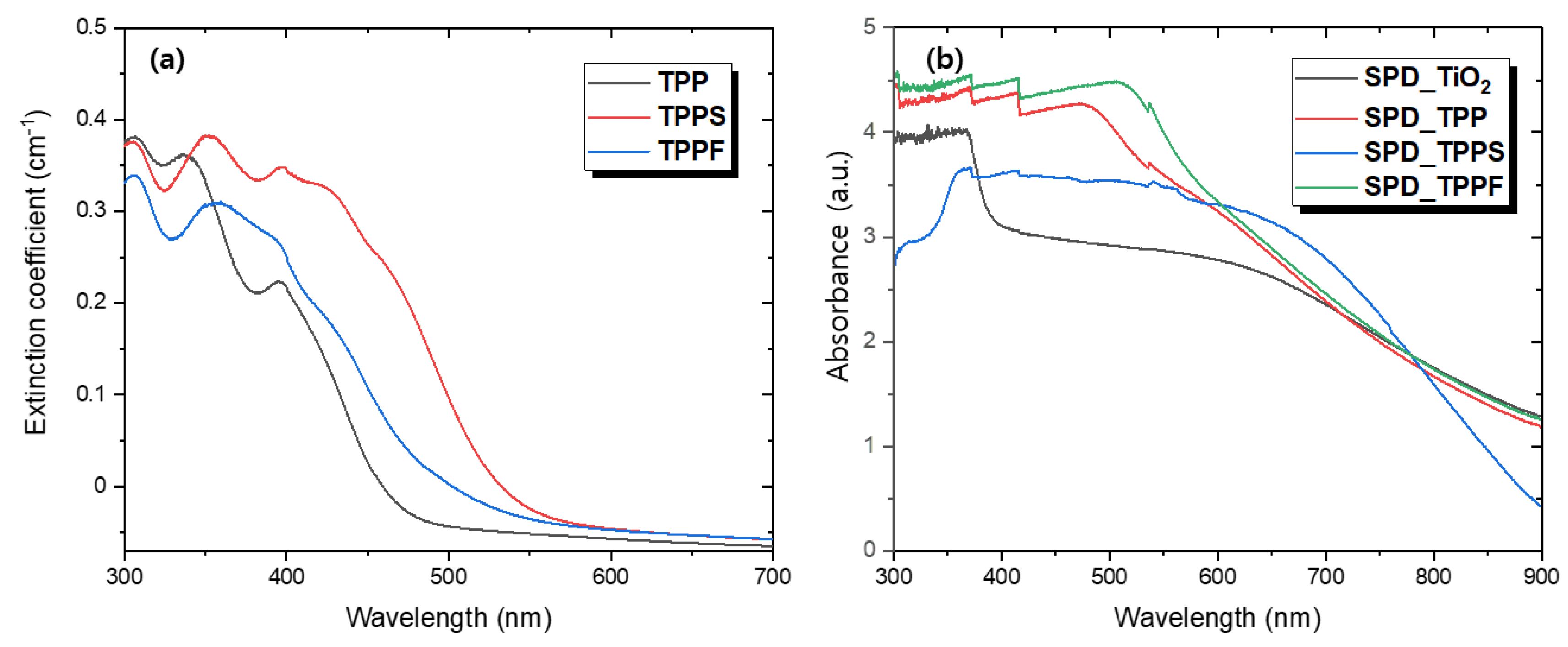
3.2. Optical Properties
3.3. Electrochemical Properties
3.4. Molecular Orbital Calculations
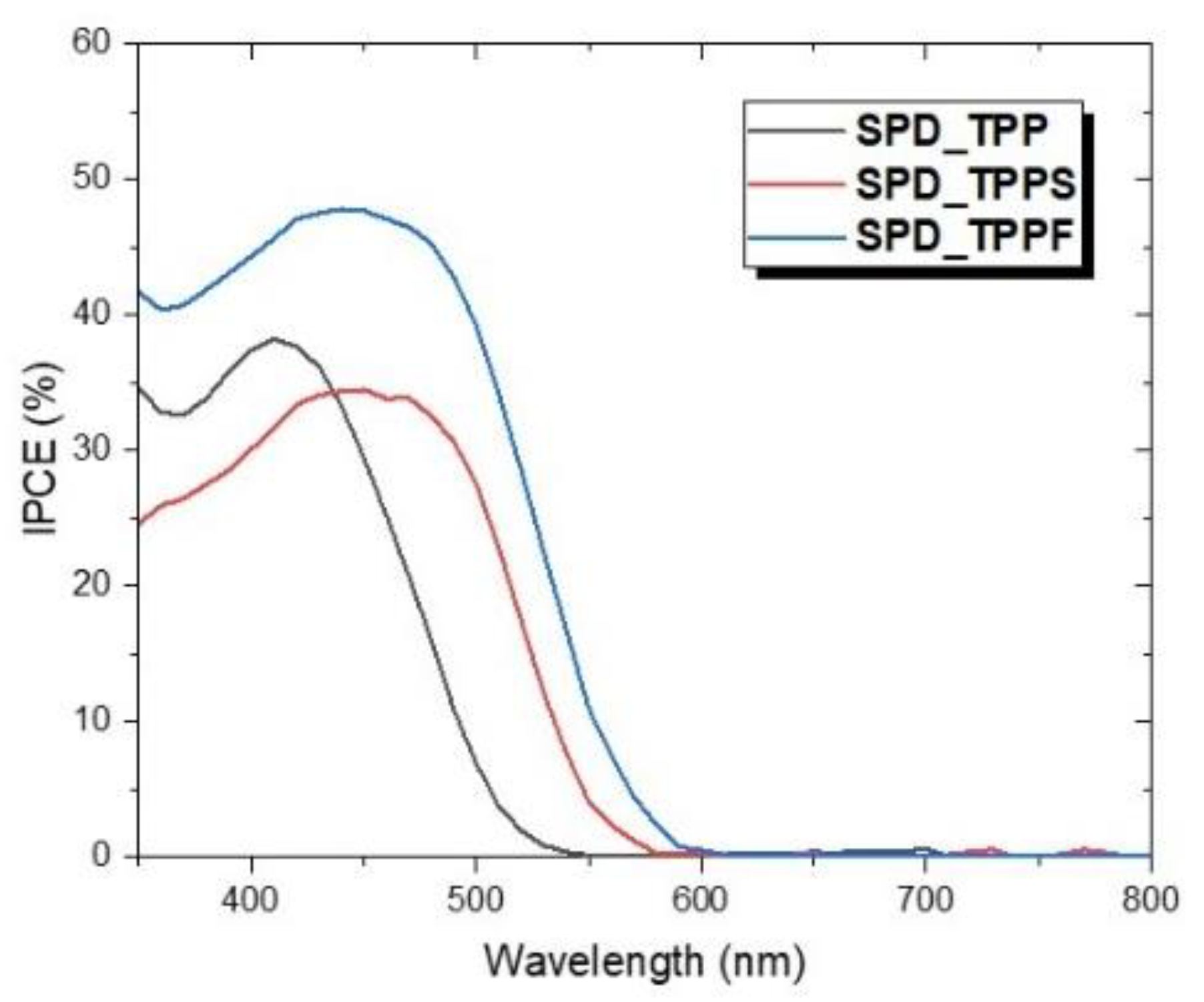
3.5. Photovoltaic Performance of DSSC Devices
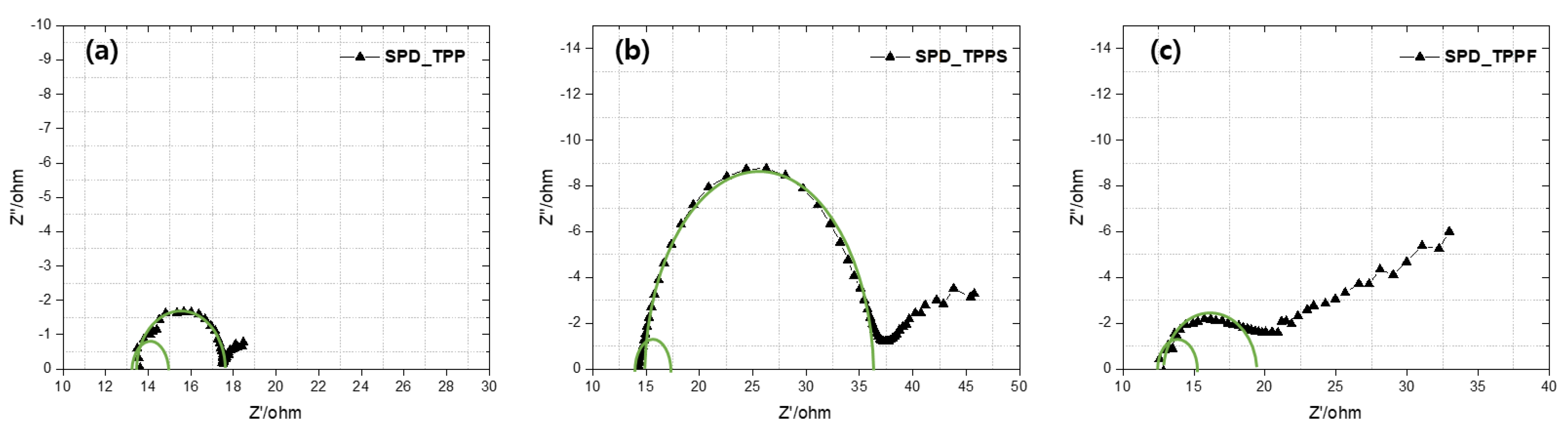
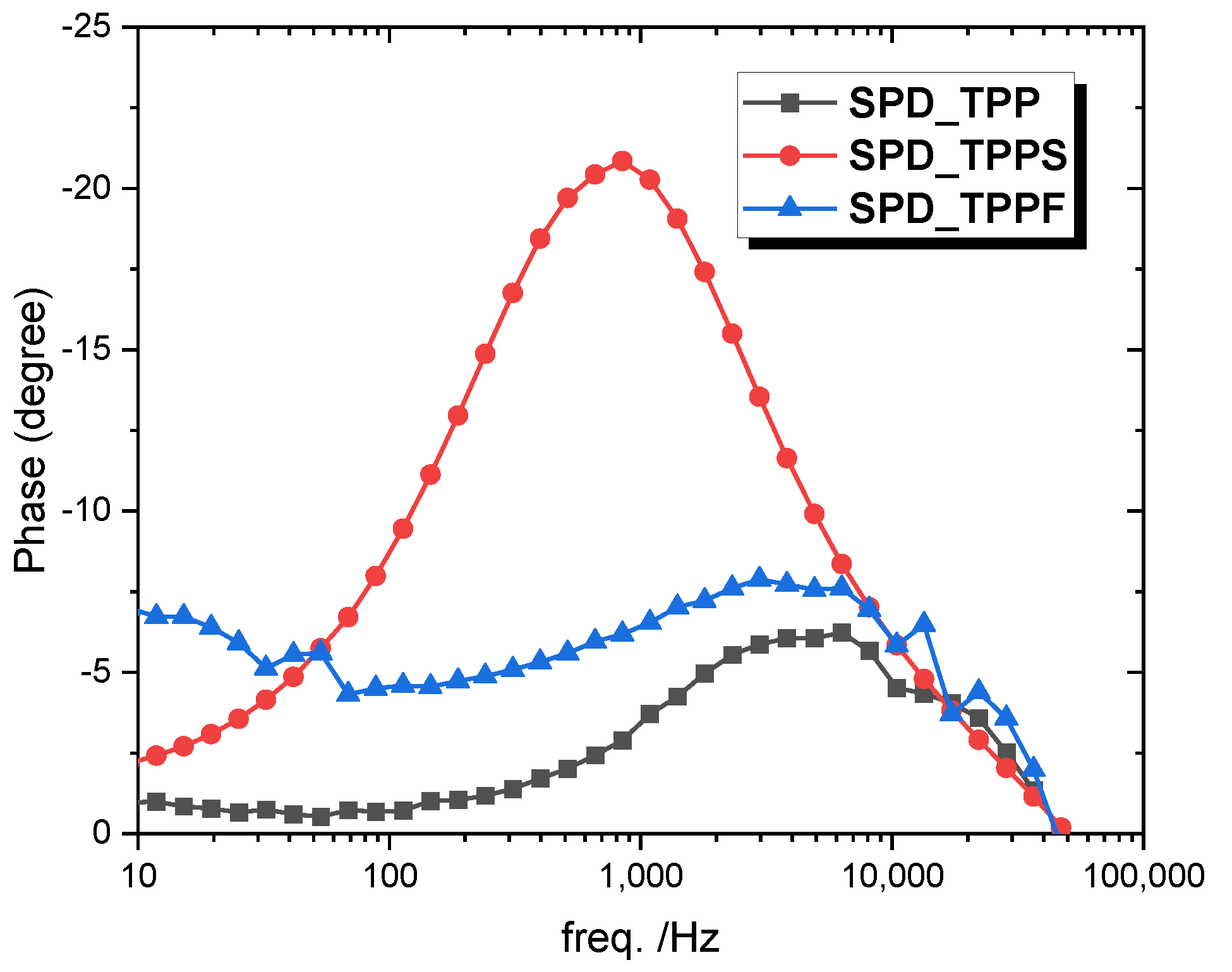
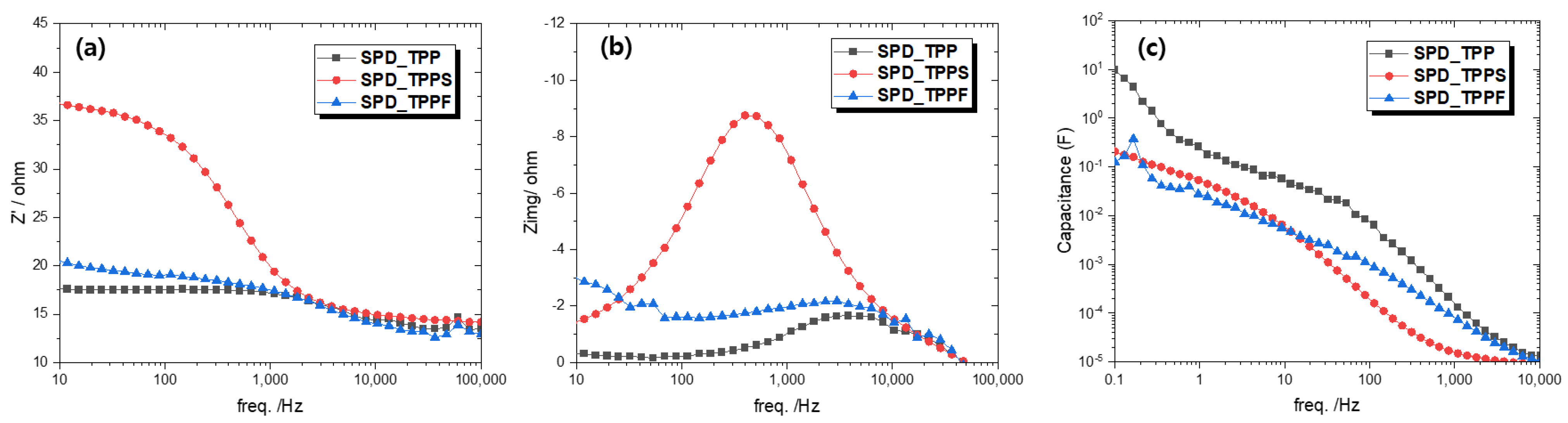
3.6. Electrochemical Impedance Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Regan, B.; Gratzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, V.; Sharma, S.S. Dye-sensitized solar cells: Fundamentals and current status. Nanoscale Res. Lett. 2018, 13, 381–427. [Google Scholar] [CrossRef]
- Nazeeruddin, K.; Baranoff, E.; Gratzel, M. Dye-sensitized solar cells: A brief overview. Sol. Energy 2011, 85, 1172–1178. [Google Scholar] [CrossRef]
- Altobello, S.; Bignozzi, C.A.; Caramori, S.; Larramona, G.; Quici, S.; Marzanni, G.; Lakhmiri, R. Sensitization of TiO2 with ruthenium complexes containing boronic acid functions. J. Photochem. Photobiol. A Chem. 2004, 166, 91–98. [Google Scholar] [CrossRef]
- Kunzmann, A.; Valero, S.E.; Sepúlveda, A.; Rico-Santacruz, M.; Lalinde, E.R.; Berenguer, J.; García-Martínez, J.M.; Guldi, D.; Serrano, E.D.; Costa, R. Hybrid dye-titania nanoparticles for superior low-temperature dye-sensitized solar cells. Adv. Energy Mater. 2018, 8, 121–212. [Google Scholar] [CrossRef]
- Chung, I.; Lee, B.; He, J.; Chang, R.P.H.; Kanatzidis, M.G. All-solid-state dye-sensitized solar cells with high efficiency. Nature 2012, 485, 486–489. [Google Scholar] [CrossRef]
- Tsai, M.C.; Wang, C.L.; Chang, C.W.; Hsu, C.W.; Hsiao, Y.H.; Liu, C.L.; Wang, C.C.; Lin, S.Y.; Lin, C.Y. A large, ultra-black, efficient and cost-effective dye-sensitized solar module approaching 12% overall efficiency under 1000 lux indoor light. Mater. Chem. A 2018, 6, 1995–2003. [Google Scholar] [CrossRef]
- Naim, W.; Novelli, V.; Nikolinakos, I.; Barbero, N.; Dzeba, I.; Grifoni, F.; Ren, Y.; Alnasser, T.; Velardo, A.; Borrelli, R.; et al. Transparent and colorless dye-sensitized solar cells exceeding 75% average visible transmittance. J. Am. Chem. Soc. 2021, 1, 409–426. [Google Scholar] [CrossRef]
- Velusamy, M.; Thomas, K.R.J.; Lin, J.T.; Hsu, Y.C.; Ho, K.C. Organic dyes incorporating low-band-gap chromophores for dye-sensitized solar cells. Org. Lett. 2005, 7, 1899–1902. [Google Scholar] [CrossRef]
- Cai, L.; Tsao, H.N.; Zhang, W.; Wang, L.; Xue, Z.; Gratzel, M.; Liu, B. Organic sensitizers with bridged triphenylamine donor units for efficient dye-sensitized solar cells. Adv. Energy Mater. 2013, 3, 200–205. [Google Scholar] [CrossRef]
- Yu, J.; Shen, T.L.; Weng, W.H.; Huang, Y.C.; Huang, C.I.; Su, W.F.; Rwei, S.P.; Ho, K.C.; Wang, L. Molecular design of interfacial modifiers for polymer-inorganic hybrid solar cells. Adv. Energy Mater. 2012, 2, 245–252. [Google Scholar] [CrossRef]
- Xiao, Y.; Su, X.; Lydia, S.V.; Lacaze, E.; Heinrich, B.; Donnio, B.; Kreher, D.; Mathevet, F.; Attias, A.J. Chemical engineering of donor–acceptor liquid crystalline dyads and triads for the controlled nanostructuration of organic semiconductors. Cryst. Eng. Commun. 2016, 18, 4787–4798. [Google Scholar] [CrossRef]
- Park, H.-H.; Meti, P.; Gong, Y.-D. Effect of conjugation on the optoelectronic properties of pyrazine-based push-pull chromophores: Aggregation-induced emission, solvatochromism, and acidochromism. Dyes Pigments 2021, 190, 109320. [Google Scholar] [CrossRef]
- Chalkias, D.A.; Charalampopoulos, C.; Aivali, S.; Andreopoulou, A.K.; Karavioti, A.; Stathatos, E. A di-carbazole-based dye as a potential sensitizer for greenhouse-integrated dye-sensitized solar cells. Energies 2021, 14, 1159. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, J.; Sakong, C.; Namgoong, J.W.; Lee, W.; Kim, D.H.; Kim, B.; Ko, M.J.; Kim, J.P. The effect of the number, position, and shape of methoxy groups in triphenylamine donors on the performance of dye-sensitized solar cells. Dyes Pigments 2015, 113, 390–401. [Google Scholar] [CrossRef]
- Tigreros, A.; Dhas, V.; Ortiz, A.; Insuasty, B.; Martín, N.; Echegoyen, L. Influence of acetylene-linked π-spacers on triphenylamine–fluorene dye sensitized solar cells performance. Sol. Energy Mater. Sol. Cells 2014, 121, 61–68. [Google Scholar] [CrossRef]
- Baheti, A.; Gajjela, S.R.; Balaya, P.; Thomas, K.R.J. Synthesis, optical, electrochemical and photovoltaic properties of organic dyes containing trifluorenylamine donors. Dyes Pigments 2015, 113, 78–86. [Google Scholar] [CrossRef]
- Misra, R.; Maragani, R.; Patelb, K.R.; Sharma, G.D. Synthesis, optical and electrochemical properties of new ferrocenyl substituted triphenylamine based donor–acceptor dyes for dye sensitized solar cells. RSC Adv. 2014, 4, 34904–34911. [Google Scholar] [CrossRef]
- Ying, W.; Yang, J.; Wielopolski, M.; Moehl, T.; Moser, J.E.; Comte, P.; Hua, J.; Zakeeruddin, S.M.; Tiana, H.; Gratzel, M. New pyrido[3,4-b]pyrazine-based sensitizers for efficient and stable dye-sensitized solar cells. Chem. Sci. 2014, 5, 206–214. [Google Scholar] [CrossRef]
- Meti, P.; Park, H.-H.; Gong, Y.-D. Recent developments in pyrazine functionalized p-conjugated materials for optoelectronic applications. J. Mater. Chem. C 2020, 8, 352–379. [Google Scholar] [CrossRef]
- Tian, H.; Yang, X.; Pan, J.; Chen, R.; Liu, M.; Zhang, Q.; Hagfeldt, A.; Sun, L. A Triphenylamine Dye Model for the Study of Intramolecular Energy Transfer and Charge Transfer in Dye-Sensitized Solar Cells. Adv. Funct. Mater. 2008, 18, 3461–3468. [Google Scholar] [CrossRef]
- Hara, K.; Sayama, K.; Ohga, Y.; Shinpo, A.; Sugab, S.; Arakawa, H. A coumarin-derivative dye sensitized nanocrystalline TiO2 solar cell having a high solar-energy conversion efficiency up to 5.6%. Chem. Commun. 2001, 6, 569–570. [Google Scholar] [CrossRef]
- Tian, H.; Yang, X.; Chen, R.; Pan, Y.; Li, L.; Hagfeldt, A.; Sun, L. Phenothiazine derivatives for efficient organic dye-sensitized solar cells. J. Chem. Commun. 2007, 36, 3741–3743. [Google Scholar] [CrossRef]
- Ning, Z.; Zhang, Q.; Wu, W.; Pei, H.; Liu, B.; Tian, H. Starburst Triarylamine Based Dyes for Efficient Dye-Sensitized Solar Cells. J. Org. Chem. 2008, 73, 3791–3797. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, S.M.; Seo, M.H.; Cho, S.E.; Hwang, W.P.; Park, S.H.; Kim, Y.G.; Lee, J.K.; Kim, M.R. Syntheses of organic dyes based on phenothiazine as photosensitizers and effects of their π-conjugated bridges on the photovoltaic performances of dye-sensitized solar cells. Macromol. Res. 2012, 20, 128–137. [Google Scholar] [CrossRef]
- Jung, I.; Lee, J.K.; Song, K.H.; Song, K.; Kang, S.O.; Ko, J.J. Synthesis and photovoltaic properties of efficient organic dyes containing the benzo[b]furan moiety for solar cells. J. Org. Chem. 2007, 72, 3652–3658. [Google Scholar] [CrossRef]
- Sayama, K.; Tsukagoshi, S.; Mori, T.; Hara, K.; Ohga, Y.; Shinpou, A.; Abe, Y.; Suga, S.; Arakawa, H. Efficient sensitization of nanocrystalline TiO2 films with cyanine and merocyanine organic dyes. Sol. Energy Mater. Sol. Cells 2003, 80, 47–71. [Google Scholar] [CrossRef]
- Horiuchi, T.; Miura, H.; Uchida, S. Highly efficient metal-free organic dyes for dye-sensitized solar cells. J. Photochem. Photobiol. A 2004, 164, 29–32. [Google Scholar] [CrossRef]
- Patrycja, M.; Michał, P.; Wojciech, M. How to Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar]
- Hagfeldt, A.; Grätzel, M. Light-induced redox reactions in nanocrystalline systems. Chem. Rev. 1995, 95, 49–68. [Google Scholar] [CrossRef]
- Asbury, J.B.; Wang, Y.-Q.; Hao, E.; Ghosh, H.N.; Lian, T. Evidences of hot excited state electron injection from sensitizer molecules to TiO2 nanocrystalline thin films. Res. Chem. Intermed. 2001, 27, 393–406. [Google Scholar] [CrossRef]
- Borbon, S.; Lugo, S.; Pourjafari, D.; Aguilar, N.P.; Oskam, G.; López, I. Open-circuit voltage (Voc) enhancement in TiO2-based DSSCs: Incorporation of ZnO nanoflowers and Au nanoparticles. ACS Omega 2020, 5, 10977–10986. [Google Scholar] [CrossRef] [PubMed]
- Kern, R.; Sastrawan, R.; Ferber, J.; Stangl, R.; Luther, J. Modeling and interpretation of electrical impedance spectra of dye solar cells operated under open-circuit conditions. Electrochim. Acta 2002, 47, 4213–4225. [Google Scholar] [CrossRef]
- Kuang, D.; Uchida, S.; Humphry-Baker, R.; Zakeeruddin, S.M.; Grätzel, M. Organic dye-sensitized ionic liquid based solar cells: Remarkable enhancement in performance through molecular design of indoline sensitizers. Angew. Chem. Int. Ed. 2008, 47, 1923–1927. [Google Scholar] [CrossRef] [PubMed]
- Jonscher, K. The ‘universal’ dielectric response. Nature 1977, 267, 673–679. [Google Scholar] [CrossRef]
- Singh, B.K.; Kumar, B. Impedance analysis and high temperature conduction mechanism of flux grown Pb(Zn1/3Nb2/3)0.91Ti0.09O3 single crystal. Cryst. Res. Technol. 2010, 45, 1003–1011. [Google Scholar] [CrossRef]
- Maier, J. Defect chemistry and ion transport in nanostructured materials: Part II. Aspects of nanoionics. Solid State Ion. 2003, 157, 327–334. [Google Scholar] [CrossRef]
- Sinclair, D.C.; West, A.R. Effect of atmosphere on the PTCR properties of BaTiO3 ceramics. J. Mater. Sci. 1994, 29, 6061–6068. [Google Scholar] [CrossRef]
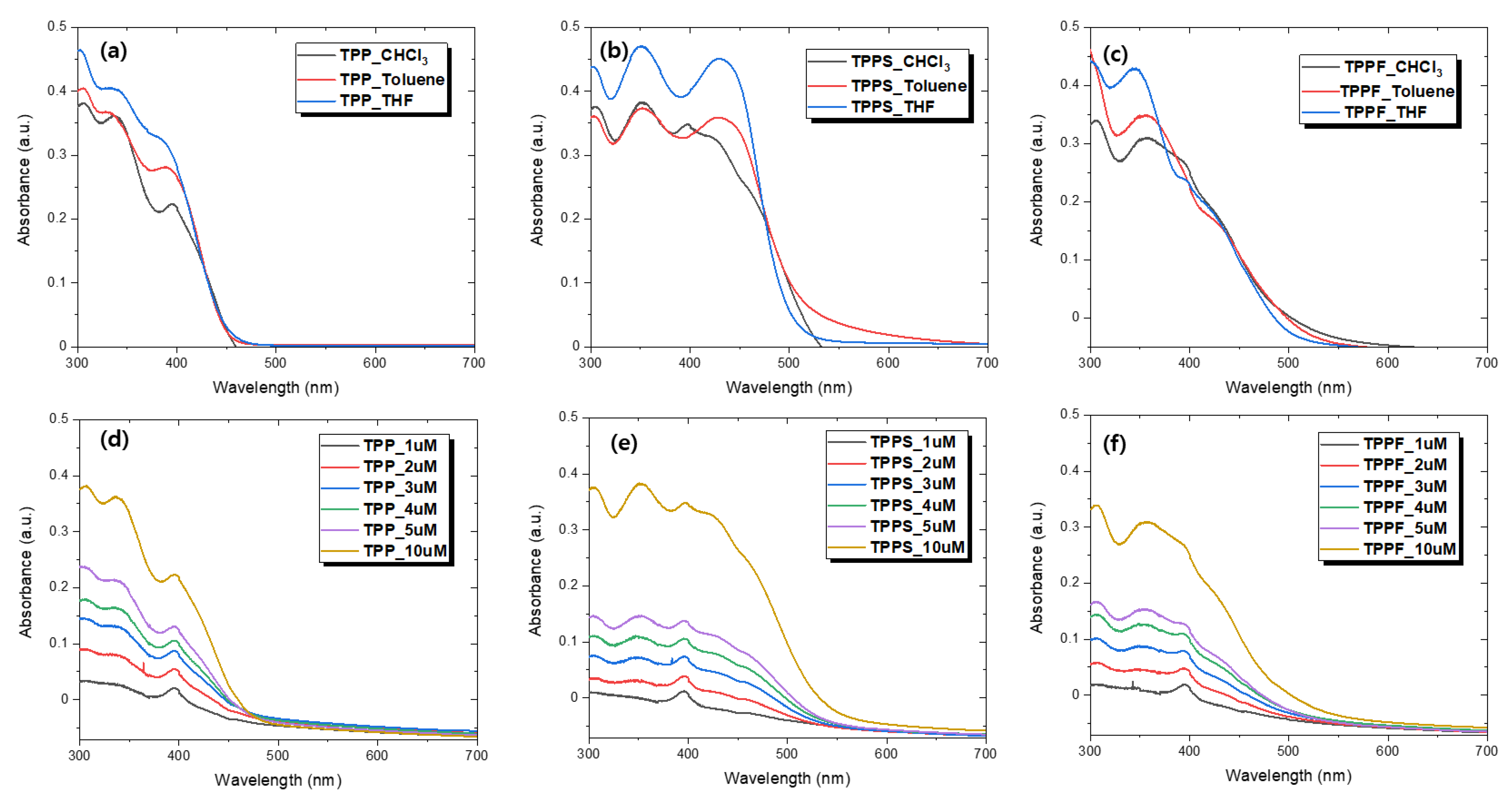

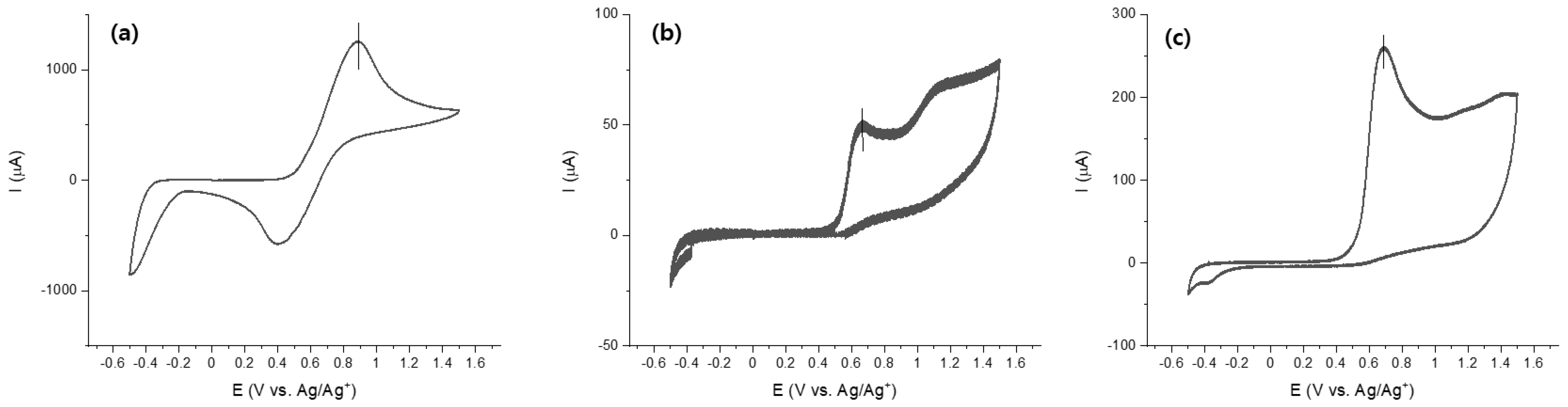


| Compound | λmax (nm) | Emission (nm) | E a (M−1cm−1) | Eoxid (V) | HOMO b/LUMO c (eV) | Optical Band Gap d (eV) | Theoretical Band Gap e (eV) |
|---|---|---|---|---|---|---|---|
| TPP | 338 f 336 g 338 h | 590 f 505 g 568 h | 21,770 | 0.89 | −5.91/−3.18 | 2.73 | 2.78 |
| TPPS | 350 f 350 g 352 h | 628 f 575 g 629 h | 37,700 | 0.65 | −5.67/−3.24 | 2.43 | 2.13 |
| TPPF | 358 f 358 g 357 h | 620 f 598 g 655 h | 27,510 | 0.68 | −5.70/−3.26 | 2.44 | 2.13 |
| Maximum IPCE (%) | Wavelength (nm) | Voc (V) | Jsc (mA/cm2) | Fill Factor | Efficiency (%) | |
|---|---|---|---|---|---|---|
| SPD_TPP | 38.19 | 410 | 0.72 | 3.84 | 0.65 | 1.8 |
| SPD_TPPS | 34.48 | 450 | 0.59 | 4.01 | 0.55 | 1.31 |
| SPD_TPPF | 47.8 | 440 | 0.69 | 5.69 | 0.67 | 2.64 |
| RS (Ω) | R1CT (Ω) | R2CT (Ω) | τe (ms) a | |
|---|---|---|---|---|
| SPD_TPP | 13.62 | 2.08 | 5.9 | 0.032 |
| SPD_TPPS | 14.36 | 5.66 | 22.56 | 0.188 |
| SPD_TPPF | 12.58 | 2.53 | 6.73 | 0.042 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-R.; Pham, T.C.; Choi, Y.; Yang, S.; Yang, H.-S.; Park, S.H.; Kang, M.; Lee, S. Syntheses and Photovoltaic Properties of New Pyrazine-Based Organic Photosensitizers for Dye-Sensitized Solar Cells. Energies 2022, 15, 5938. https://doi.org/10.3390/en15165938
Kim M-R, Pham TC, Choi Y, Yang S, Yang H-S, Park SH, Kang M, Lee S. Syntheses and Photovoltaic Properties of New Pyrazine-Based Organic Photosensitizers for Dye-Sensitized Solar Cells. Energies. 2022; 15(16):5938. https://doi.org/10.3390/en15165938
Chicago/Turabian StyleKim, Mi-Ra, Thanh Chung Pham, Yeonghwan Choi, Seah Yang, Hyun-Seock Yang, Sung Heum Park, Mijeong Kang, and Songyi Lee. 2022. "Syntheses and Photovoltaic Properties of New Pyrazine-Based Organic Photosensitizers for Dye-Sensitized Solar Cells" Energies 15, no. 16: 5938. https://doi.org/10.3390/en15165938





