Abstract
The purpose of this study is to characterize and compare the microstructural features of the main morphotypes occurring in the char obtained at 850–950 °C by fluidized bed gasification of lignite from the “Szczerców” deposit (Central Poland), and to bring new insights into the knowledge on the origin of these morphotypes. Optical microscopy and Raman spectroscopy were used. The char is composed mostly of crassinetwork and inertoid, accompanied by tenuinetwork and small amounts of fusinoid. Tenuinetwork originates mainly from textinite, crassinetwork is formed from attrinite, while inertoid results from transformation of strongly gelified macerals such as densinite and ulminite. Similarities in the microstructure of tenuinetwork and crassinetwork as well as inertoid and fusinoid are observed. Inertoid and fusinoid are composed of larger aromatic systems, with lower amount of alkyl-aryl structures, and their microstructure is better organized compared to tenuinetwork and crassinetwork. Inertoid and fusinoid differ in microscopic appearance and were formed from different starting materials, but their microstructural properties converged during gasification. Different morphological features of the network morphotypes (tenuinetwork, crassinetwork) are not reflected in the differences in their microstructural characteristics.
1. Introduction
Poland is one of the few European countries where power generation is still largely based on domestically mined bituminous coal and lignite. The possibility of using coal in processes other than direct combustion was investigated due to the abundance of lignite reserves in Poland. The main focus was on clean coal technologies and in particular the gasification process in gasifiers. Research on various aspects of coal gasification technology has been conducted for many years [1,2,3,4,5,6,7,8,9,10,11,12,13,14]. Recently, due to the urgent need to mitigate climate changes and in connection with the rise in oil and gas prices this issue has taken on particular significance. Lignite is mined on a large scale in many countries, mainly in Germany, China, Russia, United States, and Poland. The development of local coal gasification projects is also essential in order to meet the needs of individual countries, which simultaneously translates into energy security and independence from external suppliers. Lignite usability for gasification was confirmed in several studies, usually conducted on coal from China, Japan, Canada, Russia, and the USA [10,11,15,16,17,18,19].
Previous works demonstrated the importance of petrographic examination in determining the suitability of coal for gasification and in predicting its behavior during the process. Furthermore, the importance and role of individual macerals in the gasification process was highlighted [5,12,20,21,22,23,24]. The petrographic features of chars obtained by the gasification process were also investigated [12,24,25,26,27,28,29,30,31]. The evolution of char structure can be characterized by coal petrography, in particular, the percentage char determination [27].
The structure of char was found to be one of the key factors affecting its reactivity during gasification [14,32]. To characterize the chemical-structural evolution of coals during gasification, and to determine the properties of the resulting chars Raman spectroscopy was frequently used [14,27,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47]. It was found that with increasing temperature the amount of large aromatic structures increased at the cost of smaller systems (<6 fused aromatic rings) and amorphous carbon [14,27,33,34,35,42,43]. The concentration of active sites (sp2–sp3 bonding) initially increased in lower temperature, and decreased above 800 °C [27]. The course of structural changes depends also on the gasifying agent [37,38].
However, these studies focused on bulk samples, and almost no attention was paid to the individual petrographic components of chars [48]. Therefore, structural properties of the morphotypes that may affect char reactivity remain unknown. It has also not been known whether the differences in morphological features, which are the basis for identification of the char morphotypes [26], are related to different microstructural characteristics of these components.
The purpose of this study was to characterize and compare the microstructural features of the main morphotypes occurring in the char produced by fluidized bed gasification of lignite from the “Szczerców” deposit (Central Poland), and to bring new insights into the knowledge on the origin of these morphotypes. The Raman characteristics of lignite macerals before gasification were obtained previously [49].
2. Materials and Methods
2.1. Gasification
The tested material is the residue from the gasification of lignite from the “Szczerców” deposit. The deposit is situated in the southern part of the Middle Polish synclinorium, within the Kleszczów Graben in Central Poland. The gasified coal is of the Miocene age. The physico-chemical parameters of the gasified lignite from the “Szczerców” deposit are shown in Table 1, whereas its petrographic composition is given in Table 2.

Table 1.
Physico-chemical parameters of lignite from the “Szczerców” deposit [50].

Table 2.
Petrographic composition of lignite from the “Szczerców” deposit [49].
The lignite was gasified at the Institute for Chemical Processing of Coal in Zabrze, Poland, in a pilot circulating fluidized bed reactor using CO2 as the gasification agent. The other components of the gasification mixture were nitrogen and oxygen. The temperature was between 856 °C and 952 °C. The process used 97 kg of coal, which yielded 21.5 kg of char [56].
The gasification parameters and the content of combustible components in the process gases are presented in Table 3. The entire system was discussed by [56]. Table 4 shows the physical parameters and chemical composition of the resulting char. A representative channel sample of char was collected to make preparations for microscopic and spectroscopic examinations.

Table 3.
Gasification process parameters and the content of combustible components in the process gases [50].

Table 4.
Physico-chemical parameters of the studied char [50].
2.2. Petrographic Analysis
The petrographic analysis of chars was based on the procedure used to develop a coal char classification [26]. It was performed with the use of 500 equally spaced points on the surface of the polished sections, prepared following the ISO standard 7404-2:2009 [57].
2.3. Raman Spectroscopy
Raman spectroscopy investigation was carried out on polished sections, allowing for identification of char morphotypes. This was done following the previous studies [27,48]. However, based on the recommendations of Lünsdorf [58], the final polishing (with the finest slurry of 0.5 μm) was omitted to exclude the possible effect of polishing on the Raman spectra. Vitrinites are unaffected by polishing up to maximum reflectance value of 7% [58].
The Raman measurements were performed on 22 (fusinoid) to 32 (inertoid) randomly chosen spots, with a Thermo Scientific DXR Raman microscope with a 900 grooves/mm grating and a CCD detector. The smaller number of analyses executed on fusinoid, in comparison to other morphotypes, was due to low proportion of this component. The Olympus 10× (NA 0.25) objectives (spot sizes 2.1 μm and 1.1 μm, respectively) were used. Excitation was activated with a 532 nm diode laser with a maximum power of 10 mW. Measurements were conducted in a spectral range of 400–3500 cm−1, at a spectral resolution of 1 cm−1, and an area of 1 μm2. The laser power was set at 1–2 mW. The spectrometer was calibrated using a polystyrene standard. The accumulated measurement time was 30 s for each spectrum. The spectra were analyzed by peak-fitting performed in the range of 1000–1800 cm−1 using GRAMS32, based on the previous Raman studies of chars and related carbon materials [39,40,59,60,61,62]. The second derivative of the spectra was also considered to find the initial positions of the bands. Four bands (D2, G, D1, and D4) were fitted by Lorentzian curves and one (D3 band) by the Gaussian curve. The goodness of fit was checked by the χ2 test. The ID1/IG ratio was determined from the D1 and G band intensities (heights). Furthermore, the AD1/AALL, AD3/AALL, and AD4/AALL ratios were calculated from the band areas, where “AALL” denotes the sum of all band areas in a spectrum. The final Raman results given below are the arithmetic means of the values obtained from the individual spectra. The standard deviations were also calculated.
2.4. Statistical Analysis
To assess the statistical significance of the differences in the spectral parameters between the morphotypes, the analysis of variance ANOVA and the Tukey’s HSD multiple comparison test were performed. This was preceded by checking the normality of distribution by the Shapiro-Wilk test and assessing the equality of variances by the Levene test. All statistical analyses were carried out using the Statistica 13.3. software (TIBCO Software Inc., Palo Alto, CA, USA).
3. Results and Discussion
3.1. Petrographic Composition of the Char
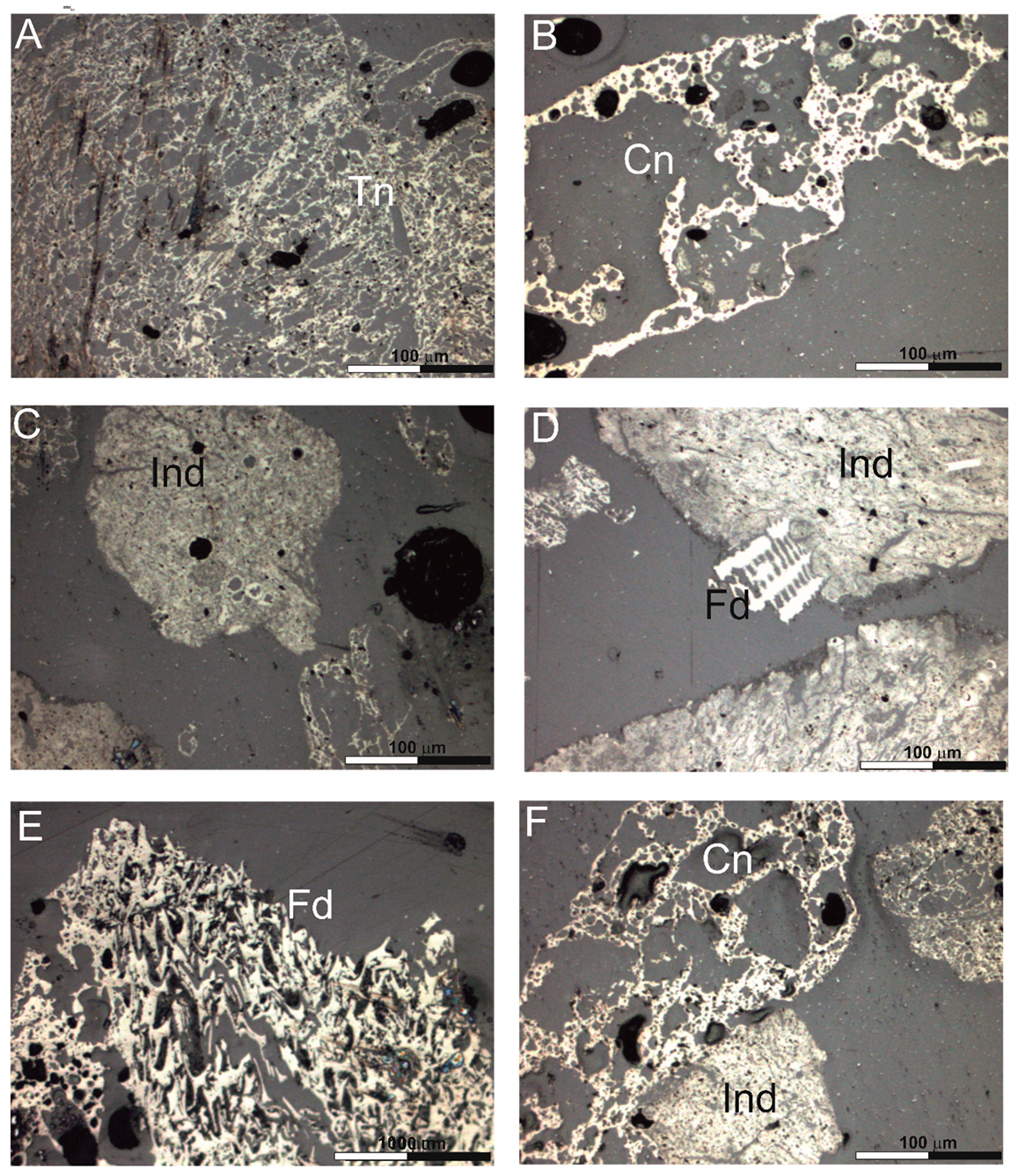
The petrographic composition of the char is dominated by inertoid (35.44% vol.) (Table 5, Figure 1C,D) and crassinetwork particles (31.59% vol.) (Table 5, Figure 1B,F). Inertoids are dense with porosities between 5 and 40% [26]. In the tested samples it can be either fused or unfused. On the other hand, commonly observed crassinetwork chars are particles with internal network structure, where most of wall area >3 μm, and the porosity is greater than 40%. However, it should be noted that, in total, chars with several types of pores formed by degassing (tenuinetwork, crassinetwork, tenuisphere, crassisphere, mixed porous) account for 52.33% by volume of all components (Table 5).

Table 5.
Petrographic composition of chars. Reprinted with permission from [29] 2016 Elsevier.
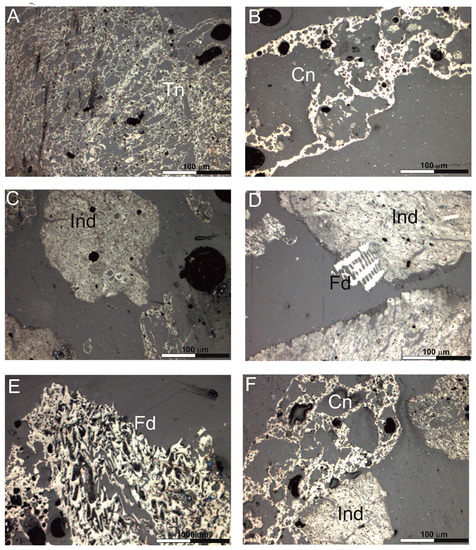
Figure 1.
Char morphotypes: (A)—tenuinetwork, (B)—crassinetwork, (C)—inertoid, (D)—inertoid and fusinoid, (E)—fusinoid, (F)—crassinetwork and inertoid. Explanations: Tn—tenuinetwork, Cn—crassinetwork, Ind—inertoid, Fd—fusinoid.
Tenuinetwork, such as crassinetwork have internal network structure, but their porosity is over 70%. In contrast to crassinetwork, in the case of tenuinetwork more than 50% of wall area is thinner than 3 μm. Sphere-type grains, tenuisphere and crassisphere, which are spherical to angular in shape and have porosity higher than 60%, are less commonly observed in the gasification residue.
In tenuisphere chars over 50% of wall area is below 3 μm, for crassisphere chars over 50% of wall area is below 3 μm. The share of fusinoid, which is characterized by inherited cellular fusinite structure, or solid particle with <5% porosity, in the tested chars is 1.70% vol., (Figure 1D,E). The inertinite group macerals almost do not change during the gasification process.
Gasification produces char that contains mineral matter. These are products of the thermal decomposition of the original mineral matter. The minerals in lignite are dominated by quartz and clay minerals (Table 2). The output coal from the “Szczerców” deposit also contains calcite and, to some extent, aragonite. Calcite, occurring in the form of lacustrine chalk in deposits, forms layers and spherical aggregates in the seam. Pyrite occurs as framboidal pyrite, but also as regular crystals and in the form of veins. The process produces loose, glassy or crystalline mixtures of silicates and aluminosilicates, and small amounts of complex mixtures of metal oxides and metalloids. Some minerals, such as pyrite, quartz, dolomite, and siderite, retain their original form during gasification. Clay minerals are also present in inertoids.
3.2. Raman Spectroscopy of the Char Morphotypes
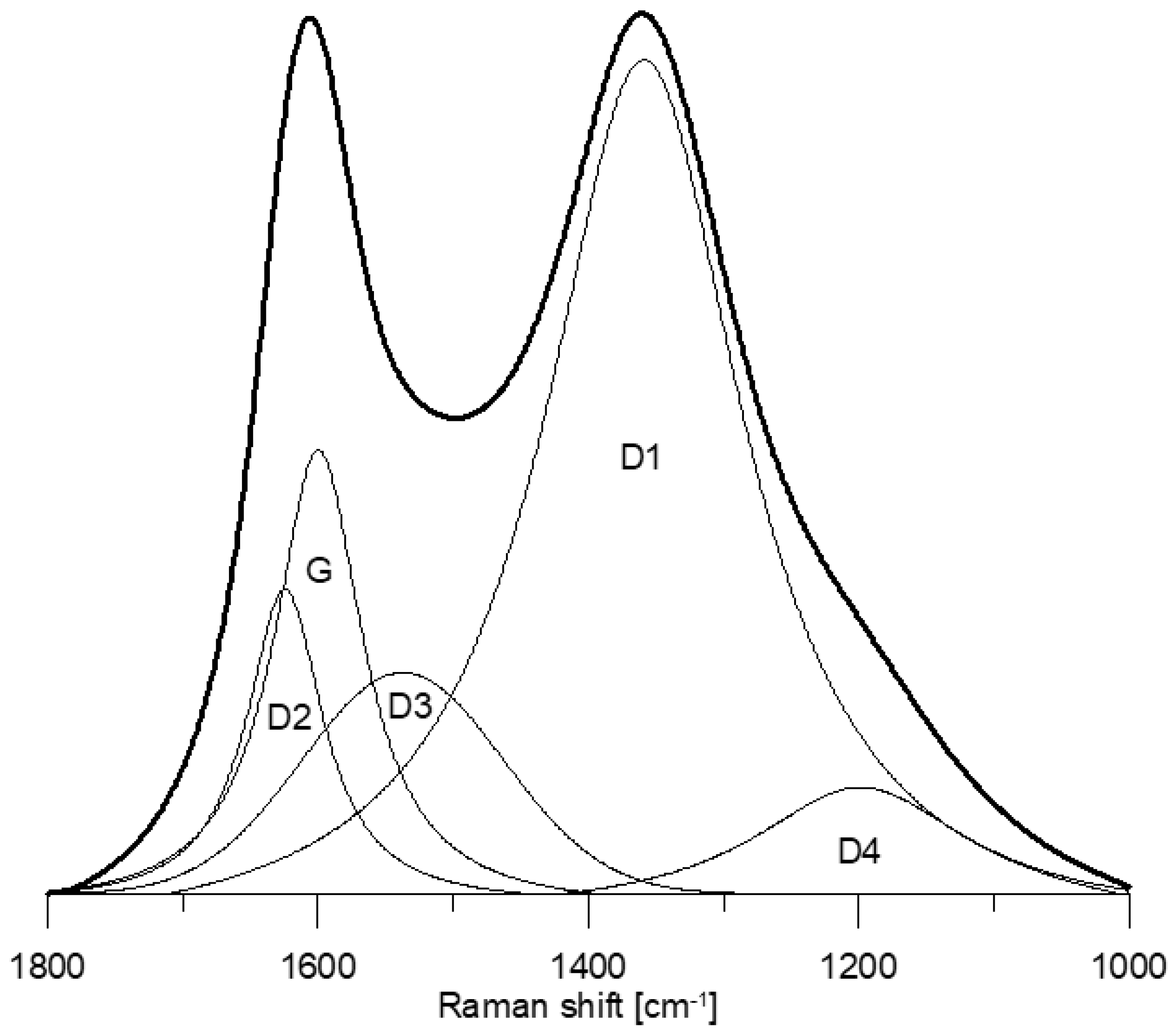
The Raman spectra of the studied morphotypes (Figure 2) show the occurrence of five bands: D2, G, D3, D1, and D4 (Figure 3). The G band (~1585 cm−1) corresponds to the graphitic lattice vibration (E2g mode) or aromatic ring breathing [34,63,64]. The D2 band (~1612 cm−1) makes a shoulder on the G band, and it is also assigned to the E2g mode [65,66]. Its intensity decreases with increasing degree of organization [67]. The D3 band (~1510–1525 cm−1) originates due to interstitial defects outside the plane of aromatic layers [68,69]. It is attributed to the occurrence of small aromatic systems composed of 3–5 rings, organic molecules or functional groups forming “amorphous” carbon phase [59,66,70]. Sometimes two [48] or three [34] bands are detected within the D3 band region. The D1 band (~1340 cm−1) is related to vibration mode A1g of graphitic lattice and assigned to in-plane defects, and the occurrence of heteroatoms [62,63,68,69] as well as aromatics with six or more fused rings [34]. The D4 band (~1180–1200 cm−1) is attributed to sp3 or sp2–sp3 carbons such as alkyl-aryl C–C structures, which have been suggested as the location of active sites [33,34,59,71,72,73]. Such sites are mostly positioned at the edges of aromatic structures [72,73,74]. Three bands within the D4 band region were also proposed [34]. The extracted spectral parameters are given in Table 6 and Table 7.
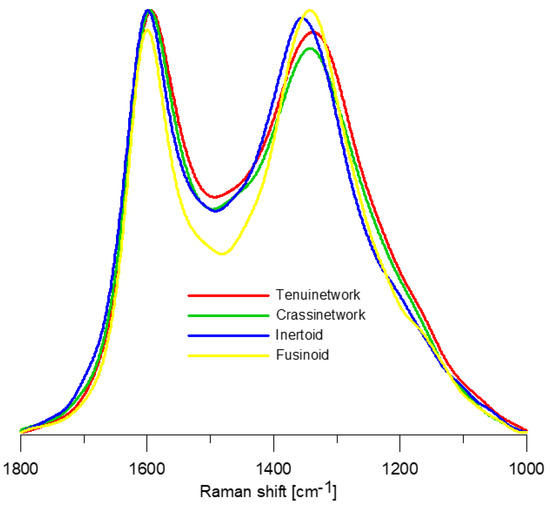
Figure 2.
Representative Raman spectra of the investigated morphotypes.
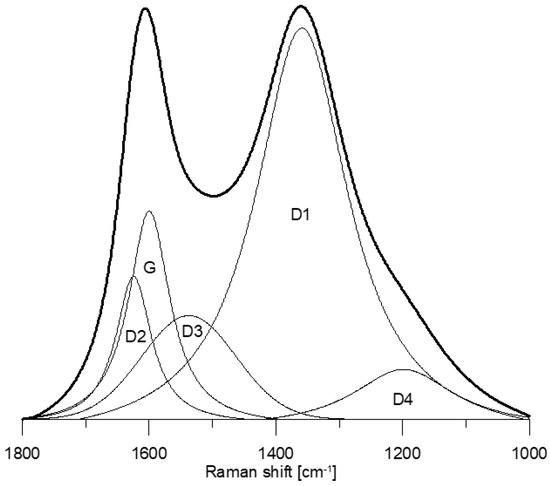
Figure 3.
Curve-fitting of a representative inertoid spectrum.

Table 6.
Position and full width at half maximum (FWHM) of the Raman bands in the spectra of the studied morphotypes.

Table 7.
Raman spectral ratios for the studied morphotypes.
The spectra, taking the occurrence of the D3 and D4 bands, and overlapping D2 and G bands, are typical for poorly organized carbonaceous material (“crystallinity” level 1 [75]).
To assess the significance of the differences in the spectral parameters between the morphotypes, the statistical analysis was performed. The Shapiro–Wilk test demonstrates that all sets of results obtained for the G band position and FWHM, D1 band FWHM, and ID1/IG, AD1/AALL and AD4/AALL ratios, for the morphotypes analyzed (tenuinetwork, crassinetwork, inertoid and fusinoid) have normal distribution, and the Levene test shows that variances are equal. Considering this, the ANOVA analysis of variance was conducted. It indicates that in all six sets of the above mentioned spectral parameters at least one mean value significantly differs from the others. This was checked by the Tukey’s HSD multiple comparison test, results of which are summarized in Table 8, Table 9, Table 10, Table 11 and Table 12.

Table 8.
Results of the Tukey’s HSD multiple comparison test for the G band position.

Table 9.
Results of the Tukey’s HSD multiple comparison test for the G band FWHM.

Table 10.
Results of the Tukey’s HSD multiple comparison test for the ID1/IG ratio.

Table 11.
Results of the Tukey’s HSD multiple comparison test for the AD1/AALL ratio.

Table 12.
Results of the Tukey’s HSD multiple comparison test for the AD4/AALL ratio.
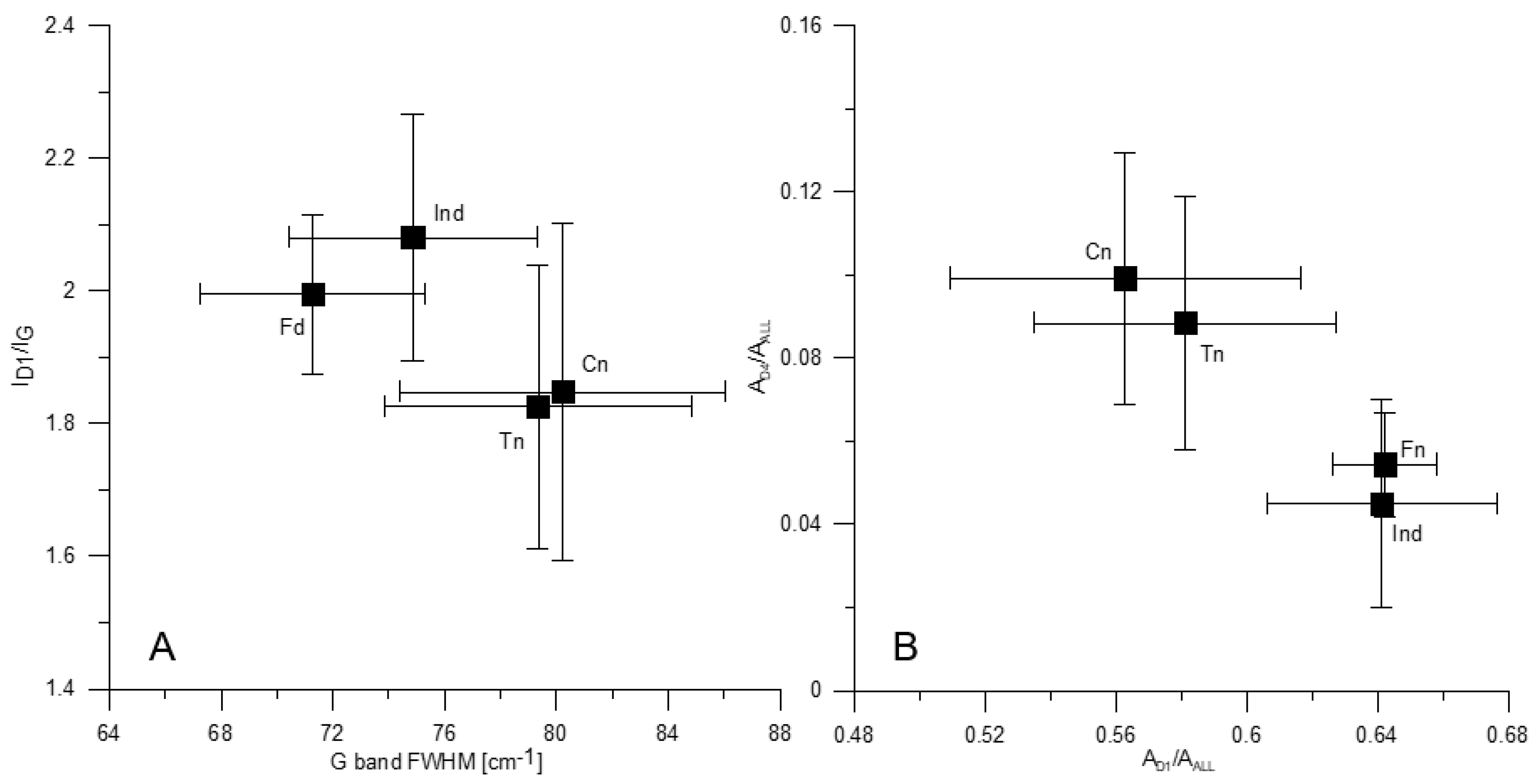
The G band position in the spectra of the tested morphotypes falls in a narrow range of 1585–1588 cm−1 (Table 6). The mean value is highest for fusinoid and differs significantly compared to the other components (Table 8). The FWHM of the G band is smaller in fusinoid (ca. 71 cm−1) and inertoid (ca. 75 cm−1) spectra than those of tenuinetwork and crassinetwork (ca. 79 cm−1 and 80 cm−1, respectively) (Table 6 and Table 9, Figure 4A), which indicates higher structural ordering of the first pair of the morphotypes [67]. Similar G band half-width as determined herein for fusinoid and inertoid was previously found in the spectra of chars from low rank coals [48], inertinite-rich coals [27,33], and inertinite concentrate chars [61,76]. Similar to what is observed in this study, lower G band FWHM in the spectra of fusinoid and dense char (inertinite-derived) than the fused char (vitrinite-derived) was communicated [27,48].
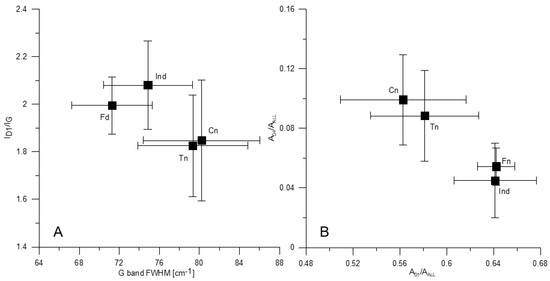
Figure 4.
The full width at half maximum (FWHM) of the G band vs. ID1/IG ratio (A) and the AD1/AALL vs. AD4/AALL ratio (B) for the tested morphotypes. Explanations as in Figure 1.
The D1 peak is centered at 1340–1344 cm−1, and its FWHM ranges from 195 cm−1 to 208 cm−1 (Table 6), which means that the size distribution of aromatic clusters does not differ much between the morphotypes studied [77]. In fusinoid, however, it is less varied than in inertoid (lower D1 band half-width), as the Tukey’s HSD test confirms (p < 0.05). Compared to this study, lower D1 band FWHM values (ca. 160–170 cm−1) were found both for the vitrinite-derived and inertinite-derived chars [27]. On the other hand, higher values were communicated for the bituminous coal chars [33]. The RBS value (i.e., the distance between the G and D1 peak) is highest in the case of fusinoid (247 cm−1).
The ID1/IG ratio is higher in the inertoid than in the tenuinetwork and crassinetwork spectra (Table 7 and Table 10, Figure 4A). The fusinoid spectra reveal higher ID1/IG ratio than those of tenuinetwork (Table 7 and Table 10, Figure 4A). As previously discussed [77,78,79,80], when the diameter of coherent domains (“crystallites”) (La) in carbonaceous materials is below 2 nm, increase in La corresponds to increasing ID1/IG ratio, contrary to the equation introduced by Tuinstra and Koenig [63]. When domains are larger than 2 nm, the increase in La is reflected by the decreasing ID1/IG ratio value. The studied char was obtained at 850–950 °C. As it is known from numerous studies, the size of coherent domains in chars and similar carbonaceous materials, formed at approximately the same temperature, does not exceed 2 nm [81,82,83,84,85,86,87]. Therefore, the higher value of the ratio is indicative for morphotypes with a larger La. An increase in ID1/IG ratio of various carbonaceous materials with temperature increasing up to 900°, and its subsequent decrease at higher temperature was previously observed [27,33,34,41,48,61,73,76,78,79,80,88,89]. This change was directly correlated with an increase in La [76,80]. The increase in the size of coherent domains results mainly from the dehydrogenation of hydroaromatics [34]. A higher ID1/IG ratio was found for fusinoid [48] and the inertinite-derived char [27] than for the vitrinite-derived char [27,48].
The higher AD1/AALL ratio for fusinoid and inertoid (Table 7 and Table 11, Figure 4B) indicates that they are more abundant in larger aromatic structures (composed of at least 6 aromatic rings) than tenuinetwork and crassinetwork. On the other hand, lower AD4/AALL ratio in fusinoid and inertoid spectra (Table 7 and Table 12, Figure 4B) shows that sp3 or sp2–sp3 bonding (active sites) is less frequent in these components than in the other two. This is because concentration of active sites decreases as the size of coherent domains increases [27,42,74]. The content of small aromatic units (3–5 rings) and amorphous carbon does not vary between the morphotypes, as it can be concluded from the AD3/AALL ratio values (Table 7).
Considering the above findings, morphotypes tested can be divided into two groups. The first includes inertoid and fusinoid, and the second comprises tenuinetwork and crassinetwork. In general, these two groups differ by the G band FWHM, and the ID1/IG, AD1/AALL and AD4/AALL ratios (Table 6, Table 7, Table 8, Table 9, Table 10, Table 11 and Table 12, Figure 4A,B). Inertoid and fusinoid and are composed of larger aromatic structures, forming larger coherent domains, with lower amount of alkyl-aryl structures. Their microstructure is better organized compared to the network morphotypes. Raman characteristics of the morphotypes within each pair do not differ significantly. The fusinoid spectra compared to those of inertoid are distinguished by the G band peak positioned at higher values. The highest standard deviations of most spectral parameters found for crassinetwork (Table 6 and Table 7) indicate that it is the most heterogeneous of all morphotypes examined.
Tenuinetwork, which has the highest porosity (>70%, [26]) of all morphotypes tested, accounts for 12.64% vol. (Table 5) of the char, and it is mainly derived from textinite (11.70% vol.) (Table 2). Textinite is a porous maceral, which contains varied amounts of humins, cellulose, and lignin as well as resins and waxes, depending on a variety (textinite A or B) [29,90,91]. During gasification it acts as a reactive component and produces substantial amounts of tar and gas, while its porosity increases [16]. Textinite belongs to the macerals, which are most heavily altered [31] but its primary cellular structure is frequently preserved [29]. Therefore, mostly thin-walled network (i.e., tenuinetwork) comes into being.
Crassinetwork constitutes 31.59% vol. (Table 5) of the char, and its porosity varies between 40% and 70% [26], whereas inertoid accounts for 35.44% vol., and its porosity is below 40%. Given the petrographic composition of the parent coal and the char (Table 2 and Table 5), and the chemical properties of lignite constituents, the explanation of the origin of these two morphotypes leads primarily to the other macerals of the huminite group, which are: attrinite (29.90% vol.), and densinite and ulminite, which together comprise 36% vol. of the char (Table 2). Attrinite, densinite, and ulminite, which are the main components of the parent coal, differ by their content of small aromatic units (3–5 rings), amorphous phase and sp2–sp3-bonded carbon, increasing from attrinite to ulminite [49]. These differences are related to the degree of gelification, as aromaticity increases with increasing gelification [90,92,93,94]. They may also result from different origin of the plant material, as attrinite may come from the soft tissues (i.e., cellulose-rich), while ulminite—from the lignin-rich (and though more aromatic) xylem [95]. Attrinite, due to its low aromaticity, is the most reactive of these three macerals. Moreover, it is often accompanied by various liptinite macerals, such as resinite or sporinite, which are abundant in aliphatic hydrocarbons, further increasing its reactivity [96]. Lignite chars obtained at 500 oC contain cavities resulting from complete decomposition of liptinite group macerals, whose shapes are similar to the morphology of these macerals [24]. Such cavities retain their shape even after heating at 950 °C [24]. These are the reasons for the formation of still very porous but a thick-walled network (i.e., crassinetwork) from attrinite. Densinite, and, especially, ulminite are more abundant in small aromatic systems [49]. They are also less porous, which makes the process of gasification more difficult, even though they are richer in active sites than attrinite [49]. Technological properties of the former maceral depend on the degree of homogenization and gelification, and worsen as gelification increases [97,98]. Densinite does not have coking properties. Ulminite produces greater amount of char than textinite during carbonization [97,98]. It accounts for the largest part of unchanged macerals during gasification [31]. Moreover, ulminite does not change its shape, and its porosity does not increase substantially [29]. This explains the transformation of densinite and ulminite into inertoid. It is usually observed that dense chars are produced from inertinite-rich coals [27,89,98]. However, inertinite content (deducting fusinite, which contributes to the formation of fusinoid) is about 2% vol., being incomparably lower than inertinoid content in the char (ca. 35% vol.). This indicates that the other macerals must have played a significant role in the formation of inertoid, with inertodetrinite (1.80% vol.) (Table 2) making only a minor contribution.
Morphotypes constituting the studied char are typical products of lignite gasification, as lignite usually generates network-type to solid char structure [31,89]. Morphology of the chars derived from the low rank coals closely reflects coal maceral composition [24]. This results from the limited thermoplasticity of such coals. An increase in heating temperature increases the proportion of the more reacted network-type morphotypes due to further chemical-structural changes [23,24,89,99].
Compared to the corresponding macerals from the parent coal [49], the huminite-derived char morphotypes are characterized by a higher content of large aromatic systems (≥6 fused rings), and the occurrence of larger coherent domains (higher ID1/IG ratio). The amount of small aromatics and amorphous carbon decreased. Regarding various types of chars (bulk samples), such changes were previously reported [14,27,33,34,42,43]. Crassinetwork and tenuinetwork are still rich in alkyl–aryl C-C structures that are not easily removed [34].
Fusinoid (1.79%), porosity of which is below 5% [26] emerges mainly from fusinite (1.0%). Alteration of chemical structure of fusinite requires high activation energy and has more limited range, which reflects inert nature of this maceral, resulting from highly cross-linked structure and low content of mobile phase [61,74,76,100]. However, in comparison to the fusinite in the parent coal [49], the content of large aromatic systems in fusinoid increased, and the amount of amorphous carbons and small aromatics decreased. The sp2–sp3 bonds in fusinoid are scarce and therefore concentration of active sites is low, as in inertoid, which is due to the presence of relatively large coherent domains, as evidenced by the ID1/IG ratio (Table 7 and Table 10, Figure 4A). These observations are consistent with the results of previous works on the microstructural alteration of fusinite under heat-treatment [48,61,76].
The Raman characteristics of inertoid and fusinoid are remarkably similar, although these morphotypes differ in morphology and they are derived from macerals having different properties. During gasification, the chemical-structural features of both morphotypes converged. Similar phenomenon was previously observed by Guedes et al. [48] who studied Raman properties of a fused char and fusinoid after heat treatment at 800 °C. In addition, chars generated at 800–1000 °C from inertinite- and vitrinite-rich coals had similar structure, differing mainly in La size, which was larger in the former char [27,42].
4. Conclusions
The studied lignite char is composed mainly of crassinetwork and inertoid, accompanied by tenuinetwork and small amounts of fusinoid. Tenuinetwork originates mostly from textinite, crassinetwork is formed from attrinite, while inertoid results from transformation of strongly gelified macerals such as densinite and ulminite. Similarities in the microstructure of tenuinetwork and crassinetwork as well as inertoid and fusinoid are observed. Inertoid and fusinoid are composed of larger aromatic systems, with lower amount of alkyl-aryl structures, and their microstructure is better organized compared to tenuinetwork and crassinetwork. Inertoid and fusinoid differ in microscopic appearance and were formed from different starting materials, but their microstructural properties converged during gasification. Different morphological features of the network morphotypes (tenuinetwork, crassinetwork) are not reflected in the differences in their microstructural characteristics.
Author Contributions
Conceptualization, R.M. and B.B.; methodology, R.M. and B.B.; software, R.M. and B.B.; validation, R.M.; formal analysis, R.M.; investigation, R.M. and B.B.; writing—original draft preparation, R.M. and B.B.; writing—review and editing, R.M. and B.B.; visualization, R.M. and B.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education of Poland (subsidies no. 06/060/BK_21/0102 and no. 16.16.140.315).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Jüntgen, H. Reactivities of carbon to steam and hydrogen and applications to technical gasification processes—A review. Carbon 1981, 19, 167–173. [Google Scholar] [CrossRef]
- Mühlen, H.J.; van Heek, K.H.; Jüntgen, H. Kinetic studies of steam gasification of char in the presence of H2, CO2 and CO. Fuel 1985, 64, 944–949. [Google Scholar] [CrossRef]
- Li, C.Z. Some Recent Advances in the Understanding of the Pyrolysis and Gasification Behaviour of Victorian Brown Coal; Elsevier: Amsterdam, The Netherlands, 2007; Volume 86, pp. 1664–1683. [Google Scholar]
- Wagner, N.J.; Coertzen, M.; Matjie, R.H.; van Dyk, J.C. Chapter 5—Coal Gasification. In Applied Coal Petrology: The Role of Petrology in Coal Utilization; Elsevier: Amsterdam, The Netherlands, 2008; pp. 119–144. [Google Scholar]
- Bell, D.A.; Towler, B.F. Coal Gasification and Its Applications; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Li, T.; Zhang, L.; Dong, L.; Li, C.-Z.Z. Effects of gasification atmosphere and temperature on char structural evolution during the gasification of Collie sub-bituminous coal. Fuel 2014, 117, 1190–1195. [Google Scholar] [CrossRef]
- Radovic, L.R.; Steczko, K.; Walker, P.L.; Jenkins, R.G. Combined effects of inorganic constituents and pyrolysis conditions on the gasification reactivity of coal chars. Fuel Process. Technol. 1985, 10, 311–326. [Google Scholar] [CrossRef]
- Takarada, T.; Tamai, Y.; Tomita, A. Reactivities of 34 coals under steam gasification. Fuel 1985, 64, 1438–1442. [Google Scholar] [CrossRef]
- Van Heek, K.H.; Mühlen, H.-J.J. Aspects of coal properties and constitution important for gasification. Fuel 1985, 64, 1405–1414. [Google Scholar] [CrossRef]
- Hashimoto, K.; Miura, K.; Ueda, T. Correlation of gasification rates of various coals measured by a rapid heating method in a steam atmosphere at relatively low temperatures. Fuel 1986, 65, 1516–1523. [Google Scholar] [CrossRef]
- Kasaoka, S.; Sakata, Y.; Shimada, M. Effects of coal carbonization conditions on rate of steam gasification of char. Fuel 1987, 66, 697–701. [Google Scholar] [CrossRef]
- Miura, K.; Hashimoto, K.; Silveston, P.L. Factors affecting the reactivity of coal chars during gasification, and indices representing reactivity. Fuel 1989, 68, 1461–1475. [Google Scholar] [CrossRef]
- Miura, K.; Makino, M.; Silveston, P.L. Correlation of gasification reactivities with char properties and pyrolysis conditions using low rank Canadian coals. Fuel 1990, 69, 580–589. [Google Scholar] [CrossRef]
- Tomita, A.; Ohtsuka, Y. Gasification and Combustion of Brown Coal. In Advances in the Science of Victorian Brown Coal; Elsevier Science: Amsterdam, The Netherlands, 2004; pp. 223–285. [Google Scholar] [CrossRef]
- Bielowicz, B. A new technological classification of low-rank coal on the basis of Polish deposits. Fuel 2012, 96, 497–510. [Google Scholar] [CrossRef]
- Bielowicz, B. Petrographic composition of Polish lignite and its possible use in a fluidized bed gasification process. Int. J. Coal Geol. 2013, 116, 236–246. [Google Scholar] [CrossRef]
- Bielowicz, B.; Kasiński, J.R.J.R. The possibility of underground gasification of lignite from Polish deposits. Int. J. Coal Geol. 2014, 131, 304–318. [Google Scholar] [CrossRef]
- Kapusta, K.; Wiatowski, M.; Stańczyk, K. An experimental ex-situ study of the suitability of a high moisture ortho-lignite for underground coal gasification (UCG) process. Fuel 2016, 179, 150–155. [Google Scholar] [CrossRef]
- Xi, J.; Liang, J.; Sheng, X.; Shi, L.; Li, S. Characteristics of lump lignite pyrolysis and the influence of temperature on lignite swelling in underground coal gasification. J. Anal. Appl. Pyrolysis 2016, 117, 228–235. [Google Scholar] [CrossRef]
- Furimsky, E.; Palmer, A.D.D.A.; Kalkreuth, W.D.D.; Cameron, A.R.R.; Kovacik, G. Prediction of coal reactivity during combustion and gasification by using petrographic data. Fuel Process. Technol. 1990, 25, 135–151. [Google Scholar] [CrossRef]
- Duxbury, J. Prediction of coal pyrolysis yields from BS volatile matter and petrographic analyses. Fuel 1997, 76, 1337–1343. [Google Scholar] [CrossRef]
- Sun, Q.; Li, W.; Chen, H.; Li, B. The CO2-gasification and kinetics of Shenmu maceral chars with and without catalyst. Fuel 2004, 83, 1787–1793. [Google Scholar] [CrossRef]
- Wagner, N.J.J.; Matjie, R.H.H.; Slaghuis, J.H.H.; van Heerden, J.H.P.H.P. Characterization of unburned carbon present in coarse gasification ash. Fuel 2008, 87, 683–691. [Google Scholar] [CrossRef]
- Guo, X.; Tang, Y.; Eble, C.F.; Wang, Y.; Li, P. Study on petrographic characteristics of devolatilization char/coke related to coal rank and coal maceral. Int. J. Coal Geol. 2020, 227, 103504. [Google Scholar] [CrossRef]
- Everson, R.C.; Neomagus, H.W.J.P.; Kasaini, H.; Njapha, D. Reaction kinetics of pulverized coal-chars derived from inertinite-rich coal discards: Gasification with carbon dioxide and steam. Fuel 2006, 85, 1076–1082. [Google Scholar] [CrossRef]
- Lester, E.; Alvarez, D.; Borrego, A.G.G.; Valentim, B.; Flores, D.; Clift, D.A.A.; Rosenberg, P.; Kwiecinska, B.; Barranco, R.; Petersen, H.I.I.; et al. The procedure used to develop a coal char classification—Commission III Combustion Working Group of the International Committee for Coal and Organic Petrology. Int. J. Coal Geol. 2010, 81, 333–342. [Google Scholar] [CrossRef]
- Chabalala, V.P.P.; Wagner, N.; Potgieter-Vermaak, S. Investigation into the evolution of char structure using Raman spectroscopy in conjunction with coal petrography; Part 1. Fuel Process. Technol. 2011, 92, 750–756. [Google Scholar] [CrossRef]
- Oboirien, B.O.; Engelbrecht, A.D.; North, B.C.; Du Cann, V.M.; Falcon, R. Textural properties of chars as determined by petrographic analysis: Comparison between air-blown, oxygen-blown and oxygen-enriched gasification. Fuel 2012, 101, 16–22. [Google Scholar] [CrossRef]
- Bielowicz, B. Petrographic characteristics of lignite gasification chars. Int. J. Coal Geol. 2016, 168, 146–161. [Google Scholar] [CrossRef]
- Bielowicz, B. Change of the petrographic composition of lignite during the ex-situ lignite gasification. Fuel 2017, 206, 219–229. [Google Scholar] [CrossRef]
- Bielowicz, B.; Raszowski, M.; Maciejończyk, N. Petrographic composition of the ex-situ lignite gasification residues. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 1762–1779. [Google Scholar] [CrossRef]
- Guo, X.; Tay, H.L.; Zhang, S.; Li, C.Z. Changes in char structure during the gasification of a Victorian brown coal in steam and oxygen at 800 °C. Energy Fuels 2008, 22, 4034–4038. [Google Scholar] [CrossRef]
- Qi, J.; Fan, C.; Li, S. Characteristics of lignite char derived from oxy-pyrolysis. Fuel 2021, 291, 120261. [Google Scholar] [CrossRef]
- Roberts, M.J.; Everson, R.C.; Neomagus, H.W.J.P.; Van Niekerk, D.; Mathews, J.P.; Branken, D.J. Influence of maceral composition on the structure, properties and behaviour of chars derived from South African coals. Fuel 2015, 142, 9–20. [Google Scholar] [CrossRef]
- Xu, X.Q.; Wang, Y.G.; Chen, Z.D.; Chen, X.J.; Zhang, H.Y.; Bai, L.; Zhang, S. Variations in char structure and reactivity due to the pyrolysis and in-situ gasification using Shengli brown coal. J. Anal. Appl. Pyrolysis 2015, 115, 233–241. [Google Scholar] [CrossRef]
- Yu, J.; Guo, Q.; Ding, L.; Gong, Y.; Yu, G. Study on the effect of inherent AAEM on char structure evolution during coal pyrolysis by in-situ Raman and TG. Fuel 2021, 292, 120406. [Google Scholar] [CrossRef]
- Li, L.; Tong, S.; Duan, L.; Zhao, C.; Shi, Z. Effect of CO2 and H2O on lignite char structure and reactivity in a fluidized bed reactor. Fuel Process. Technol. 2021, 211, 106564. [Google Scholar] [CrossRef]
- Liang, C.; Wang, X.; Lyu, Q. Experimental investigation on fluidized modification in gasification of preheated coal using oxygen and steam. Fuel 2021, 304, 121375. [Google Scholar] [CrossRef]
- Sun, J.; Feng, H.; Kou, J.; Jin, H.; Chen, Y.; Guo, L. Experimental investigation on carbon microstructure for coal gasification in supercritical water. Fuel 2021, 306, 121675. [Google Scholar] [CrossRef]
- Bar-Ziv, E.; Zaida, A.; Salatino, P.; Senneca, O. Diagnostics of carbon gasification by raman microprobe spectroscopy. Proc. Combust. Inst. 2000, 28, 2369–2374. [Google Scholar] [CrossRef]
- Li, T.; Zhang, L.; Dong, L.; Zhang, S.; Qiu, P.; Wang, S.; Li, C.-Z.Z. Effects of gasification temperature and atmosphere on char structural evolution and AAEM retention during the gasification of Loy Yang brown coal. Fuel Process. Technol. 2017, 159, 48–54. [Google Scholar] [CrossRef]
- Tay, H.L.; Li, C.Z. Changes in char reactivity and structure during the gasification of a Victorian brown coal: Comparison between gasification in O2 and CO2. In Fuel Processing Technology; Elsevier: Amsterdam, The Netherlands, 2010; Volume 91, pp. 800–804. [Google Scholar]
- Tay, H.-L.L.; Kajitani, S.; Wang, S.; Li, C.-Z.Z. A preliminary Raman spectroscopic perspective for the roles of catalysts during char gasification. Fuel 2014, 121, 165–172. [Google Scholar] [CrossRef]
- Zhang, S.; Min, Z.; Tay, H.-L.L.; Asadullah, M.; Li, C.-Z.Z. Effects of volatile–char interactions on the evolution of char structure during the gasification of Victorian brown coal in steam. Fuel 2011, 90, 1529–1535. [Google Scholar] [CrossRef]
- Li, X.; Hayashi, J.-I.; Li, C.-Z. FT-Raman spectroscopic study of the evolution of char structure during the pyrolysis of a Victorian brown coal. Fuel 2006, 85, 1700–1707. [Google Scholar] [CrossRef]
- Zhang, X.P.; Zhang, C.; Tan, P.; Li, X.; Fang, Q.Y.; Chen, G. Effects of hydrothermal upgrading on the physicochemical structure and gasification characteristics of Zhundong coal. Fuel Process. Technol. 2018, 172, 200–208. [Google Scholar] [CrossRef]
- Qi, X.; Guo, X.; Xue, L.; Zheng, C. Effect of iron on Shenfu coal char structure and its influence on gasification reactivity. J. Anal. Appl. Pyrolysis 2014, 110, 401–407. [Google Scholar] [CrossRef]
- Guedes, A.; Valentim, B.; Prieto, A.C.C.; Noronha, F. Raman spectroscopy of coal macerals and fluidized bed char morphotypes. Fuel 2012, 97, 443–449. [Google Scholar] [CrossRef]
- Bielowicz, B.; Morga, R. Micro-Raman Spectroscopy of Selected Macerals of the Huminite Group: An Example from the Szczerców Lignite Deposit (Central Poland). Energies 2021, 14, 281. [Google Scholar] [CrossRef]
- Chmielniak, T.; Sobolewski, A.; Tomaszewicz, G. CO2-Enhanced coal gasification. Experience of the Institute for Chemical Processing of Coal. Przem. Chem. 2015, 94, 442–448. [Google Scholar]
- ISO 589:2008; Hard Coal—Determination of Total Moisture. Available online: https://www.iso.org/standard/45370.html (accessed on 28 October 2021).
- ISO 1171; Solid Mineral Fuels—Determination of Ash. 2010. Available online: https://www.iso.org/standard/55944.html (accessed on 28 October 2021).
- ISO 19579:2006; Solid Mineral Fuels—Determination of Sulfur by IR Spectrometry. Available online: https://www.iso.org/standard/39113.html (accessed on 28 October 2021).
- ISO 1928:2020; Coal and Coke—Determination of Gross Calorific Value. Available online: https://www.iso.org/standard/75883.html (accessed on 28 October 2021).
- ISO 562:2010; Hard coal and Coke—Determination of Volatile Matter. Available online: https://www.iso.org/standard/55943.html (accessed on 17 June 2020).
- ISO 29541:2010; Solid mineral fuels—Determination of Total Carbon, Hydrogen and Nitrogen Content—Instrumental Method. Available online: https://www.iso.org/standard/45546.html (accessed on 28 October 2021).
- ISO 7404-2; Methods for the Petrographic Analysis of Coals—Part 2: Methods of Preparing Coal Samples. 2009. Available online: https://www.iso.org/standard/42798.html (accessed on 28 October 2021).
- Lünsdorf, N.K. Raman spectroscopy of dispersed vitrinite—Methodical aspects and correlation with reflectance. Int. J. Coal Geol. 2016, 153, 75–86. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Sheng, C. Char structure characterised by Raman spectroscopy and its correlations with combustion reactivity. Fuel 2007, 86, 2316–2324. [Google Scholar] [CrossRef]
- Morga, R. Micro-Raman spectroscopy of carbonized semifusinite and fusinite. Int. J. Coal Geol. 2011, 87, 253–267. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, Y.; Liu, Z.; Ding, H.; Huang, X.; Zheng, C. Study on the evolution of the char structure during hydrogasification process using Raman spectroscopy. Fuel 2015, 157, 97–106. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef] [Green Version]
- Nemanich, R.J.; Solin, S.A. First- and second-order Raman scattering from finite-size crystals of graphite. Phys. Rev. B 1979, 20, 392–401. [Google Scholar] [CrossRef]
- Green, P.D.; Johnson, C.A.; Thomas, K.M. Applications of laser Raman microprobe spectroscopy to the characterization of coals and cokes. Fuel 1983, 62, 1013–1023. [Google Scholar] [CrossRef]
- Cuesta, A.; Dhamelincourt, P.; Laureyns, J.; Martínez-Alonso, A.; Tascón, J.M.D. Raman microprobe studies on carbon materials. Carbon 1994, 32, 1523–1532. [Google Scholar] [CrossRef]
- Beyssac, O.; Goffé, B.; Petitet, J.P.; Froigneux, E.; Moreau, M.; Rouzaud, J.N. On the characterization of disordered and heterogeneous carbonaceous materials by Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2003, 59, 2267–2276. [Google Scholar] [CrossRef]
- Rouzaud, J.N.; Oberlin, A.; Beny-Bassez, C. Carbon films: Structure and microtexture (optical and electron microscopy, Raman spectroscopy). Thin Solid Film. 1983, 105, 75–96. [Google Scholar] [CrossRef]
- Beny-Bassez, C.; Rouzaud, J.N.; Bassez, C.; Rouzaud, J.N. Characterization of carbonaceous materials by correlated electron and optical microscopy and Raman microspectrometry. Scanning Electron Microsc. 1985, 1, 119–132. [Google Scholar]
- Jawhari, T.; Roid, A.; Casado, J. Raman spectroscopic characterization of some commercially available carbon black materials. Carbon 1995, 33, 1561–1565. [Google Scholar] [CrossRef]
- Schwan, J.; Ulrich, S.; Batori, V.; Ehrhardt, H.; Silva, S.R.P. Raman spectroscopy on amorphous carbon films. J. Appl. Phys. 1996, 80, 440–447. [Google Scholar] [CrossRef] [Green Version]
- Livneh, T.; Bar-Ziv, E.; Senneca, O.; Salatino, P. Evolution of Reactivity of Highly Porous Chars from Raman Microscopy. Combust. Sci. Technol. 2000, 153, 65–82. [Google Scholar] [CrossRef]
- Zaida, A.; Bar-Ziv, E.; Radovic, L.R.; Lee, Y.-J. Further development of Raman Microprobe spectroscopy for characterization of char reactivity. Proc. Combust. Inst. 2007, 31, 1881–1887. [Google Scholar] [CrossRef]
- Borrego, A.G.; Alvarez, D.; Menéndez, R. Effects of Inertinite Content in Coal on Char Structure and Combustion. Energy Fuels 1997, 11, 702–708. [Google Scholar] [CrossRef]
- Lünsdorf, N.K.; Dunkl, I.; Schmidt, B.C.; Rantitsch, G.; von Eynatten, H. Towards a Higher Comparability of Geothermometric Data Obtained by Raman Spectroscopy of Carbonaceous Material. Part 2: A Revised Geothermometer. Geostand. Geoanal. Res. 2017, 41, 593–612. [Google Scholar] [CrossRef]
- Morga, R. Changes of Semifusinite and Fusinite Microstructure during Carbonization Inferred from the Raman Spectroscopy Examination; Monograph. In Polish, English abstract; Silesian University of Technology Publishing House: Gliwice, Poland, 2013. [Google Scholar]
- Ferrari, A.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B Condens. Matter Mater. Phys. 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Dillon, R.O.; Woollam, J.A.; Katkanant, V. Use of Raman scattering to investigate disorder and crystallite formation in as-deposited and annealed carbon films. Phys. Rev. B 1984, 29, 3482. [Google Scholar] [CrossRef]
- Prawer, S.; Ninio, F.; Blanchonette, I. Raman spectroscopic investigation of ion-beam-irradiated glassy carbon. J. Appl. Phys. 1998, 68, 2361. [Google Scholar] [CrossRef]
- Zickler, G.A.; Smarsly, B.; Gierlinger, N.; Peterlik, H.; Paris, O. A reconsideration of the relationship between the crystallite size La of carbons determined by X-ray diffraction and Raman spectroscopy. Carbon 2006, 44, 3239–3246. [Google Scholar] [CrossRef]
- Johnson, C.A.; Patrick, J.W.; Mark Thomas, K. Characterization of coal chars by Raman spectroscopy, X-ray diffraction and reflectance measurements. Fuel 1986, 65, 1284–1290. [Google Scholar] [CrossRef]
- Lu, L.; Sahajwalla, V.; Harris, D. Characteristics of chars prepared from various pulverized coals at different temperatures using drop-tube furnace. Energy Fuels 2000, 14, 869–876. [Google Scholar] [CrossRef]
- Sharma, A.; Kyotani, T.; Tomita, A. Quantitative evaluation of structural transformations in raw coals on heat-treatment using HRTEM technique. Fuel 2001, 80, 1467–1473. [Google Scholar] [CrossRef]
- Feng, B.; Bhatia, S.K.; Barry, J.C. Structural ordering of coal char during heat treatment and its impact on reactivity. Carbon 2002, 40, 481–496. [Google Scholar] [CrossRef]
- Feng, B.; Bhatia, S.K.; Barry, J.C. Variation of the Crystalline Structure of Coal Char during Gasification. Energy Fuels 2003, 17, 744–754. [Google Scholar] [CrossRef]
- Matsuoka, K.; Akahane, T.; Aso, H.; Sharma, A.; Tomita, A. The size of polyaromatic layer of coal char estimated from elemental analysis data. Fuel 2008, 87, 539–545. [Google Scholar] [CrossRef]
- Everson, R.C.; Okolo, G.N.; Neomagus, H.W.J.P.; Dos Santos, J.M. X-ray diffraction parameters and reaction rate modeling for gasification and combustion of chars derived from inertinite-rich coals. Fuel 2013, 109, 148–156. [Google Scholar] [CrossRef]
- Morga, R.; Jelonek, I.; Kruszewska, K.; Szulik, W. Relationships between quality of coals, resulting cokes, and micro-Raman spectral characteristics of these cokes. Int. J. Coal Geol. 2015, 144, 130–137. [Google Scholar] [CrossRef]
- Yu, J.; Lucas, J.A.; Wall, T.F. Formation of the structure of chars during devolatilization of pulverized coal and its thermoproperties: A review. Prog. Energy Combust. Sci. 2007, 33, 135–170. [Google Scholar] [CrossRef]
- Sýkorová, I.; Pickel, W.; Christanis, K.; Wolf, M.; Taylor, G.H.; Flores, D. Classification of huminite—ICCP System 1994. Int. J. Coal Geol. 2005, 62, 85–106. [Google Scholar] [CrossRef]
- Russell, N.J. Gelification of Victorian tertiary soft brown coal wood. I. Relationship between chemical composition and microscopic appearance and variation in the degree of gelification. Int. J. Coal Geol. 1984, 4, 99–118. [Google Scholar] [CrossRef]
- Wagner, M. The character of the IR absorption in the spectral rang 1700–1500 cm−1 of some macerals of the huminite group of brown coal. Bull. L’Academie Pol. Des Sci. 1981, 29, 321–330. [Google Scholar]
- Drobniak, A.; Mastalerz, M. Chemical evolution of Miocene wood: Example from the Belchatow brown coal deposit, central Poland. Int. J. Coal Geol. 2006, 66, 157–178. [Google Scholar] [CrossRef]
- Mastalerz, M.; Hower, J.C.; Taulbee, D.N. Variations in chemistry of macerals as refl ected by micro-scale analysis of a Spanish coal. Geol. Acta 2013, 11, 483–493. [Google Scholar] [CrossRef]
- Kalaitzidis, S.; Georgakopoulos, A.; Christanis, K.; Iordanidis, A. Early coalification features as approached by solid state 13C CP/MAS NMR spectroscopy. Geochim. Cosmochim. Acta 2006, 70, 947–959. [Google Scholar] [CrossRef]
- Taylor, G.H.; Teichmuller, M.; Davis, A.; Diessel, C.F.K.; Littke, R.; Robert, P. Organic Petrology: A New Handbook Incorporating Some Revised Parts of Stach’s Textbook of Coal Petrology; Gebrüder Borntraeger: Berlin, Germany, 1998; ISBN 9783443010362. [Google Scholar]
- Sontag, E.; Süss, M. Beispiele petrologischer Untersuchungen zur Klärung rohstoffabhängiger verfahrenstechnischer Probleme der Braunkphlenveredlung. Bergbautechnik 1969, 19, 255–260, 376–381. [Google Scholar]
- Jones, R.B.B.; McCourt, C.B.B.; Morley, C.; King, K. Maceral and rank influences on the morphology of coal char. Fuel 1985, 64, 1460–1467. [Google Scholar] [CrossRef]
- Bailey, J.G.G.; Tate, A.; Diessel, C.F.K.F.K.; Wall, T.F.F. A char morphology system with applications to coal combustion. Fuel 1990, 69, 225–239. [Google Scholar] [CrossRef]
- Maroto-Valer, M.M.; Taulbee, D.N.; Andrésen, J.M.; Hower, J.C.; Snape, C.E. The Role of Semifusinite in Plasticity Development for a Coking Coal. Energy Fuels 1998, 12, 1040–1046. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).