Techno-Economic Analysis of Hydrogen Storage Technologies for Railway Engineering: A Review
Abstract
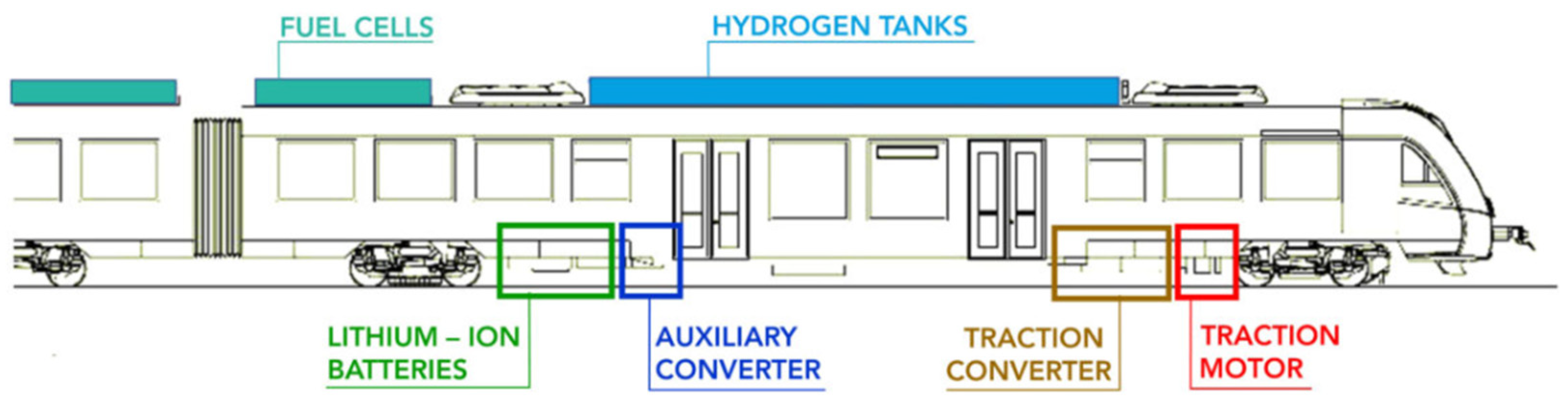
:1. Introduction
2. Compressed Hydrogen Storage
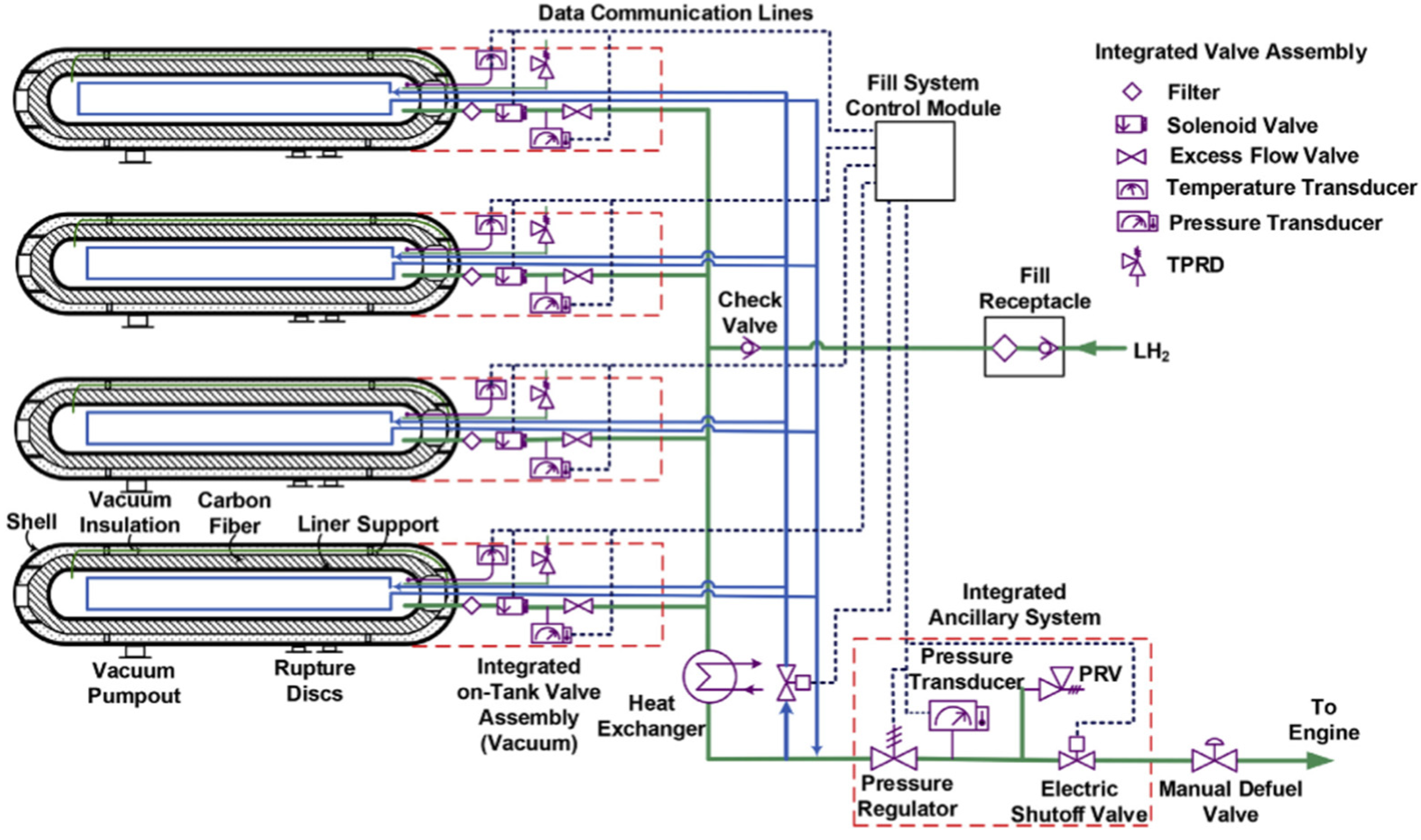
3. Liquid Hydrogen Storage
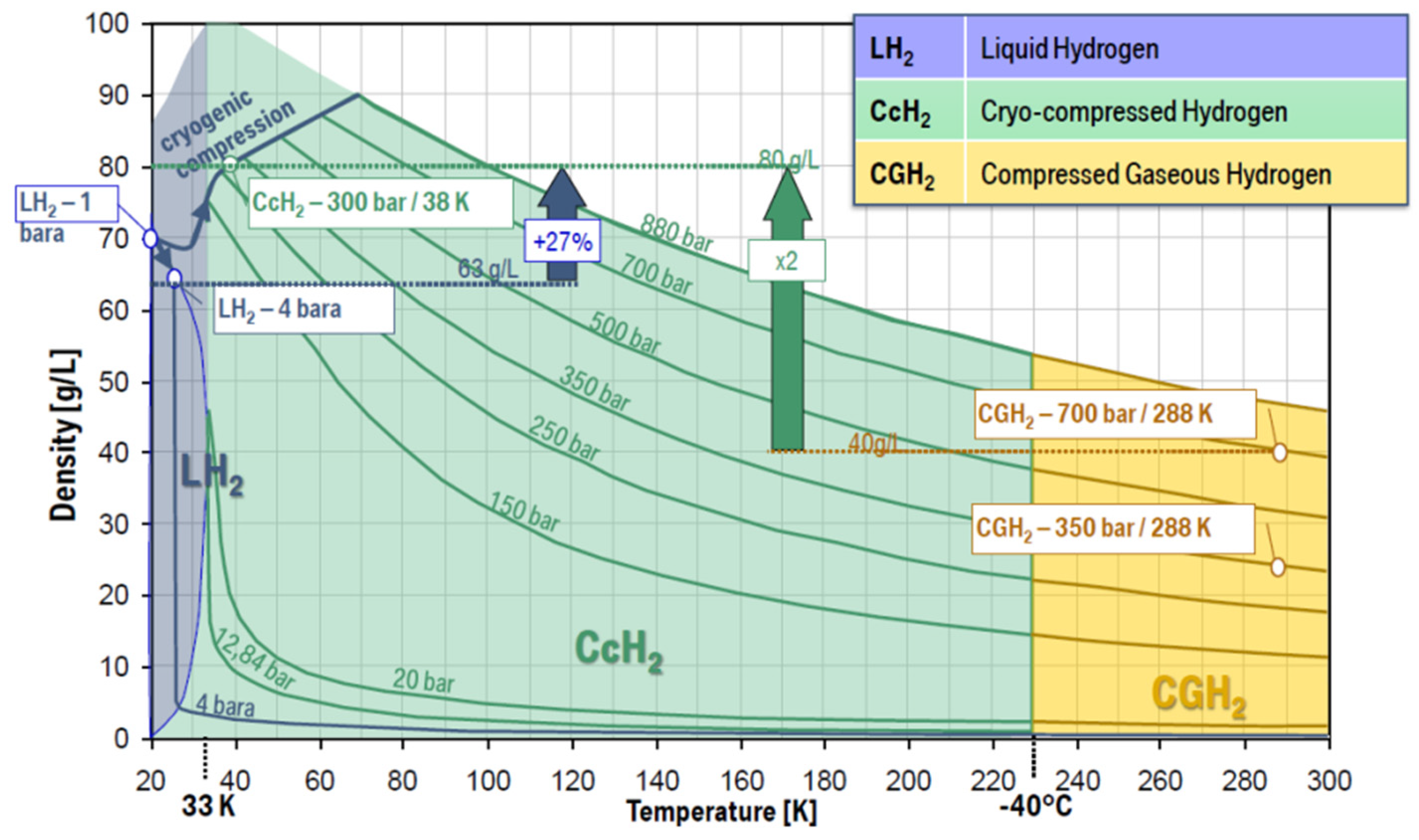
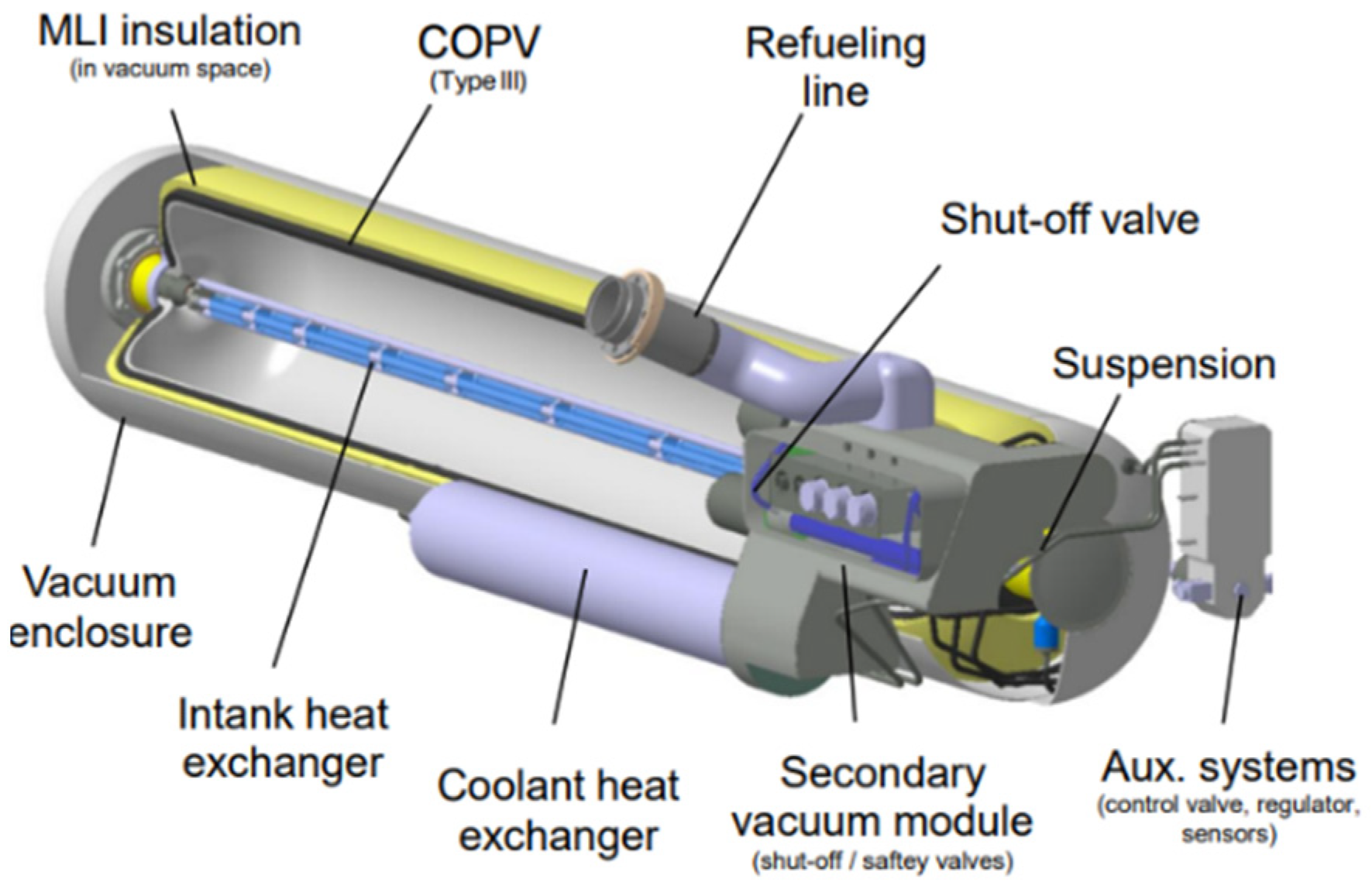
4. Cryo-Compressed Hydrogen Storage
5. Liquid Organic Hydrogen Carriers (LOHCs)
6. Ammonia
7. Metal Hydride-Based Storage
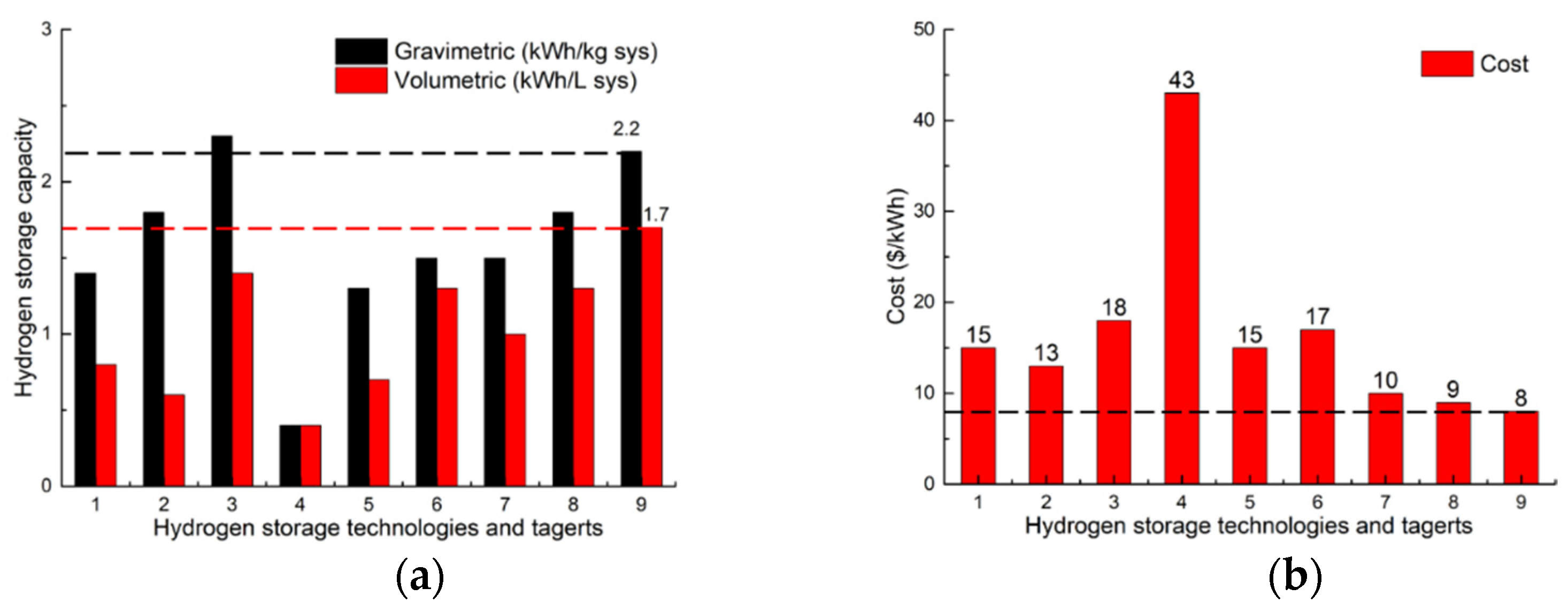
8. Overall Comparison
- For physical hydrogen storage technologies, it can be forecasted that they will be the most popular onboard storage technology for trains in the next few years, since CH2 storage can mostly meet the demands for train operation and is the most mature now. If higher requirements in terms of operation range and train power are raised, especially for long-haul freight trains, more attention should be paid to LH2 and CcH2 because of their high hydrogen storage capacities and relatively simple procedures before H2 enters fuel cell stacks. Their supporting technologies are being studied and becoming mature.
- For material-based storage technologies, they are attractive for being able to absorb or adsorb quantities of hydrogen. They are capable for hydrogen transport as hydrogen carriers. Impediments of their train-mounted application are the dehydrogenation and hydrogenation process because controlled energy flow and refuelling speed are important evaluative criteria for mobility. Onboard thermal management design would be an emphasis for a material-based storage system.
- Notably, ammonia has an extremely high energy capacity, along with low cost and a mature production chain. It has various application approaches according to different scenarios, which gives flexible choice for NH3-based vehicles. It has the viability to become the energy source for locomotives with relevant technological advances, especially the catalyst for decomposition and SOFC technology.
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geels, F.W.; Sovacool, B.K.; Schwanen, T.; Sorrell, S. Sociotechnical transitions for deep decarbonization. Science 2017, 357, 1242–1244. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wang, H.; Wang, L.; Chen, W. Decarbonization of China’s transportation sector: In light of national mitigation toward the Paris Agreement goals. Energy 2018, 155, 853–864. [Google Scholar] [CrossRef]
- Cullen, D.A.; Neyerlin, K.C.; Ahluwalia, R.K.; Mukundan, R.; More, K.L.; Borup, R.L.; Weber, A.Z.; Myers, D.J.; Kusoglu, A. New roads and challenges for fuel cells in heavy-duty transportation. Nat. Energy 2021, 6, 462–474. [Google Scholar] [CrossRef]
- Khosravi, A.; Koury, R.N.N.; Machado, L.; Pabon, J.J.G. Energy, exergy and economic analysis of a hybrid renewable energy with hydrogen storage system. Energy 2018, 148, 1087–1102. [Google Scholar] [CrossRef]
- Atteridge, W.J.; Lloyd, S.A. Thoughts on use of hydrogen to power railway trains. Proc. Inst. Mech. Eng. Part A J. Power Energy 2021, 235, 306–316. [Google Scholar] [CrossRef]
- Hoffrichter, A.; Hillmansen, S.; Roberts, C. Conceptual propulsion system design for a hydrogen-powered regional train. IET Electr. Syst. Transp. 2016, 6, 56–66. [Google Scholar] [CrossRef]
- Jones, W.D. Hydrogen On Track. IEEE Spectr. 2006, 43, 10–13. [Google Scholar] [CrossRef]
- Washing, E.; Pulugurtha, S. Well-to-Wheel Analysis of Electric and Hydrogen Light Rail. J. Public Transp. 2015, 18, 74–88. [Google Scholar] [CrossRef]
- Gallas, D.; Stobnicki, P. Adoption of Modern Hydrogen Technologies in Rail Transport. J. Ecol. Eng. 2022, 23, 84–91. [Google Scholar] [CrossRef]
- Sun, Y.; Anwar, M.; Hassan, N.M.S.; Spiryagin, M.; Cole, C. A review of hydrogen technologies and engineering solutions for railway vehicle design and operations. Railw. Eng. Sci. 2021, 29, 212–232. [Google Scholar] [CrossRef]
- Miller, A.R.; Peters, J.; Smith, B.E.; Velev, O.A. Analysis of fuel cell hybrid locomotives. J. Power Sources 2006, 157, 855–861. [Google Scholar] [CrossRef]
- Hoffrichter, A.; Hillmansen, S.; Roberts, C. Review and assessment of hydrogen propelled railway vehicles. In Proceedings of the IET Conference on Railway Traction Systems, Birmingham, UK, 13–15 April 2010. [Google Scholar]
- Hoffrichter, A. Hydrogen-Rail (Hydrail) Development; H2@ Rail Workshop: Lansing, MI, USA, 2019; pp. 1–4.
- Yan, Y.; Li, Q.; Chen, W.; Su, B.; Liu, J.; Ma, L. Optimal energy management and control in multimode equivalent energy consumption of fuel cell/supercapacitor of hybrid electric tram. IEEE Trans. Ind. Electron. 2018, 66, 6065–6076. [Google Scholar] [CrossRef]
- Siwiec, J. Use of Hydrogen Fuel Cells in Rail Transport. Probl. Kolejnictwa 2021, 190, 113–117. [Google Scholar] [CrossRef]
- Calvert, C.; Allan, J.; Amor, P.; Hillmansen, S.; Roberts, C.; Weston, P. Concept development and testing of the UK’s first hydrogen-hybrid train (HydroFLEX). Railw. Eng. Sci. 2021, 29, 248–257. [Google Scholar] [CrossRef]
- Ogawa, K.; Yoneyama, T.; Sudo, T.; Kashiwagi, T.; Yamamoto, T. Performance improvement of fuel cell hybrid powered test railway vehicle. Q. Rep. RTRI 2021, 62, 16–21. [Google Scholar] [CrossRef]
- Durbin, D.J.; Malardier-Jugroot, C. Review of hydrogen storage techniques for on board vehicle applications. Int. J. Hydrogen Energy 2013, 38, 14595–14617. [Google Scholar] [CrossRef]
- Hassan, I.A.; Ramadan, H.S.; Saleh, M.A.; Hissel, D. Hydrogen storage technologies for stationary and mobile applications: Review, analysis and perspectives. Renew. Sustain. Energy Rev. 2021, 149, 111311. [Google Scholar] [CrossRef]
- Lahnaoui, A.; Wulf, C.; Heinrichs, H.; Dalmazzone, D. Optimizing hydrogen transportation system for mobility via compressed hydrogen trucks. Int. J. Hydrogen Energy 2019, 44, 19302–19312. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Kayfeci, M.; Keçebaş, A. Hydrogen storage. In Solar Hydrogen Production; Elsevier: Amsterdam, The Netherlands, 2019; pp. 85–110. [Google Scholar]
- Xu, X.; Xu, H.; Zheng, J.; Chen, L.; Wang, J. A high-efficiency liquid hydrogen storage system cooled by a fuel-cell-driven refrigerator for hydrogen combustion heat recovery. Energy Convers. Manag. 2020, 226, 113496. [Google Scholar] [CrossRef]
- Ahluwalia, R.K.; Peng, J.K.; Roh, H.S.; Hua, T.Q.; Houchins, C.; James, B.D. Supercritical cryo-compressed hydrogen storage for fuel cell electric buses. Int. J. Hydrogen Energy 2018, 43, 10215–10231. [Google Scholar] [CrossRef]
- James, B.D.; Houchins, C.; Huya-Kouadio, J.M.; DeSantis, D.A. Hydrogen Storage System Cost Analysis; Strategic Analysis Inc.: Arlington, VA, USA, 2016. [Google Scholar]
- Rusman, N.A.A.; Dahari, M. A review on the current progress of metal hydrides material for solid-state hydrogen storage applications. Int. J. Hydrogen Energy 2016, 41, 12108–12126. [Google Scholar] [CrossRef]
- Ley, M.B.; Jepsen, L.H.; Lee, Y.-S.; Cho, Y.W.; Von Colbe, J.M.B.; Dornheim, M.; Rokni, M.; Jensen, J.O.; Sloth, M.; Filinchuk, Y. Complex hydrides for hydrogen storage–new perspectives. Mater. Today 2014, 17, 122–128. [Google Scholar] [CrossRef]
- Ponthieu, M.; Fernández, J.; Cuevas, F.; Bodega, J.; Ares, J.R.; Adeva, P.; Sánchez, C. Thermodynamics and reaction pathways of hydrogen sorption in Mg6 (Pd, TM)(TM = Ag, Cu and Ni) pseudo-binary compounds. Int. J. Hydrogen Energy 2014, 39, 18291–18301. [Google Scholar] [CrossRef]
- Liu, T.; Wang, C.; Wu, Y. Mg-based nanocomposites with improved hydrogen storage performances. Int. J. Hydrogen Energy 2014, 39, 14262–14274. [Google Scholar] [CrossRef]
- Smythe, N.C.; Gordon, J.C. Ammonia borane as a hydrogen carrier: Dehydrogenation and regeneration. Eur. J. Inorg. Chem. 2010, 2010, 509–521. [Google Scholar] [CrossRef]
- Macfarlane, D.R.; Cherepanov, P.V.; Choi, J.; Suryanto, B.H.R.; Hodgetts, R.Y.; Bakker, J.M.; Ferrero Vallana, F.M.; Simonov, A.N. A Roadmap to the Ammonia Economy. Joule 2020, 4, 1186–1205. [Google Scholar] [CrossRef]
- Alagharu, V.; Palanki, S.; West, K.N. Analysis of ammonia decomposition reactor to generate hydrogen for fuel cell applications. J. Power Sources 2010, 195, 829–833. [Google Scholar] [CrossRef]
- Davoodabadi, A.; Mahmoudi, A.; Ghasemi, H. The potential of hydrogen hydrate as a future hydrogen storage medium. Iscience 2021, 24, 101907. [Google Scholar] [CrossRef]
- USDoE. Hydrogen Sorption Center of Excellence (HSCoE) Final Report. Available online: https://www.energy.gov/eere/fuelcells/downloads/hydrogen-sorption-center-excellence-hscoe-final-report (accessed on 7 March 2014).
- Xia, Y.; Yang, Z.; Zhu, Y. Porous carbon-based materials for hydrogen storage: Advancement and challenges. J. Mater. Chem. A 2013, 1, 9365. [Google Scholar] [CrossRef]
- Ramirez-Vidal, P.; Sdanghi, G.; Celzard, A.; Fierro, V. High hydrogen release by cryo-adsorption and compression on porous materials. Int. J. Hydrogen Energy 2022, 47, 8892–8915. [Google Scholar] [CrossRef]
- Ahluwalia, R.; Peng, J. Automotive hydrogen storage system using cryo-adsorption on activated carbon. Int. J. Hydrogen Energy 2009, 34, 5476–5487. [Google Scholar] [CrossRef]
- Stamatakis, E.; Zoulias, E.; Tzamalis, G.; Massina, Z.; Analytis, V.; Christodoulou, C.; Stubos, A. Metal hydride hydrogen compressors: Current developments & early markets. Renew. Energy 2018, 127, 850–862. [Google Scholar]
- Tarasov, B.P.; Fursikov, P.V.; Volodin, A.A.; Bocharnikov, M.S.; Shimkus, Y.Y.; Kashin, A.M.; Yartys, V.A.; Chidziva, S.; Pasupathi, S.; Lototskyy, M.V. Metal hydride hydrogen storage and compression systems for energy storage technologies. Int. J. Hydrogen Energy 2021, 46, 13647–13657. [Google Scholar] [CrossRef]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen Storage for Mobility: A Review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef]
- Li, M.; Bai, Y.; Zhang, C.; Song, Y.; Jiang, S.; Grouset, D.; Zhang, M. Review on the research of hydrogen storage system fast refueling in fuel cell vehicle. Int. J. Hydrogen Energy 2019, 44, 10677–10693. [Google Scholar] [CrossRef]
- Hua, T.Q.; Ahluwalia, R.K.; Peng, J.K.; Kromer, M.; Lasher, S.; McKenney, K.; Law, K.; Sinha, J. Technical assessment of compressed hydrogen storage tank systems for automotive applications. Int. J. Hydrogen Energy 2011, 36, 3037–3049. [Google Scholar] [CrossRef]
- USDoE. DOE Technical Targets for Onboard Hydrogen Storage for Light-Duty Vehicles. Available online: https://www.energy.gov/eere/fuelcells/doe-technical-targets-onboard-hydrogen-storage-light-duty-vehicles (accessed on 1 November 2021).
- James, B.D.; Houchins, C. 700 Bar Type IV H2 Pressure Vessel Cost Projections. Available online: https://www.energy.gov/sites/prod/files/2016/09/f33/fcto_h2_storage_700bar_workshop_2_james.pdf (accessed on 24 August 2016).
- Din, T.; Hillmansen, S. Energy consumption and carbon dioxide emissions analysis for a concept design of a hydrogen hybrid railway vehicle. IET Electr. Syst. Transp. 2018, 8, 112–121. [Google Scholar] [CrossRef]
- Gallucci, M. Hydrogen trains roll into service: A new hybrid locomotive signals a growing push for zero-emission rail technologies—[News]. IEEE Spectr. 2019, 56, 6–7. [Google Scholar] [CrossRef]
- Ku, B.-Y. September 2021 Land Transportation News [Transportation Systems]. IEEE Veh. Technol. Mag. 2021, 16, 14–17. [Google Scholar] [CrossRef]
- Fedele, E.; Iannuzzi, D.; Del Pizzo, A. Onboard energy storage in rail transport: Review of real applications and techno-economic assessments. IET Electr. Syst. Transp. 2021, 11, 279–309. [Google Scholar] [CrossRef]
- Alstom. Successful Year and a Half of Trial Operation of the World’s First Two Hydrogen Trains, Next Project Phase Begins. Available online: https://www.alstom.com/press-releases-news/2020/5/successful-year-andhalf-trial-operation-worlds-first-two-hydrogen (accessed on 19 May 2020).
- Stetson, N.T.; McWhorter, S.; Ahn, C.C. Compendium of Hydrogen Energy: Hydrogen Storage, Distribution and Infrastructure; Woodhead Publishing: Sawston, UK, 2015; Volume 2, pp. 3–25. [Google Scholar]
- Yanxing, Z.; Maoqiong, G.; Yuan, Z.; Xueqiang, D.; Jun, S. Thermodynamics analysis of hydrogen storage based on compressed gaseous hydrogen, liquid hydrogen and cryo-compressed hydrogen. Int. J. Hydrogen Energy 2019, 44, 16833–16840. [Google Scholar] [CrossRef]
- Ahluwalia, R.; Hua, T.; Peng, J.; Kumar, R. System level analysis of hydrogen storage options. In DOE Hydrogen Program Annual Review; DOE: Washington, DC, USA, 2010. [Google Scholar]
- Ahluwalia, R.; Hua, T.; Peng, J.; Kumar, R. System level analysis of hydrogen storage options. In DOE Hydrogen Program Annual Review; DOE: Washington, DC, USA, 2020. [Google Scholar]
- Aziz, M.; Oda, T.; Kashiwagi, T. Comparison of liquid hydrogen, methylcyclohexane and ammonia on energy efficiency and economy. Energy Procedia 2019, 158, 4086–4091. [Google Scholar] [CrossRef]
- Peng, J.K.; Ahluwalia, R.K. Enhanced dormancy due to para-to-ortho hydrogen conversion in insulated cryogenic pressure vessels for automotive applications. Int. J. Hydrogen Energy 2013, 38, 13664–13672. [Google Scholar]
- Agarwal, R.; Dondapati, R.S. Numerical investigation on hydrodynamic characteristics of two-phase flow with liquid hydrogen through cryogenic feed lines at terrestrial and microgravity. Appl. Therm. Eng. 2020, 173, 115240. [Google Scholar] [CrossRef]
- Thomas, J.M.; Edwards, P.P.; Dobson, P.J.; Owen, G.P. Decarbonising energy: The developing international activity in hydrogen technologies and fuel cells. J. Energy Chem. 2020, 51, 405–415. [Google Scholar] [CrossRef]
- Kang, D.; Yun, S.; Kim, B.-K. Review of the Liquid Hydrogen Storage Tank and Insulation System for the High-Power Locomotive. Energies 2022, 15, 4357. [Google Scholar] [CrossRef]
- Siebel, T. Pressure in the Hydrogen Tank. ATZ Worldw. 2021, 123, 8–13. [Google Scholar]
- Rao, A.G.; Yin, F.; Werij, H. Energy Transition in Aviation: The Role of Cryogenic Fuels. Aerospace 2020, 7, 181. [Google Scholar] [CrossRef]
- Gupta, R.K.; Basile, A.; Veziroglu, T.N. Compendium of Hydrogen Energy; Woodhead Publishing: Cambridge, UK, 2016; Volume 2. [Google Scholar]
- Barthélémy, H.; Weber, M.; Barbier, F. Hydrogen storage: Recent improvements and industrial perspectives. Int. J. Hydrogen Energy 2017, 42, 7254–7262. [Google Scholar] [CrossRef]
- Moreno-Blanco, J.; Petitpas, G.; Espinosa-Loza, F.; Elizalde-Blancas, F.; Martinez-Frias, J.; Aceves, S.M. The storage performance of automotive cryo-compressed hydrogen vessels. Int. J. Hydrogen Energy 2019, 44, 16841–16851. [Google Scholar] [CrossRef]
- Ahluwalia, R.K.; Hua, T.Q.; Peng, J.K.; Lasher, S.; McKenney, K.; Sinha, J.; Gardiner, M. Technical assessment of cryo-compressed hydrogen storage tank systems for automotive applications. Int. J. Hydrogen Energy 2010, 35, 4171–4184. [Google Scholar] [CrossRef]
- Shafiei, E.; Davidsdottir, B.; Leaver, J.; Stefansson, H.; Asgeirsson, E.I. Comparative analysis of hydrogen, biofuels and electricity transitional pathways to sustainable transport in a renewable-based energy system. Energy 2015, 83, 614–627. [Google Scholar] [CrossRef]
- Zhan, X.; Yan, Y.; Wei, W.; Dongke, S.; Zhonghua, N. Supply system of cryo-compressed hydrogen for fuel cell stacks on heavy duty trucks. Int. J. Hydrogen Energy 2020, 45, 12921–12931. [Google Scholar]
- Yan, Y.; Xu, Z.; Han, F.; Wang, Z.; Ni, Z. Energy control of providing cryo-compressed hydrogen for the heavy-duty trucks driving. Energy 2021, 242, 122817. [Google Scholar] [CrossRef]
- Chen, L.; Xiao, R.; Cheng, C.; Tian, G.; Chen, S.; Hou, Y. Thermodynamic analysis of the para-to-ortho hydrogen conversion in cryo-compressed hydrogen vessels for automotive applications. Int. J. Hydrogen Energy 2020, 45, 24928–24937. [Google Scholar] [CrossRef]
- Ahluwalia, R.K.; Papadias, D.; Peng, J.-K.; Krause, T. Total Cost of Ownership for Line Haul, Yard Switchers and Regional Passenger Locomotives –Preliminary Results. In Proceedings of the 2019 US DOE Hydrogen Program Annual Merit Review, Crystal City, VA, USA, 29 April–1 May 2019. [Google Scholar]
- Aceves, S.; Brunner, T. Hydrogen Storage Tests for Cryo-Compressed Vessels, CRADA No. TC02119.0; Lawrence Livermore National Lab.(LLNL): Livermore, CA, USA, 2021.
- Petitpas, G. 1,000+ Cycles of a 350 Bar Prototype Cryo-Compressed Pressure Vessel; Lawrence Livermore National Lab.(LLNL): Livermore, CA, USA, 2018.
- James, B.D.; Houchins, C.; Huya-Kouadio, J.; DeSantis, D. Hydrogen Storage Cost Analysis. In Proceedings of the US DOE Hydrogen and Fuel Cells Program Annual Merit Review, Washington, DC, USA, 13–15 June 2018. [Google Scholar]
- Geiling, J.; Steinberger, M.; Ortner, F.; Seyfried, R.; Nuß, A.; Uhrig, F.; Lange, C.; Öchsner, R.; Wasserscheid, P.; März, M.; et al. Combined dynamic operation of PEM fuel cell and continuous dehydrogenation of perhydro-dibenzyltoluene. Int. J. Hydrogen Energy 2021, 46, 35662–35677. [Google Scholar] [CrossRef]
- Rao, P.C.; Yoon, M. Potential liquid-organic hydrogen carrier (LOHC) systems: A review on recent progress. Energies 2020, 13, 6040. [Google Scholar] [CrossRef]
- Niermann, M.; Timmerberg, S.; Drünert, S.; Kaltschmitt, M. Liquid Organic Hydrogen Carriers and alternatives for international transport of renewable hydrogen. Renew. Sustain. Energy Rev. 2021, 135, 110171. [Google Scholar] [CrossRef]
- Preuster, P.; Papp, C.; Wasserscheid, P. Liquid organic hydrogen carriers (LOHCs): Toward a hydrogen-free hydrogen economy. Acc. Chem. Res. 2017, 50, 74–85. [Google Scholar] [CrossRef]
- Gonda, M.; Ohshima, M.A.; Kurokawa, H.; Miura, H. Toluene hydrogenation over Pd and Pt catalysts as a model hydrogen storage process using low grade hydrogen containing catalyst inhibitors. Int. J. Hydrogen Energy 2014, 39, 16339–16346. [Google Scholar] [CrossRef]
- Mizuno, Y.; Ishimoto, Y.; Sakai, S.; Sakata, K. Economic analysis on international hydrogen energy carrier supply chains. J. Jpn. Soc. Energy Resour. 2016, 38, 11–17. [Google Scholar]
- Wijayanta, A.T.; Oda, T.; Purnomo, C.W.; Kashiwagi, T.; Aziz, M. Liquid hydrogen, methylcyclohexane, and ammonia as potential hydrogen storage: Comparison review. Int. J. Hydrogen Energy 2019, 44, 15026–15044. [Google Scholar] [CrossRef]
- Hurskainen, M.; Ihonen, J. Techno-economic feasibility of road transport of hydrogen using liquid organic hydrogen carriers. Int. J. Hydrogen Energy 2020, 45, 32098–32112. [Google Scholar] [CrossRef]
- Aakko-Saksa, P.T.; Cook, C.; Kiviaho, J.; Repo, T. Liquid organic hydrogen carriers for transportation and storing of renewable energy—Review and discussion. J. Power Sources 2018, 396, 803–823. [Google Scholar] [CrossRef]
- Fikrt, A.; Brehmer, R.; Milella, V.-O.; Müller, K.; Bösmann, A.; Preuster, P.; Alt, N.; Schlücker, E.; Wasserscheid, P.; Arlt, W. Dynamic power supply by hydrogen bound to a liquid organic hydrogen carrier. Appl. Energy 2017, 194, 1–8. [Google Scholar] [CrossRef]
- Ezzat, M.; Dincer, I. Comparative assessments of two integrated systems with/without fuel cells utilizing liquefied ammonia as a fuel for vehicular applications. Int. J. Hydrogen Energy 2018, 43, 4597–4608. [Google Scholar] [CrossRef]
- Ezzat, M.; Dincer, I. Development and assessment of a new hybrid vehicle with ammonia and hydrogen. Appl. Energy 2018, 219, 226–239. [Google Scholar] [CrossRef]
- Al-Hamed, K.H.; Dincer, I. A novel ammonia solid oxide fuel cell-based powering system with on-board hydrogen production for clean locomotives. Energy 2021, 220, 119771. [Google Scholar] [CrossRef]
- Al-Hamed, K.H.; Dincer, I. Investigation of an integrated powering system for clean locomotives with solid-oxide fuel cell with heat recovery organic Rankine cycle. Energy Convers. Manag. 2020, 219, 112857. [Google Scholar] [CrossRef]
- Lamb, K.E.; Dolan, M.D.; Kennedy, D.F. Ammonia for hydrogen storage; A review of catalytic ammonia decomposition and hydrogen separation and purification. Int. J. Hydrogen Energy 2019, 44, 3580–3593. [Google Scholar] [CrossRef]
- Lan, R.; Irvine, J.T.; Tao, S. Ammonia and related chemicals as potential indirect hydrogen storage materials. Int. J. Hydrogen Energy 2012, 37, 1482–1494. [Google Scholar] [CrossRef]
- Ezzat, M.F.; Dincer, I. Energy and exergy analyses of a novel ammonia combined power plant operating with gas turbine and solid oxide fuel cell systems. Energy 2020, 194, 116750. [Google Scholar] [CrossRef]
- Barelli, L.; Bidini, G.; Cinti, G. Operation of a Solid Oxide Fuel Cell Based Power System with Ammonia as a Fuel: Experimental Test and System Design. Energies 2020, 13, 6173. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.I.; Bowen, P. Ammonia for power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- Chai, W.S.; Bao, Y.; Jin, P.; Tang, G.; Zhou, L. A review on ammonia, ammonia-hydrogen and ammonia-methane fuels. Renew. Sustain. Energy Rev. 2021, 147, 111254. [Google Scholar] [CrossRef]
- Afif, A.; Radenahmad, N.; Cheok, Q.; Shams, S.; Kim, J.H.; Azad, A.K. Ammonia-fed fuel cells: A comprehensive review. Renew. Sustain. Energy Rev. 2016, 60, 822–835. [Google Scholar] [CrossRef]
- James, B.D.; DeSantis, D.A. Manufacturing Cost and Installed Price Analysis of Stationary Fuel Cell Systems; Strategic Analysis Inc.: Arlington, VA, USA, 2015. [Google Scholar]
- Stanescu, A.; Mocioi, N.; Dimitrescu, A. Hybrid Propulsion Train with Energy Storage in Metal Hydrides. In Proceedings of the Electric Vehicles International Conference (EV), Bucharest, Romania, 3–4 October 2019; IEEE: Bucharest, Romania, 2019; pp. 1–4. [Google Scholar]
- Davids, M.; Lototskyy, M.; Malinowski, M.; Van Schalkwyk, D.; Parsons, A.; Pasupathi, S.; Swanepoel, D.; van Niekerk, T. Metal hydride hydrogen storage tank for light fuel cell vehicle. Int. J. Hydrogen Energy 2019, 44, 29263–29272. [Google Scholar] [CrossRef]
- Lototskyy, M.; Tolj, I.; Klochko, Y.; Davids, M.W.; Swanepoel, D.; Linkov, V. Metal hydride hydrogen storage tank for fuel cell utility vehicles. Int. J. Hydrogen Energy 2020, 45, 7958–7967. [Google Scholar] [CrossRef]
- Falahati, H.; Barz, D.P.J. Evaluation of hydrogen sorption models for AB5-type metal alloys by employing a gravimetric technique. Int. J. Hydrogen Energy 2013, 38, 8838–8851. [Google Scholar] [CrossRef]
- Szajek, A.; Jurczyk, M.; Okońska, I.; Smardz, K.; Jankowska, E.; Smardz, L. Electrochemical and electronic properties of nanocrystalline Mg-based hydrogen storage materials. J. Alloys Compd. 2007, 436, 345–350. [Google Scholar] [CrossRef]
- Wang, P.; Kang, X.-d. Hydrogen-rich boron-containing materials for hydrogen storage. Dalton Trans. 2008, 5400–5413. [Google Scholar] [CrossRef] [PubMed]
- Züttel, A.; Wenger, P.; Rentsch, S.; Sudan, P.; Mauron, P.; Emmenegger, C. LiBH4 a new hydrogen storage material. J. Power Sources 2003, 118, 1–7. [Google Scholar] [CrossRef]
- Gross, K.; Sandrock, G.; Thomas, G. Dynamic in situ X-ray diffraction of catalyzed alanates. J. Alloys Compd. 2002, 330, 691–695. [Google Scholar] [CrossRef]
- Jensen, C.; Gross, K. Development of catalytically enhanced sodium aluminum hydride as a hydrogen-storage material. Appl. Phys. A 2001, 72, 213–219. [Google Scholar] [CrossRef]
- Urbanczyk, R.; Peinecke, K.; Felderhoff, M.; Hauschild, K.; Kersten, W.; Peil, S.; Bathen, D. Aluminium alloy based hydrogen storage tank operated with sodium aluminium hexahydride Na3AlH6. Int. J. Hydrogen Energy 2014, 39, 17118–17128. [Google Scholar] [CrossRef]
- USDoE. System Projection Graphs. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-storage-engineering-center-excellence#graphs (accessed on 8 March 2022).
- De Castro, J.F.R.; Santos, S.F.; Costa, A.L.M.; Yavari, A.R.; Botta F, W.J.; Ishikawa, T.T. Structural characterization and dehydrogenation behavior of Mg–5 at.%Nb nano-composite processed by reactive milling. J. Alloys Compd. 2004, 376, 251–256. [Google Scholar] [CrossRef]
- Kang, X.-D.; Luo, J.-H.; Wang, P. Efficient and highly rapid hydrogen release from ball-milled 3NH3BH3/MMgH3 (M = Na, K, Rb) mixtures at low temperatures. Int. J. Hydrogen Energy 2012, 37, 4259–4266. [Google Scholar] [CrossRef]
- USDoE. Hydrogen Storage Tech Team Roadmap. Available online: https://www.energy.gov/sites/prod/files/2017/08/f36/hstt_roadmap_July2017.pdf (accessed on 30 July 2017).
- USDoE. DOE Hydrogen and Fuel Cells Program Record#15013. Available online: https://www.hydrogen.energy.gov/pdfs/15013_onboard_storage_performance_cost.pdf (accessed on 30 September 2015).
- USDoE. System Level Analysis of Hydrogen Storage Options. Available online: https://www.hydrogen.energy.gov/pdfs/review17/st001_ahluwalia_2017_o.pdf (accessed on 5 June 2017).
- USDoE. Hydrogen Storage Engineering Center of Excellence-Savannah River National Laboratory (Anton). Available online: https://www.hydrogen.energy.gov/pdfs/review16/st004_anton_2016_o.pdf (accessed on 9 June 2016).
- USDoE. Department of Energy Hydrogen Program Plan. Available online: https://www.hydrogen.energy.gov/roadmaps_vision.html (accessed on 30 November 2020).



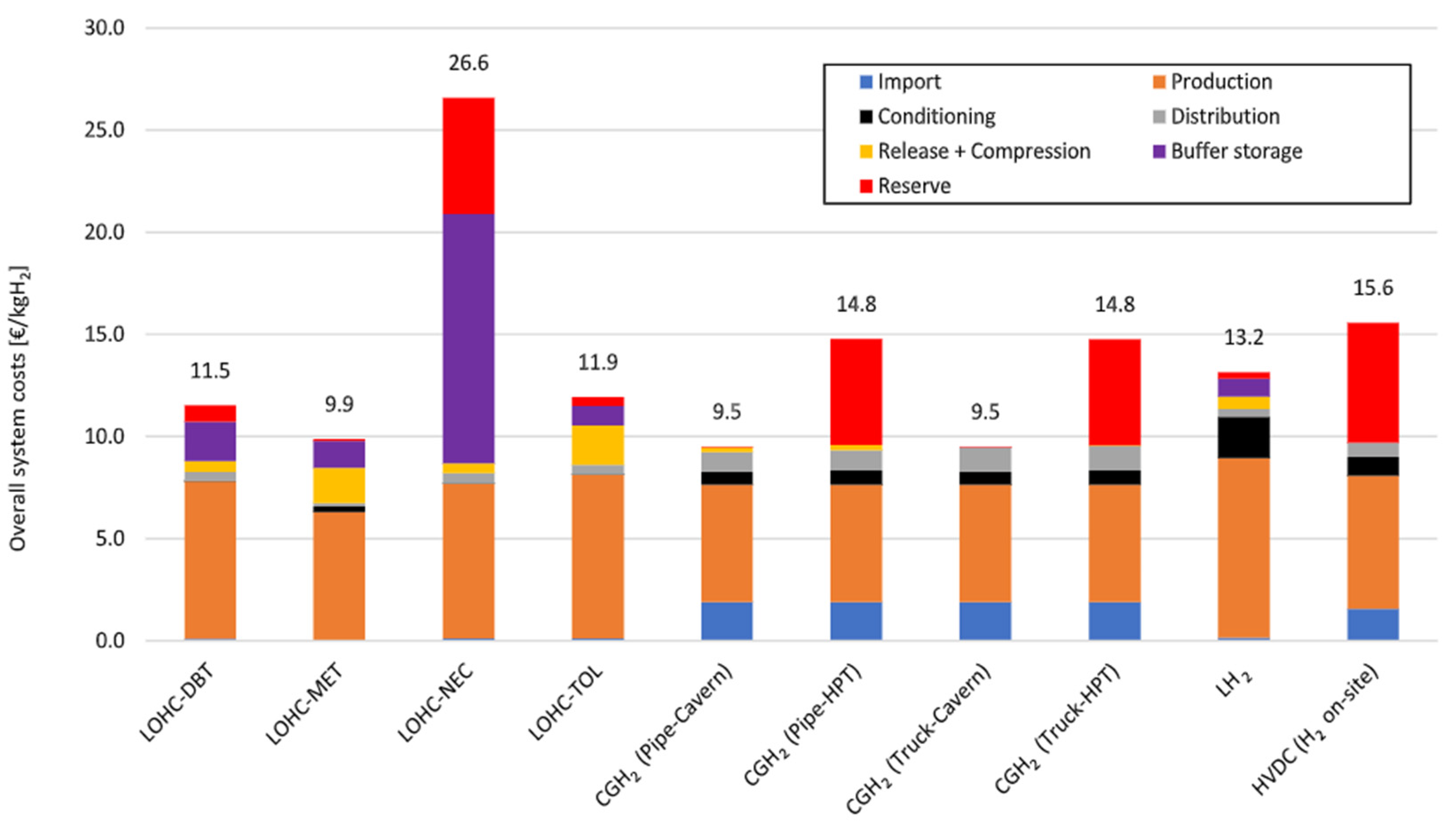
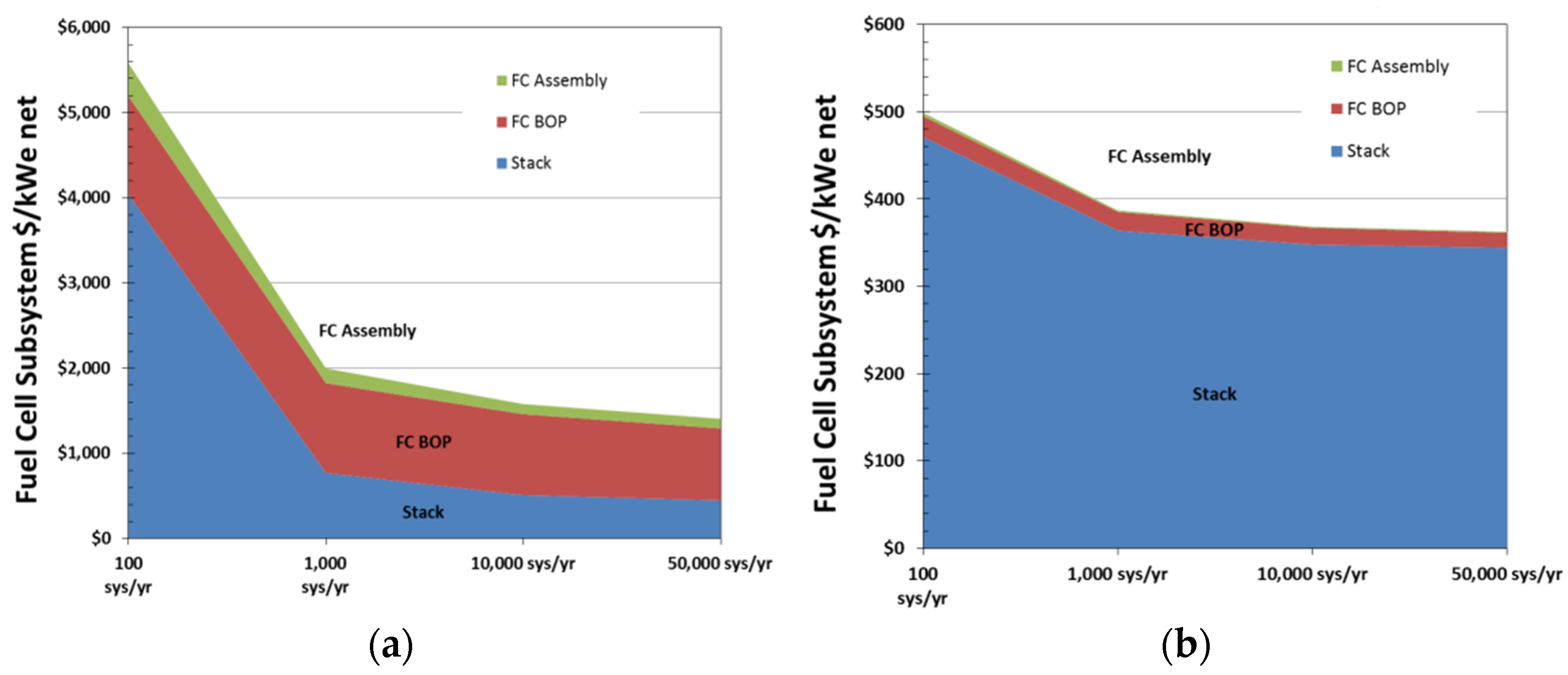
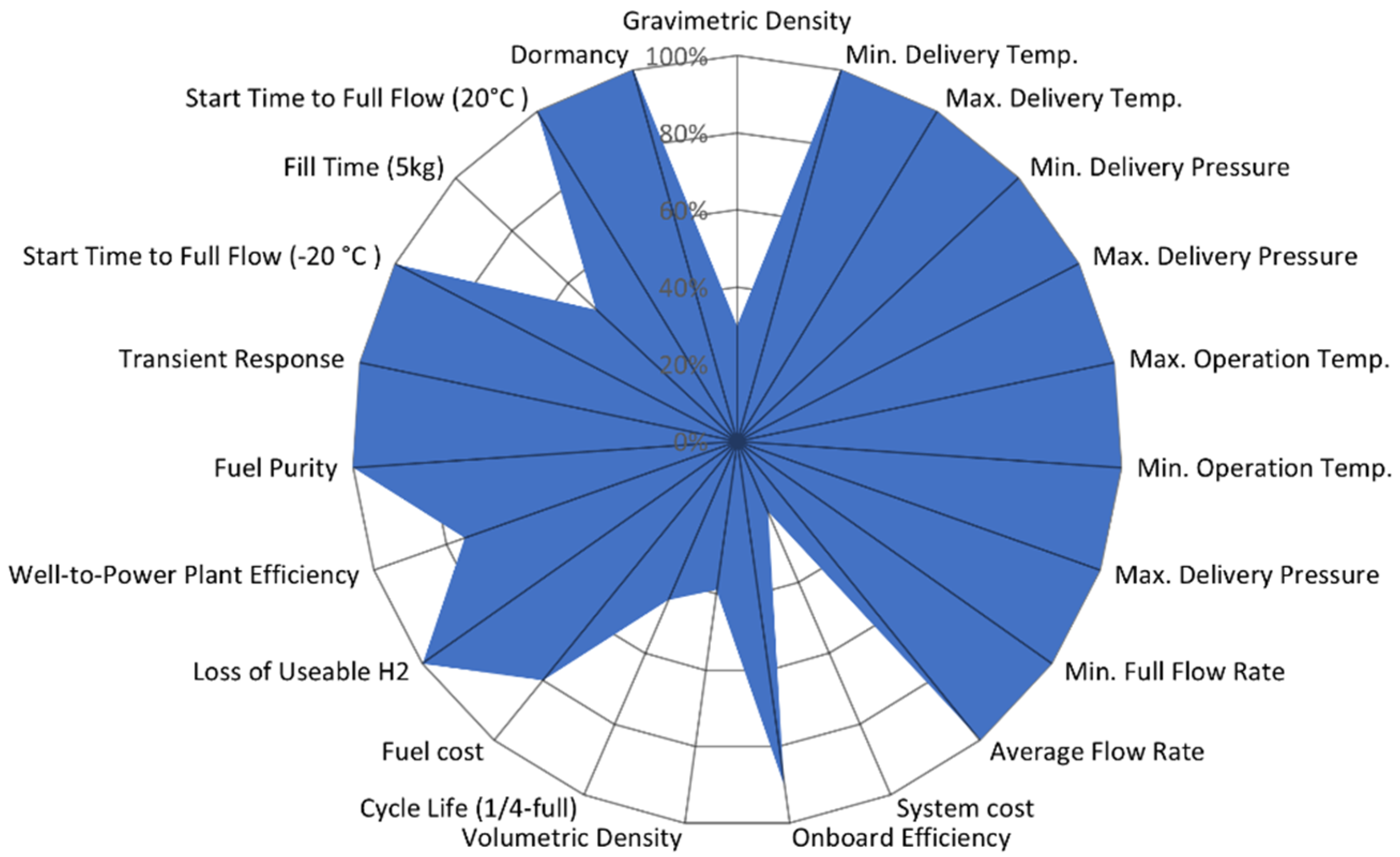
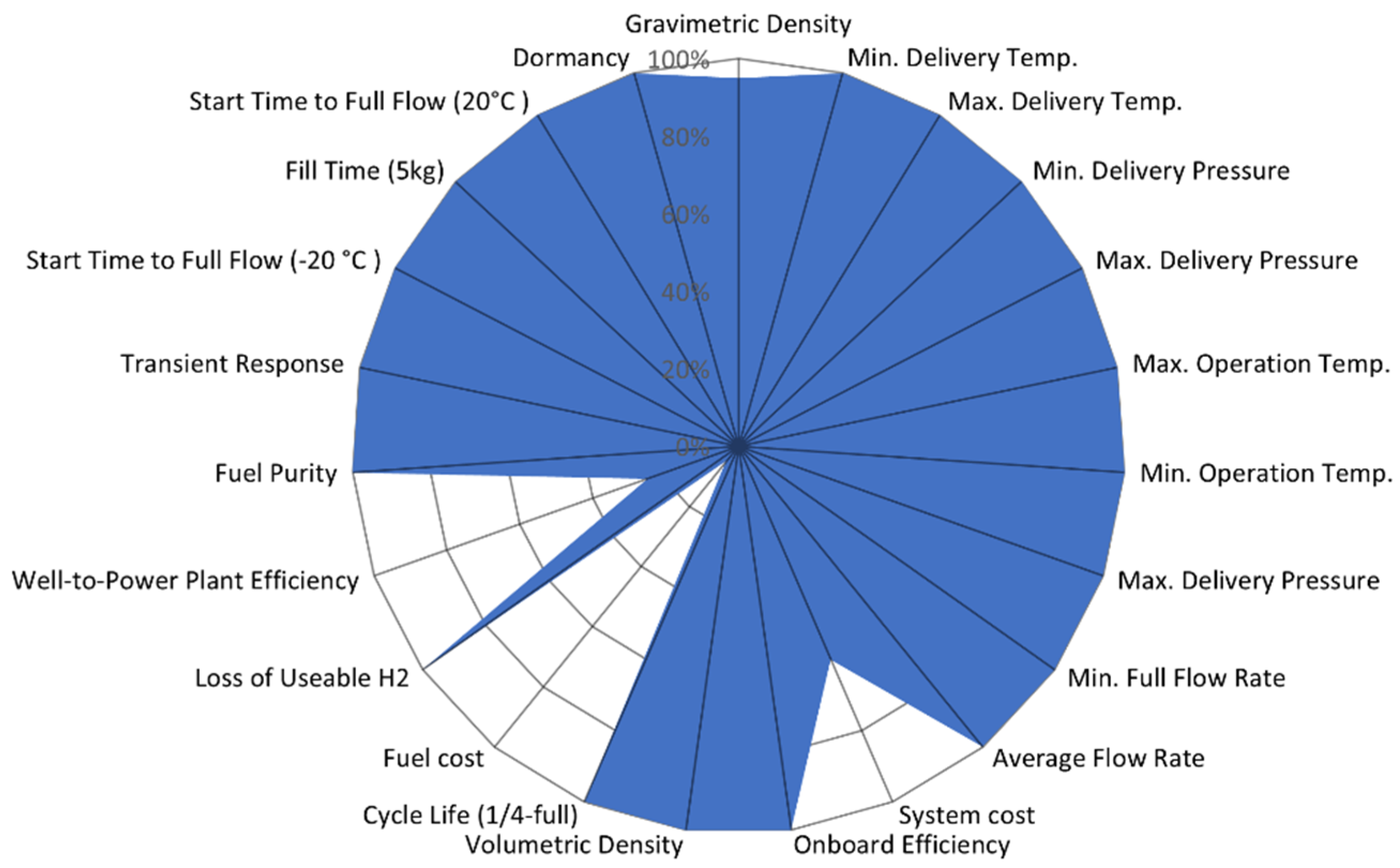
| Type | Materials | Features | Typical Pressure (MPa) | Cost (USD/kg) | Gravimetric Density (wt%) |
|---|---|---|---|---|---|
| I | All-metal construction | Heavy, internal corrosion | 17.5–20 | 83 | 1.7 |
| II | All-metal hoop-wrapped composite cylinders | Heavy, short life due to internal corrosion | 20–30 | 86 | 2.1 |
| III | Fully wrapped composite cylinders with metallic liners | Lightness, high burst pressure, no permeation, galvanic corrosion between liner and fibre (CF) | 35–70 | 567 | 5–5.5 |
| IV | All-composite construction | Lightness, lower burst pressure. High durability against repeated charging. Simple manufacturability | 35–70 | 633 | 5–5.7 (Toyota data) |
| Performance and Cost Metric | Units | 35 MPa | 70 MPa | 2020 Targets | 2025 Targets | Ultimate |
|---|---|---|---|---|---|---|
| System gravimetric capacity | Wt % | 5.5 | 5.2 | 4.5 | 5.5 | 6.5 |
| System volumetric capacity | g-H2/L | 17.6 | 26.3 | 30 | 40 | 50 |
| Storage system cost | USD/kWh | 15.4 | 18.7 | 10 | 9 | 8 |
| WTT efficiency (LHV) | % | 56.5 | 54.2 | 60 | 60 | 60 |
| Hydrogen-Powered Trains | HydroFLEX 1.0 | CRRC Datong | Coradia iLint |
|---|---|---|---|
| Manufacturer | Porterbrook and University of Birmingham, UK, 2019 | CRRC, China, 2021 | Alstom, Germany, 2018 |
| Type | Passenger locomotive | Freight locomotive | Passenger train |
| Hydrogen storage method | 35 MPa CH2 vessel | 35 MPa CH2 vessel | 35 MPa CH2 vessel |
| Fuel cell | PEMFC (400 kW) | PEMFC (400 kW) | PEMFC |
| Auxiliary power | Battery (400 kW) | Battery (1000 kW) | Battery |
| Pathway | H2 Production | Storage (Plant) | Liquefaction | Terminal | Transmission | Distribution | Dispensing (LDV) | Total Cost |
|---|---|---|---|---|---|---|---|---|
| CA(CH2) | 1.64 | 0.23 | - | 1.14 | - | 0.89 | 2.27 | 6.17 |
| CA (LH2) | 1.64 | - | 2.86 | 0.31 | - | 0.30 | 1.94 | 7.05 |
| TX to CA (LH2) | 0.89 | 0.31 | 2.15 | 0.33 | 1.10 | 0.30 | 1.94 | 7.02 |
| 350 Bar CcH2 | 500 Bar CcH2 | 700 Bar CcH2 | 350 Bar CH2 | Cold-CH2 | |
|---|---|---|---|---|---|
| Liner | 1.03 | 1.01 | 0.99 | 0.21 | 1.58 |
| Composite | 3.25 | 4.70 | 7.12 | 9.79 | 8.86 |
| Insulation and containment vessel | 3.48 | 3.21 | 2.92 | 0.00 | 3.05 |
| BOP | 3.84 | 3.85 | 3.85 | 3.25 | 3.45 |
| Assembly and other | 0.04 | 0.04 | 0.04 | 0.12 | 0.04 |
| System cost (USD/kWh) | 11.65 [−2.32, +2.90] | 12.82 [−2.32, +2.90] | 14.92 [−2.78, +3.61] | 13.38 [−3.44, +5.73] | 16.97 [−0.81, +1.59] |
| Properties | Toluene-MCH | Naphthalene-Decalin | Benzene-Cyclohexane | DBT-PDBT | ||||
|---|---|---|---|---|---|---|---|---|
| Toluene | MCH | Naphthalene | Decalin | Benzene | Cyclohexane | DBT | PDBT | |
| Physical | ||||||||
| Chemical formula | C7H8 | C7H14 | C10H8 | C10H18 | C6H6 | C6H12 | C21H20 | C21H38 |
| density | 867 | 0.769 | 0.975 | 0.896 | 0.874 | 0.779 | 1.010 | 1.057 |
| Melting point | −95 | −127 | 80.3 | Cis −43.0 Trans −30.4 | 5.5 | 6.5 | −30 | −34 |
| Boiling point | 111 | 101 | 218 | Cis −94.6 Trans 185.5 | 80 | 81 | 278 | 395 |
| Phase under ambient cond. | Liquid | Liquid | Solid | Liquid | Liquid | Liquid | Liquid | Liquid |
| Gravimetric density (wt%) | 6.16 | 7.29 | 7.2 | 6.2 | ||||
| Volumetric density(kg/m3) | 47.4 | 65.4 | 55.9 | 57 | ||||
| Heat of reaction (kJ/mol) | 204.8 | 319.5(cis), 332.5(trans) | 205.9 | 588.5 | ||||
| Temperature (°C) | 200–300 | 150–250 | 150–250 | 180 | ||||
| Pressure (bar) | 10–50 | 20–50 | 10–50 | 10–50 | ||||
| Dehydrogenation with selected catalyst | ||||||||
| Temperature (°C) | 250–350 | 300–350 | 330 | 260–320 | ||||
| Pressure (bar) | 1–5 | 1–4 | 1–4 | 1–5 | ||||
| Advantages | Both liquid in wide range temperature | -Relatively high H2 content | -Relatively high H2 content | -Higher intrinsic safety -Good thermal stability | ||||
| Challenges | -Irritative inflammable -Volatile | -Different phase (difficulties in storage and transportation) -High energy for dehydrogenation -Volatile | -High melting point (possibility of phase change) -Toxic | -High energy for dehydrogenation | ||||
| Property | Unit | Value |
|---|---|---|
| Molecular weight | g/mol | 7.03 |
| Gravimetric H2 capacity | wt% | 17.7 |
| Volumetric H2 capacity | kg-H2/m−3 | 120.3 |
| Storage condition | bar or K | 10 or 240 |
| H2 release temperature | K | 600–1200 |
| Regeneration temperature | K | 650–900 |
| Ignition temperature | K | 924 |
| Price | USD/kg (USD/kWh) | 0.3 (0.058) |
| Type | Component | Hydrogen Storage Capacity (wt%) | Temperature (K) | Pressure (MPa) |
|---|---|---|---|---|
| Intermetallic hydrides | LaNi5H6 | 1.37 | 295 | 0.1 |
| FeTiH2 | 1.89 | 185 | 0.1 | |
| Mg2NiH4 | 3.59 | 255 | 0.1 | |
| ZrMn2H2 | 1.77 | 440 | 0.1 | |
| Complex hydrides | LiBH4 nanocomposite | 6.5 | 573 | - |
| LiBH4 + SiO2 | 13.5 | 373 | 5.0 | |
| NaAlH4 + 1.0 mol% TiCl3 | 5.6 | 323–383 | - | |
| NaAlH4 + 4.0 mol% Ti | 4.8 | 373 | - | |
| NaAlH4 + 1.0 mol% Ti | 5.6 | 443/423 | 15.4 | |
| Na3AlH6 + 2.0 mol% TiCl3 | 2.1 | 473/543 | 6.0 | |
| NaAlH4 + porous carbon | 7.0 | 673 | 10.0 | |
| NaAlH4 + none-porous carbon | 6.3 | 673 | 10.0 |
| Physical Storage Systems | Material-Based Storage System | ||||
|---|---|---|---|---|---|
| Barriers | Compressed | Cold/Cryo-Compressed | Metal Hydride | Sorbent-Based | Chemical Storage |
| Materials of Construction | ● | ● | ● | ● | ● |
| Balance-of-Plant Cost | ● | ● | ● | ● | ● |
| Thermal Management | ● | ● | ● | ● | ● |
| Tank Cost | ● | ● | ● | ● | |
| Tank Mass | ● | ● | ● | ● | |
| Off-board Energy Efficiency | ● | ● | ● | ● | |
| Heat Transfer Systems | ● | ● | ● | ||
| Material Gravimetric Capacity | ● | ● | ● | ||
| Material Volumetric Capacity | ● | ● | ● | ||
| Reaction Thermodynamics | ● | ● | ● | ||
| Cryogenic Tank Operation | ● | ● | |||
| High Temperature Tank Operation | ● | ● | |||
| Carbon Fibre Cost | ● | ● | |||
| Material Thermal Conductivity | ● | ● | |||
| Fuel Purity | ● | ● | |||
| Kinetics | ● | ● | |||
| Reactor Design | ● | ||||
| Material Handling | ● | ||||
| Hydrogen Storage Technology | Advantages | Disadvantages | Current State | TRL | |
|---|---|---|---|---|---|
| Physical Storage Methods | Compressed hydrogen | -Relatively mature -Many types of storage tanks for different areas -Purity | -Storage density needs to be improved -Cost needs to be reduced | -Successfully used for trains -Mass production | 8/9 |
| Liquid hydrogen | -High volumetric capacity -Purity -Relatively low utilisation cost | -High liquification cost -Short dormancy time (boil-off) | -Mostly used for military and aerospace -Prototype trucks -Being tested for trains in KR and JP | 6/7 | |
| Cryo-compressed hydrogen | -High hydrogen capacity -Long dormancy time -Relatively low cost | -Low maturity | -Prototype cars -Onboard simulation for trains by DOE | 4/5 | |
| Material-based (chemical) storage methods | Metal hydrides | -Some types have high storage capacity -Able to absorb large quantities of hydrogen -Multi-role | -Harsh operation conditions -Refuelling time -High cost for onboard use | -Prototype vehicles -Mostly discussed for hydrogen transportation | 4/5 |
| Liquid organic hydrogen carriers (LOHC) | -High hydrogen capacity -Relatively low cost | -Complex catalytic conditions -Low hydrogen releasing speed -Toxic by-product -Unavoidable purification | -Being tested for trains by Siemens -Mostly discussed for hydrogen transportation | 6/7 | |
| Ammonia | -Mature production chain -Low cost -Multiple use routes | -By-product NOx -Toxic -Expensive DA-SOFC -Dehydrogenation cost | -Successfully used for marine applications (direct combustion) -Lab stage (SOFC route) | 6/7 (direct combustion) 4/5 (SOFC route) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Zhao, N.; Hillmansen, S.; Roberts, C.; Yan, Y. Techno-Economic Analysis of Hydrogen Storage Technologies for Railway Engineering: A Review. Energies 2022, 15, 6467. https://doi.org/10.3390/en15176467
Xu Z, Zhao N, Hillmansen S, Roberts C, Yan Y. Techno-Economic Analysis of Hydrogen Storage Technologies for Railway Engineering: A Review. Energies. 2022; 15(17):6467. https://doi.org/10.3390/en15176467
Chicago/Turabian StyleXu, Zhan, Ning Zhao, Stuart Hillmansen, Clive Roberts, and Yan Yan. 2022. "Techno-Economic Analysis of Hydrogen Storage Technologies for Railway Engineering: A Review" Energies 15, no. 17: 6467. https://doi.org/10.3390/en15176467
APA StyleXu, Z., Zhao, N., Hillmansen, S., Roberts, C., & Yan, Y. (2022). Techno-Economic Analysis of Hydrogen Storage Technologies for Railway Engineering: A Review. Energies, 15(17), 6467. https://doi.org/10.3390/en15176467








