Improved Oil Recovery from Carbonate Reservoirs by Tuning Injection Seawater Composition
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.1.1. Rock Mineralogy
2.1.2. Brine Properties
2.1.3. Crude Oil
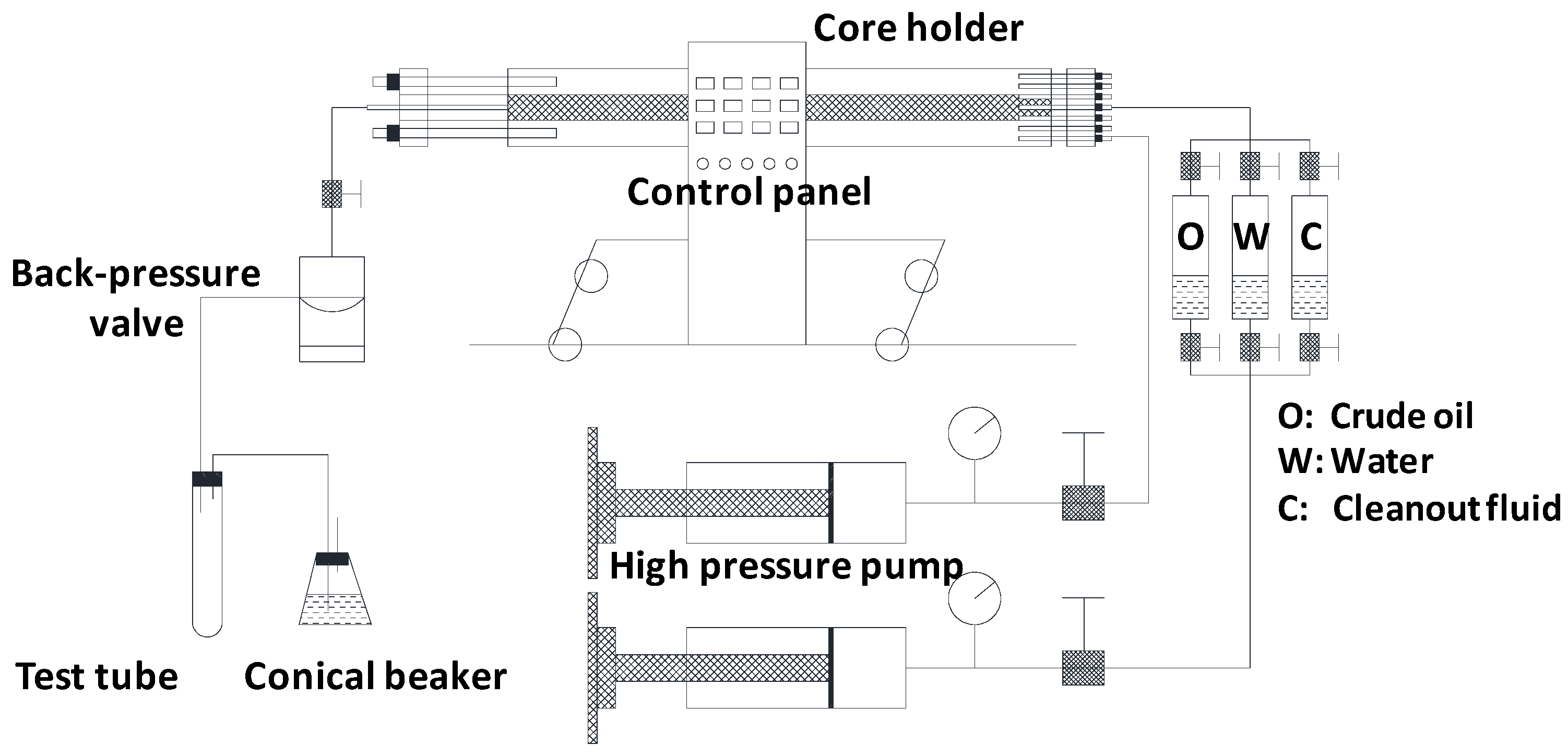
2.2. Experimental Setup and Procedure
2.2.1. Zeta Potential Measurements
2.2.2. Force-Measuring Technique
2.2.3. Core-Flooding Experiments
2.2.4. Micro-CT Experiments
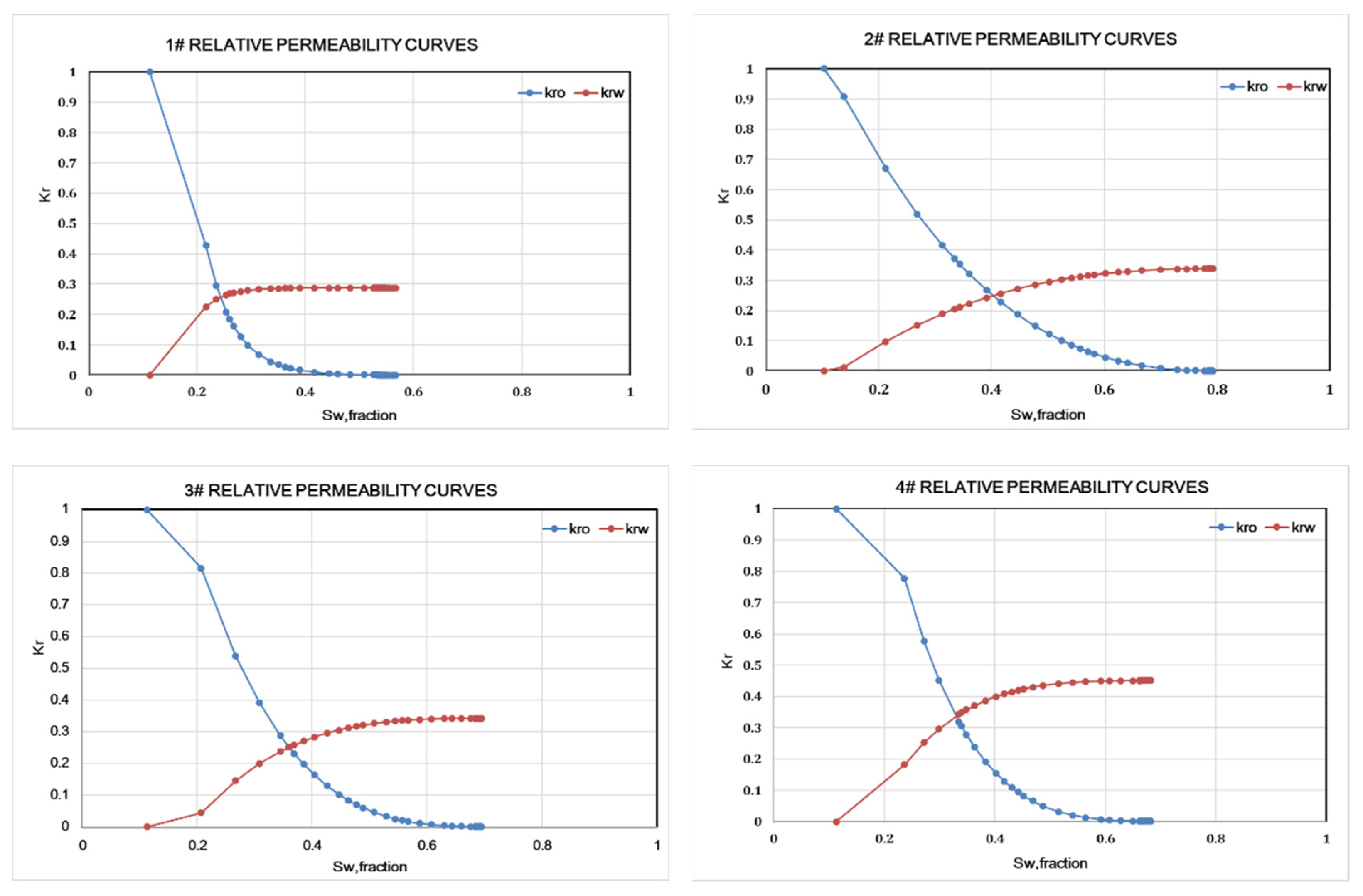
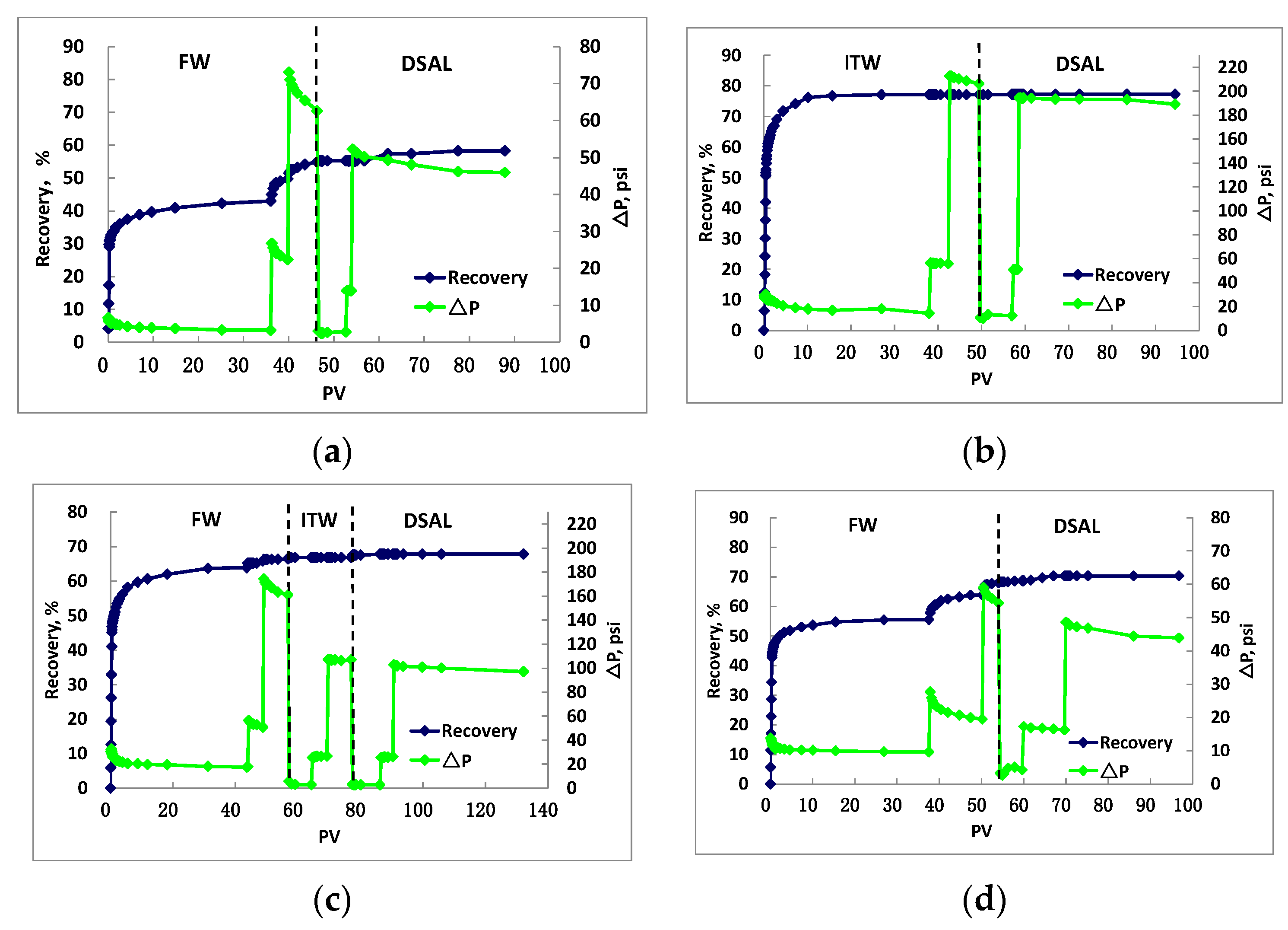
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klemme, H.D. Effective petroleum source rocks of the world; stratigraphic distribution and controlling depositional factors. Aapg. Bull. 1991, 75, 1809–1851. [Google Scholar]
- Sari, A.; Chen, Y.; Xie, Q.; Saeedi, A. Low salinity water flooding in high acidic oil reservoirs: Impact of pH on wettability of carbonate reservoirs. J. Mol. Liq. 2019, 281, 444–450. [Google Scholar] [CrossRef]
- Al-Shalabi, E.W.; Sepehrnoori, K. A comprehensive review of low salinity/engineered water injections and their applications in sandstone and carbonate rocks. J. Pet. Sci. Eng. 2016, 139, 137–161. [Google Scholar] [CrossRef]
- Mogensen, K.; Masalmeh, S. A review of EOR techniques for carbonate reservoirs in challenging geological settings. J. Pet. Sci. Eng. 2020, 195, 107889. [Google Scholar] [CrossRef]
- Lashkarbolooki, M.; Riazi, M.; Hajibagheri, F.; Ayatollahi, S. Low salinity injection into asphaltenic-carbonate oil reservoir, mechanistical study. J. Mol. Liq. 2016, 216, 377–386. [Google Scholar] [CrossRef]
- Azizollah, K.; Sharifov, A.R.; Torba, D.I. Experimental and modeling study of asphaltene adsorption onto the reservoir rocks. Pet. Sci. Technol. 2018, 36, 1–8. [Google Scholar]
- Koleini, M.M.; Badizad, M.H.; Kargozarfard, Z.; Ayatollahi, S. The impact of salinity on ionic characteristics of thin brine film wetting carbonate minerals: An atomistic insight. Colloids Surf. A 2019, 571, 27–35. [Google Scholar] [CrossRef]
- Song, J.; Zeng, Y.; Wang, L.; Duan, X.; Puerto, M.; Chapman, W.G.; Biswal, S.L.; Hirasaki, G.J. Surface complexation modeling of calcite zeta potential measurements in brines with mixed potential determining ions (Ca2+, CO32−, Mg2+, SO42−) for characterizing carbonate wettability. J. Colloid Interface Sci. 2017, 506, 169–179. [Google Scholar] [CrossRef]
- Rezaeidoust, A.; Puntervold, T.; Strand, S.; Austad, T. Smart water as wettability modifier in carbonate and sandstone: A discussion of similarities/differences in the chemical mechanisms. Energy Fuels 2009, 23, 4479–4485. [Google Scholar] [CrossRef]
- Tang, G.Q.; Morrow, N.R. Influence of brine composition and fines migration on crude oil/brine/rock interactions and oil recovery. J. Pet. Sci. Eng. 1999, 24, 99–111. [Google Scholar] [CrossRef]
- Lager, A.; Webb, K.J.; Black, C.J.J.; Singleton, M.; Sorbie, K.S. Low salinity oil recovery—An experimental investigation. Petrophysics 2008, 49, 28–35. [Google Scholar]
- Xie, Q.; Liu, Y.; Wu, J.; Liu, Q. Ions tuning water flooding experiments and interpretation by thermodynamics of wettability. J. Pet. Sci. Eng. 2014, 124, 350–358. [Google Scholar] [CrossRef]
- Wu, J.; Fanghui, L.; Yang, H.; Xu, S.; Xie, Q.; Zhang, M.; Chen, T.; Hu, G.; Wang, J. Effect of specific functional groups on oil adhesion from mica substrate: Implications for low salinity effect. J. Ind. Eng. Chem. 2017, 56, 342–349. [Google Scholar] [CrossRef]
- Liu, F.; Yang, H.; Wang, J.; Zhang, M.; Chen, T.; Hu, G.; Zhang, W.; Wu, J.; Xu, S.; Wu, X. Salinity-dependent adhesion of model molecules of crude oil at quartz surface with different wettability. Fuel 2018, 223, 401–407. [Google Scholar] [CrossRef]
- Mcguire, P.; Chatham, J.; Paskvan, F.; Sommer, D.; Carini, F. Low salinity oil recovery: An exciting new eor opportunity for Alaska’s north slope. In Proceedings of the SPE Western Regional Meeting, Irvine, CA, USA, 30 March–1 April 2005. [Google Scholar]
- Al-Nofli, K.; Pourafshary, P.; Mosavat, N.; Shafiei, A. Effect of initial wettability on performance of smart water flooding in carbonate reservoirsan experimental investigation with IOR implications. Energies 2018, 11, 1394. [Google Scholar] [CrossRef]
- Hassan, M.; Keya, A.L.; Berg, S.; Nasralla, R. Electrokinetics of carbonate/brine interface in low-salinity waterflooding: Effect of brine salinity, composition, rock type, and pH on zeta-potential and a surface-complexation model. SPE J. 2017, 22, 53–68. [Google Scholar]
- Hadia, N.J.; Ashraf, A.; Tweheyo, M.T.; Torsæter, O. Laboratory investigation on effects of initial wettabilities on performance of low salinity waterflooding. J. Pet. Sci. Eng. 2013, 105, 18–25. [Google Scholar] [CrossRef]
- Yang, G.; Chen, T.; Zhao, J.; Yu, D.; Liu, F.; Wang, D.; Fan, M.; Chen, W.; Zhang, J.; Yang, H.; et al. Desorption mechanism of asphaltenes in the presence of electrolyte and the Extended Derjaguin–Landau–Verwey–Overbeek theory. Energy Fuels 2015, 29, 4272–4280. [Google Scholar] [CrossRef]
- Wu, J.; Liu, F.; Chen, G.; Wu, X.; Ma, D.; Liu, Q.; Xu, S.; Huang, S.; Chen, T.; Zhang, W.; et al. Effect of ionic strength on the interfacial forces between oil/brine/rock interfaces: A chemical force microscopy study. Energy Fuels 2016, 30, 273–280. [Google Scholar] [CrossRef]
- Xiong, Y.; Cao, T.; Chen, Q.; Li, Z.; Yang, Y.; Xu, S.; Yuan, S.; Sjöblom, J.; Xu, Z. Adsorption of a polyaromatic compound on silica surfaces from organic solvents studied by molecular dynamics simulation and afm imaging. J. Phys. Chem. C 2017, 121, 5020–5028. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Q.; Harbottle, D.; Sjoblom, J.; Xu, Z.; Zeng, H. Molecular interactions of a polyaromatic surfactant C5Pe in aqueous solutions studied by a surface forces apparatus. J. Phys. Chem. B 2012, 116, 11187–11196. [Google Scholar] [CrossRef] [PubMed]
- Higaki, Y.; Hatae, K.; Ishikawa, T.; Takanohashi, T.; Hayashi, J.I.; Takahara, A. Adsorption and desorption behavior of asphaltene on polymer-brush-immobilized surfaces. ACS Appl. Mater. Interfaces 2014, 6, 20385. [Google Scholar] [CrossRef] [PubMed]
- Nordgard, E.L.; Landsem, E.; Sjoblom, J. Langmuir films of asphaltene model compounds and their fluorescent properties. Langmuir 2008, 24, 8742–8751. [Google Scholar] [CrossRef] [PubMed]
- Liu, F. Molecular interaction between asphaltene and quartz with different surface wettability: A combined study of experimental measurement and theoretical calculation. Fuel 2019, 258, 115937. [Google Scholar] [CrossRef]
- Sari, A.; Xie, Q.; Chen, Y.; Saeedi, A.; Pooryousefy, E. Drivers of low salinity effect in carbonate reservoirs. Energy Fuels 2017, 31, 8951–8958. [Google Scholar] [CrossRef]
- Nasralla, R.A.; Nasr-El-Din, H.A. Impact of cation type and concentration in injected brine on oil recovery in sandstone reservoirs. J. Pet. Sci. Eng. 2014, 122, 384–395. [Google Scholar] [CrossRef]
- Jackson, M.; Al-Mahrouqi, D.; Vinogradov, J. Zeta potential in oil-water-carbonate systems and its impact on oil recovery during controlled salinity water-flooding. Sci. Rep. 2016, 6, 37363. [Google Scholar] [CrossRef]
- Yang, H.; Duan, H.; Wu, X.; Wang, M.; Chen, T.; Liu, F.; Huang, S.; Zhang, W.; Chen, G.; Yu, D. Self-assembly behavior of ultrahighly charged amphiphilic polyelectrolyte on solid surfaces. Langmuir 2016, 32, 11485–11491. [Google Scholar] [CrossRef]
- Lorenz, B.; Ceccato, M.; Andersson, M.P.; Dobberschütz, S.; Stipp, S. Salinity-dependent adhesion response properties of aluminosilicate (K-Feldspar) surfaces. Energy Fuels 2017, 31, 4670–4680. [Google Scholar] [CrossRef]
- Hilner, E.; Andersson, M.P.; Hassenkam, T.; Matthiesen, J.; Salino, P.A.; Stipp, S. The effect of ionic strength on oil adhesion in sandstone—The search for the low salinity mechanism. Sci. Rep. 2015, 5, 9933. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, F.; Yang, H.; Zhu, Y.; Wang, X.; Hua, Z. Effect of ion type on the interaction between polar model oil and mica substrate: A chemical force microscopy study. Energy Fuels 2018, 32, 10486–10492. [Google Scholar] [CrossRef]

| Brine | Injection Water Composition (mg/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cl− | SO42− | CO32− | HCO3− | Na+ | K+ | Mg2+ | Ca2+ | Sr2+ | TDS | |
| FW | 140,980.80 | 312.50 | 53.20 | 60,919.75 | 1117.52 | 1524.44 | 16,506.20 | 0.00 | 338.68 | 221,753.09 |
| SW | 23,573.00 | 3458.00 | 0.00 | 154.00 | 13,050.00 | 468.00 | 1561.00 | 513.03 | 0.00 | 42,777.03 |
| ITW-1 | 23,366.90 | 3362.26 | 19.63 | 113.07 | 13,434.90 | 424.17 | 1535.28 | 431.08 | 0.00 | 42,687.29 |
| ITW-2 | 22,094.90 | 3430.19 | 19.63 | 106.42 | 13,682.20 | 435.98 | 1571.86 | 387.14 | 0.00 | 41,728.32 |
| ITW-3 | 22,160.30 | 3468.12 | 19.63 | 93.12 | 13,157.60 | 435.70 | 1517.75 | 339.68 | 0.00 | 41,191.90 |
| ITW-4 | 20,827.90 | 3379.59 | 19.63 | 93.12 | 12,935.00 | 423.07 | 1468.51 | 305.00 | 0.00 | 39,451.82 |
| Volatiles/% | Saturates/% | Aromatics/% | Resin/% | Asphaltene/% | Acid Number mg KOH/g |
|---|---|---|---|---|---|
| 7.19 | 63.64 | 17.46 | 10.23 | 1.48 | 0.116 |
| Cores | Depth/m | Porosity/% | Air Permeability/mD | L/cm | D/cm | Flooding Sequence |
|---|---|---|---|---|---|---|
| 1# | 2957.11 | 25.1 | 12.5 | 7.023 | 3.770 | FW → DSAL |
| 2# | 2962.58 | 23.5 | 3.42 | 7.035 | 3.764 | ITW → DSAL |
| 3# | 2968.83 | 21.2 | 3.63 | 6.990 | 3.765 | FW → ITW → DSAL |
| 4# | 2957.05 | 24.8 | 11.6 | 6.902 | 3.768 | FW → DSAL |
| Cores | Experiment Scheme | Experimental Procedures and Conditions |
|---|---|---|
| 1# | FW flooding (0.1–0.5–2.0 mL/min) + DSAL flooding (0.1–0.5–2.0 mL/min) | (1) Swi is about 10% (2) Temp. 90 °C (3) Dead oil aging for 4 weeks in cores (4) Back pressure is 2980 psi (20.5 MPa), and confining pressure is 300 psi more (5) Dead oil displaced 5 PV at 0.1 mL/min before water was injected (6) Water displaced at 0.1 mL/min (7) When no extra production is observed, increase to 0.5 mL/min, and then to 2.0 mL/min |
| 2# | ITW flooding (0.1–0.5–2.0 mL/min) + DSAL flooding (0.1–0.5–2.0 mL/min) | |
| 3# | FW flooding (0.1–0.5–2.0 mL/min) + ITW flooding (0.1–0.5–2.0 mL/min) + DSAL flooding (0.1–0.5–2.0 mL/min) | |
| 4# | FW flooding (0.1–0.5–2.0 mL/min) + DSAL flooding (0.1–0.5–2.0 mL/min) |
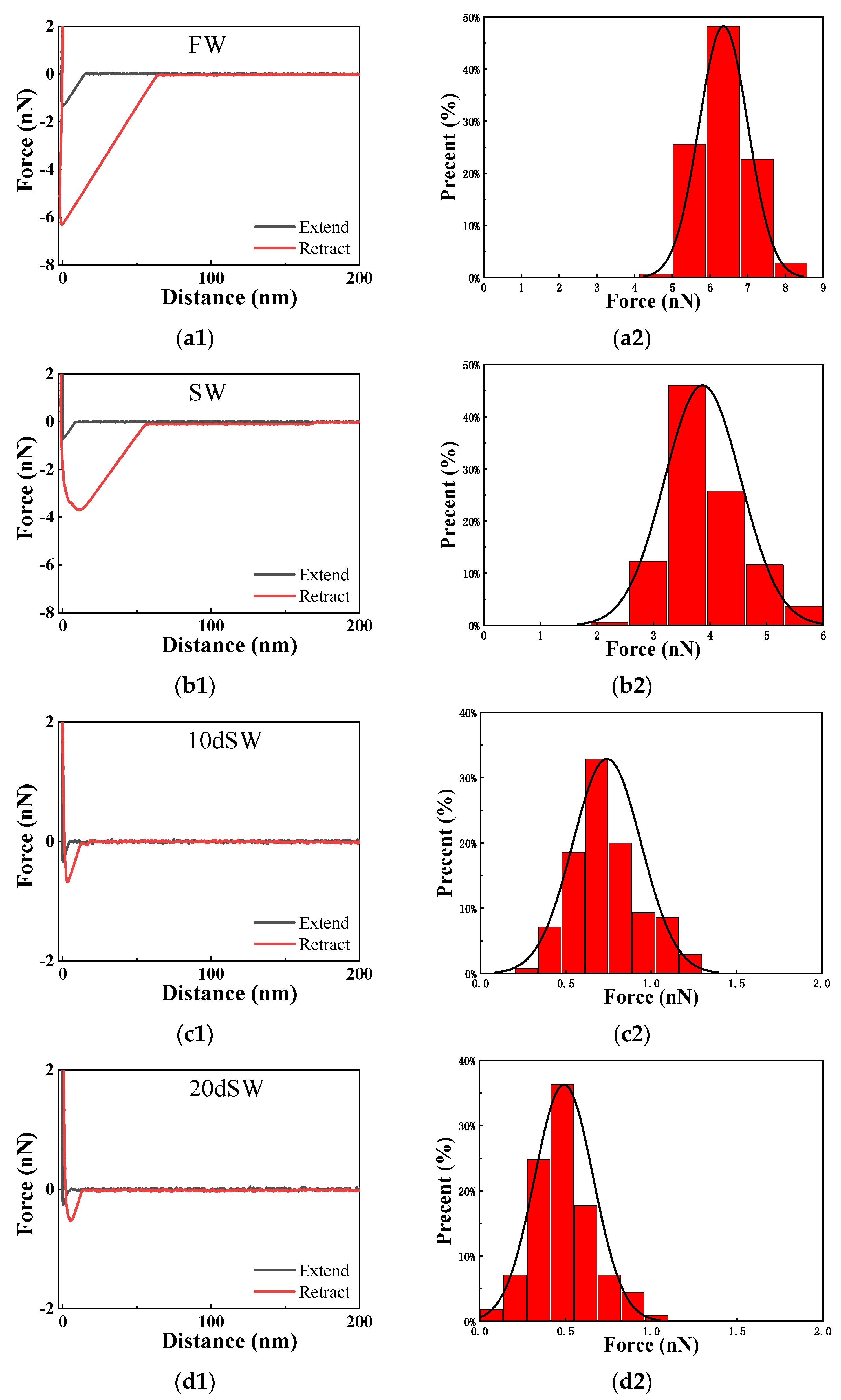
| Brine | Zetaoil/brine/mV | Zetabrine/rock/mV | Zetap/mV | Average Adhesion Force/nN |
|---|---|---|---|---|
| FW | 0.81 ± 0.22 | −2.89 ± 0.43 | 2.08 | 6.36 ± 0.65 |
| SW | −7.62 ± 0.37 | −5.72 ± 0.34 | 13.34 | 3.87 ± 0.68 |
| 10d SW | −13.62 ± 0.48 | −9.77 ± 0.18 | 23.39 | 0.74 ± 0.20 |
| 20d SW | −32.00 ± 0.62 | −11.75 ± 0.45 | 43.75 | 0.49 ± 0.17 |
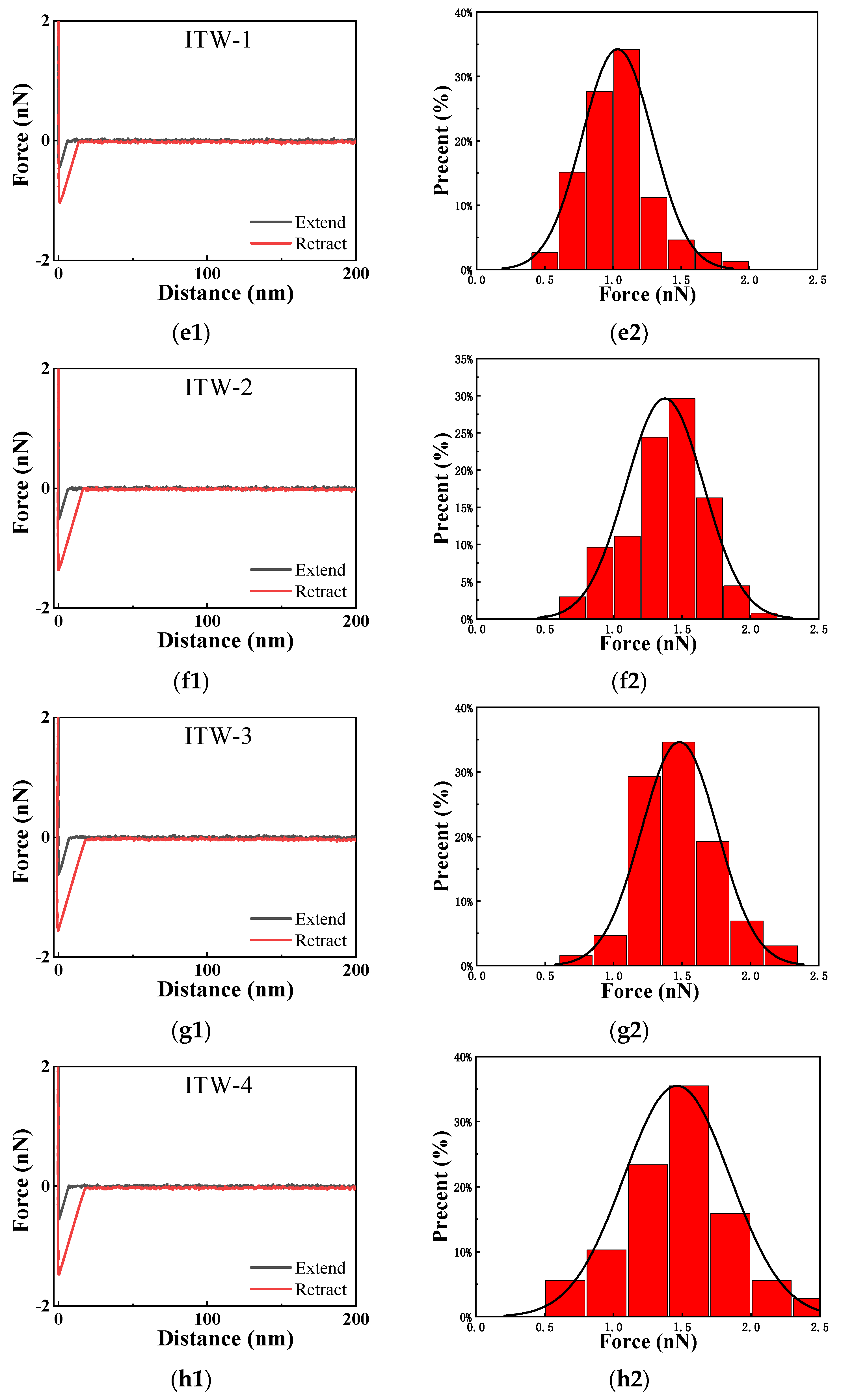
| ITW-1 | −12.01 ± 0.27 | −6.55 ± 0.11 | 18.56 | 1.03 ± 0.26 |
| ITW-2 | −8.10 ± 0.20 | −6.97 ± 0.11 | 15.07 | 1.37 ± 0.28 |
| ITW-3 | −5.69 ± 0.44 | −6.65 ± 0.33 | 12.34 | 1.48 ± 0.27 |
| ITW-4 | −8.17 ± 0.37 | −6.82 ± 0.35 | 14.99 | 1.46 ± 0.39 |
| Cores | Swi/% | Ka/10−3 μm2 | Kw/10−3 μm2 | Ko/10−3 μm2 | Water-Free Recovery Factor/% | FW Cumulative Recovery % | ITW Cumulative Recovery % | DSAL Cumulative Recovery % | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.1 | 0.5 | 2.0 | 0.1 | 0.5 | 2.0 | 0.1 | 0.5 | 2.0 | ||||||
| 1# | 11.03 | 12.50 | 5.12 | 2.60 | 29.1 | 43.0 | 49.7 | 54.9 | / | / | / | 55.3 | 55.3 | 58.3 |
| 2# | 10.28 | 3.42 | 1.31 | 0.81 | 50.7 | / | / | / | 77.1 | 77.1 | 77.1 | 77.2 | 77.2 | 77.2 |
| 3# | 12.07 | 3.63 | 1.70 | 0.98 | 45.0 | 65.2 | 65.9 | 66.4 | 66.8 | 66.8 | 66.8 | 67.5 | 67.8 | 67.8 |
| 4# | 10.90 | 11.60 | 4.93 | 3.11 | 42.7 | 55.6 | 63.8 | 68.0 | / | / | / | 68.7 | 70.3 | 70.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Liu, F.; Yang, S.; Han, H.; Chen, X.; Yang, H. Improved Oil Recovery from Carbonate Reservoirs by Tuning Injection Seawater Composition. Energies 2022, 15, 6405. https://doi.org/10.3390/en15176405
Wu J, Liu F, Yang S, Han H, Chen X, Yang H. Improved Oil Recovery from Carbonate Reservoirs by Tuning Injection Seawater Composition. Energies. 2022; 15(17):6405. https://doi.org/10.3390/en15176405
Chicago/Turabian StyleWu, Jiazhong, Fanghui Liu, Siyu Yang, Haishui Han, Xinglong Chen, and Hui Yang. 2022. "Improved Oil Recovery from Carbonate Reservoirs by Tuning Injection Seawater Composition" Energies 15, no. 17: 6405. https://doi.org/10.3390/en15176405
APA StyleWu, J., Liu, F., Yang, S., Han, H., Chen, X., & Yang, H. (2022). Improved Oil Recovery from Carbonate Reservoirs by Tuning Injection Seawater Composition. Energies, 15(17), 6405. https://doi.org/10.3390/en15176405






