Synthesis of 3D Nanonetwork Si Structures via Direct Ultrafast Pulsed Nanostructure Formation Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Analysis
2.3. Statistical Analysis
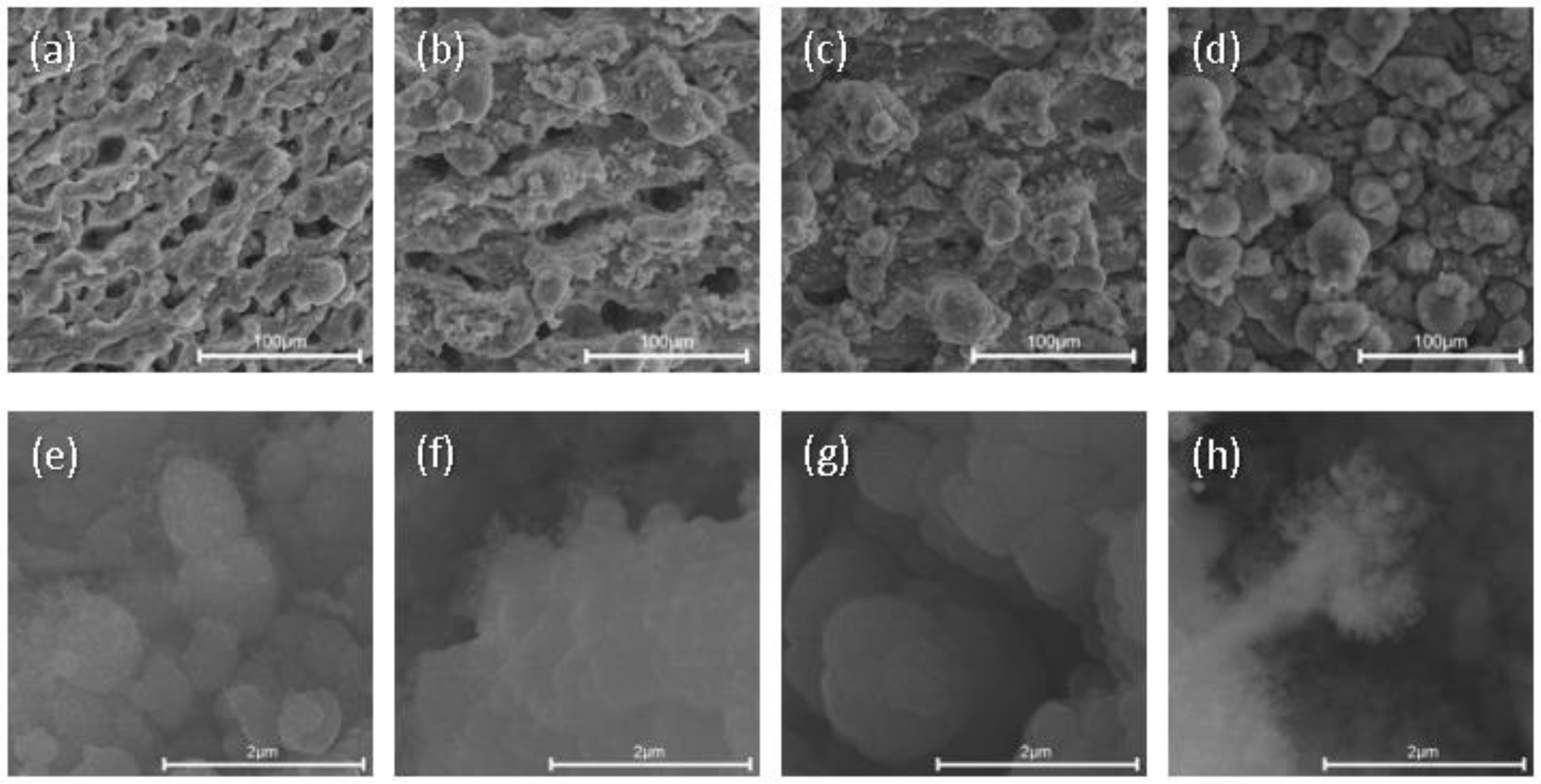
3. Results and Discussion
3.1. SEM
3.2. Optical Properties
3.2.1. Bandgap
3.2.2. Optical Conductivity
3.2.3. Dielectric Constant
3.3. Electrical Properties
Critical Breakdown Field
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parra, J.; Olivares, I.; Brimont, A.; Sanchis, P. Toward nonvolatile switching in silicon photonic devices. Laser Photonics Rev. 2021, 15, 2000501. [Google Scholar] [CrossRef]
- Kim, C.U.; Jung, E.D.; Noh, Y.W.; Seo, S.K.; Choi, Y.; Park, H.; Song, M.H.; Choi, K.J. Strategy for large-scale monolithic Perovskite/Silicon tandem solar cell: A review of recent progress. EcoMat 2021, 3, e12084. [Google Scholar] [CrossRef]
- Singh, N.; Xin, M.; Li, N.; Vermeulen, D.; Ruocco, A.; Magden, E.S.; Shtyrkova, K.; Ippen, E.; Kärtner, F.X.; Watts, M.R. Silicon photonics optical frequency synthesizer. Laser Photonics Rev. 2020, 14, 1900449. [Google Scholar] [CrossRef]
- Du, G.; Li, L.; Zhu, H.; Lu, L.; Zhou, X.; Gu, Z.; Zhang, S.T.; Yang, X.; Wang, J.; Yang, L. High-performance hole-selective V2OX/SiOX/NiOX contact for crystalline silicon solar cells. EcoMat 2022, 4, e12175. [Google Scholar] [CrossRef]
- Han, X.; Jiang, Y.; Frigg, A.; Xiao, H.; Zhang, P.; Nguyen, T.G.; Boes, A.; Yang, J.; Ren, G.; Su, Y. Mode and Polarization-Division Multiplexing Based on Silicon Nitride Loaded Lithium Niobate on Insulator Platform. Laser Photonics Rev. 2022, 16, 2100529. [Google Scholar] [CrossRef]
- Liao, K.; Chen, Y.; Yu, Z.C.; Hu, X.Y.; Wang, X.Y. All-optical computing based on convolutional neural networks. Opto-Electron. Adv. 2021, 4, 200060. [Google Scholar] [CrossRef]
- Asakawa, K.; Sugimoto, Y.; Nakamura, S. Silicon photonics for telecom and data-com applications. Opto-Electron. Adv. 2020, 3, 200011. [Google Scholar] [CrossRef]
- Fang, C.Z.; Yang, Q.Y.; Yuan, Q.C.; Gan, X.T.; Zhao, J.L. High-Q resonances governed by the quasi-bound states in the continuum in all-dielectric metasurfaces. Opto-Electron. Adv. 2021, 4, 200030. [Google Scholar] [CrossRef]
- Hassan, M.; Fakhri, M.; Adnan, S. 2-D of Nano Photonic Silicon Fabrication for Sensing Application. Dig. J. Nanomater. Biostructures 2019, 14, 873–878. [Google Scholar]
- Matsumoto, T.; Suzuki, J.-i.; Ohnuma, M.; Kanemitsu, Y.; Masumoto, Y. Evidence of quantum size effect in nanocrystalline silicon by optical absorption. Phys. Rev. B 2001, 63, 195322. [Google Scholar] [CrossRef]
- Jamwal, N.S.; Kiani, A. Gallium Oxide Nanostructures: A Review of Synthesis, Properties and Applications. Nanomaterials 2022, 12, 2061. [Google Scholar] [CrossRef] [PubMed]
- Ossicini, S. The optoelectronic properties of silicon nanostructures: The role of the interfaces. In Proceedings of the 2001 International Semiconductor Conference: CAS 2001 Proceedings (Cat. No. 01TH8547), Sinaia, Romania, 9–13 October 2001; IEEE: Piscataway, NJ, USA, 2001; pp. 23–26. [Google Scholar]
- Sina, M.; Orouji, A.A.; Ramezani, Z. Achieving a Considerable Output Power Density in SOI MESFETs Using Silicon Dioxide Engineering. Silicon 2021, 1–8. [Google Scholar] [CrossRef]
- Abid, I.; Canato, E.; Meneghini, M.; Meneghesso, G.; Cheng, K.; Medjdoub, F. GaN-on-silicon transistors with reduced current collapse and improved blocking voltage by means of local substrate removal. Appl. Phys. Express 2021, 14, 036501. [Google Scholar] [CrossRef]
- Duan, J.; Xiang, J.; Zhou, L.; Wang, X.; Ma, X.; Wang, W. Electron mobility in silicon nanowires using nonlinear surface roughness scattering model. Jpn. J. Appl. Phys. 2020, 59, 034002. [Google Scholar] [CrossRef]
- Puglisi, R.A.; Bongiorno, C.; Caccamo, S.; Fazio, E.; Mannino, G.; Neri, F.; Scalese, S.; Spucches, D.; La Magna, A. Chemical vapor deposition growth of silicon nanowires with diameter smaller than 5 nm. ACS Omega 2019, 4, 17967–17971. [Google Scholar] [CrossRef]
- Sharma, S.; Kamins, T.; Williams, R.S. Synthesis of thin silicon nanowires using gold-catalyzed chemical vapor deposition. Appl. Phys. A 2005, 80, 1225–1229. [Google Scholar] [CrossRef]
- Tsakalakos, L. Nanostructures for photovoltaics. Mater. Sci. Eng. R Rep. 2008, 62, 175–189. [Google Scholar] [CrossRef]
- Yoo, J.-K.; Kim, J.; Lee, H.; Choi, J.; Choi, M.-J.; Sim, D.M.; Jung, Y.S.; Kang, K. Porous silicon nanowires for lithium rechargeable batteries. Nanotechnology 2013, 24, 424008. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Joshi, M.; Rani, E.; Ingale, A.; Srivastava, A.; Kukreja, L. On red-shift of UV photoluminescence with decreasing size of silicon nanoparticles embedded in SiO2 matrix grown by pulsed laser deposition. J. Lumin. 2014, 154, 178–184. [Google Scholar] [CrossRef]
- Chambonneau, M.; Grojo, D.; Tokel, O.; Ilday, F.Ö.; Tzortzakis, S.; Nolte, S. In-Volume Laser Direct Writing of Silicon—Challenges and Opportunities. Laser Photonics Rev. 2021, 15, 2100140. [Google Scholar] [CrossRef]
- Paladiya, C.; Kiani, A. Synthesis of Silicon Nano-fibrous (SiNf) thin film with controlled thickness and electrical resistivity. Results Phys. 2019, 12, 1319–1328. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV–Vis spectra. ACS Publ. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Parmar, A.; Tripathi, S.; Goyal, N. Optical Study of Ge1Sb2Te4 and GeSbTe thin films. Mater. Res. Express 2019, 6, 046417. [Google Scholar] [CrossRef]
- Joshi, S.; Kiani, A. Hybrid artificial neural networks and analytical model for prediction of optical constants and bandgap energy of 3D nanonetwork silicon structures. Opto-Electron. Adv. 2021, 4, 210039-1. [Google Scholar] [CrossRef]
- Chukwuocha, E.O.; Onyeaju, M.C.; Harry, T.S. Theoretical studies on the effect of confinement on quantum dots using the brus equation. World J. Condens. Matter Phys. 2012, 2, 19097. [Google Scholar] [CrossRef]
- Wang, C.; Shim, M.; Guyot-Sionnest, P. Electrochromic nanocrystal quantum dots. Science 2001, 291, 2390–2392. [Google Scholar] [CrossRef]
- Beaudoin, M.; Meunier, M.; Arsenault, C. Blueshift of the optical band gap: Implications for the quantum confinement effect in a-Si: H/a-SiN x: H multilayers. Phys. Rev. B 1993, 47, 2197. [Google Scholar] [CrossRef]
- Sharma, I.; Tripathi, S.; Barman, P. An optical study of a-Ge20Se80− xInx thin films in sub-band gap region. J. Phys. D Appl. Phys. 2007, 40, 4460. [Google Scholar] [CrossRef]
- Löper, P. Silicon Nanostructures for Photovoltaics. Available online: https://www.reiner-lemoine-stiftung.de/pdf/dissertationen/Dissertation-Philipp_Loeper.pdf (accessed on 13 July 2022).
- Kalyanaraman, S.; Shajinshinu, P.; Vijayalakshmi, S. Refractive index, band gap energy, dielectric constant and polarizability calculations of ferroelectric Ethylenediaminium Tetrachlorozincate crystal. J. Phys. Chem. Solids 2015, 86, 108–113. [Google Scholar] [CrossRef]
- El-Desoky, M.M.; Ali, M.A.; Afifi, G.; Imam, H.; Al-Assiri, M.S. Effects of Annealing Temperatures on the Structural and Dielectric Properties of ZnO Nanoparticles. Silicon 2018, 10, 301–307. [Google Scholar] [CrossRef]
- Zunger, A.; Wang, L.-W. Theory of silicon nanostructures. Appl. Surf. Sci. 1996, 102, 350–359. [Google Scholar] [CrossRef]
- Muench, W.V.; Pfaffeneder, I. Breakdown field in vapor-grown silicon carbide p-n junctions. J. Appl. Phys. 1977, 48, 4831–4833. [Google Scholar] [CrossRef]
- Hwang, J.-S.; Lin, H.; Lin, K.; Zhang, X. Terahertz radiation from InAlAs and GaAs surface intrinsic-N+ structures and the critical electric fields of semiconductors. Appl. Phys. Lett. 2005, 87, 121107. [Google Scholar] [CrossRef]
- Rex, J.P.; Yam, F.K.; San Lim, H. The influence of deposition temperature on the structural, morphological and optical properties of micro-size structures of beta-Ga2O3. Results Phys. 2019, 14, 102475. [Google Scholar] [CrossRef]














| Sample | Frequency (kHz) | Pulse Interval (S) | Power (W) | Pulse Energy (J) | Bandgap (eV) |
|---|---|---|---|---|---|
| A1 | 600 | 1.67 × 10−6 | 15 | 1.67 × 10−4 | 1.45 |
| A2 | 800 | 1.25 × 10−6 | 15 | 1.25 × 10−4 | 1.44 |
| A3 | 1000 | 1.00 × 10−6 | 15 | 1.00 × 10−4 | 1.36 |
| A4 | 1200 | 1.67 × 10−6 | 15 | 8.33 × 10−4 | 1.3 |
| Sample | Frequency (kHz) | Pulse Interval (S) | Power (W) | Pulse Energy (J) | Bandgap (eV) |
|---|---|---|---|---|---|
| S1 | 600 | 1.67 × 10−6 | 10 | 1.11 × 10−4 | 1.36 |
| S2 | 800 | 1.25 × 10−6 | 13.3 | 1.11 × 10−4 | 1.38 |
| S3 | 1000 | 1.00 × 10−6 | 16.7 | 1.11 × 10−4 | 1.39 |
| S4 | 1200 | 8.33 × 10−7 | 20 | 1.11 × 10−4 | 1.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamwal, N.S.; Kiani, A. Synthesis of 3D Nanonetwork Si Structures via Direct Ultrafast Pulsed Nanostructure Formation Technique. Energies 2022, 15, 6005. https://doi.org/10.3390/en15166005
Jamwal NS, Kiani A. Synthesis of 3D Nanonetwork Si Structures via Direct Ultrafast Pulsed Nanostructure Formation Technique. Energies. 2022; 15(16):6005. https://doi.org/10.3390/en15166005
Chicago/Turabian StyleJamwal, Nishant Singh, and Amirkianoosh Kiani. 2022. "Synthesis of 3D Nanonetwork Si Structures via Direct Ultrafast Pulsed Nanostructure Formation Technique" Energies 15, no. 16: 6005. https://doi.org/10.3390/en15166005







