Abstract
Silica is considered one of the most prevalent components in the Earth’s shell and is synthesized for use in technological applications. Nevertheless, new methods for finding a better, cheaper, and more ecologically friendly supply of silica with less energy consumption are unavoidable. This study investigates whether nanopowders made from waste with a great silica amount (fly ash and glass) can be utilized as fillers in an epoxy glue to enhance its characteristics. Four different contents (5, 10, 15, and 20 wt%) of nano–fly ash, nanoglass, and nanosilica powder were introduced into the samples. Fourier transform infrared analysis, differential scanning calorimetry analysis, viscosity testing, and microhardness testing were conducted for nanoglass/epoxy and nano–fly ash/epoxy samples, which were compared with the silica/epoxy samples. Results indicated that the nanoglass and nano–fly ash powder have the same impact as nanosilica on the characteristics of epoxy. The hardness and viscosity of epoxy increased with the increase in the added filler. At 20 wt%, the hardness value of the nanoglass/epoxy composites was greater than that of the nanosilica/epoxy and fly ash/epoxy composites by about 15% and 7%, respectively. The results also indicated that the highest viscosity values were obtained when using nano–fly ash powder of 20 wt%. Furthermore, the modification of the epoxy by the nanoparticles had no significant effect on the values of the glass transition temperatures.
1. Introduction
In most instances, composites are produced by mixing two different engineering materials, one of which is a continuous matrix surrounding the dispersed phase. The characteristics of composites are determined by the component phases’ characteristics, their relative quantities, and the shape and size of the dispersed phase [1,2]. Recently, there has been a focus on developing improved materials by incorporating nanoreinforcements into various matrices to improve physical, thermal, and mechanical characteristics [3,4]. The substantial change in viscoelastic characteristics of molten polymers is among the essential impacts of nanofiller inclusion. In contrast to micron-sized particles, filler size reduction to the nanometric scale may significantly change the rheology and dynamics of filled polymers [5,6,7,8].
Epoxy resin is among the most extensively utilized and adaptable compounds [9,10]. Composites, electric insulators, construction, flooring, coatings, and adhesives are just a few applications. On curing, its strongly cross-linked structure improves specific characteristics, including low creep, high modulus, excellent adhesion strength, economics, low friction coefficient, long component service life, good toughness, shear strength, and good performance at higher temperatures [11,12,13]. However, the material is brittle and has low fracture initiation resistance due to its extremely cross-linked structure (thermosetting polymer). A nanophase is introduced into the matrix to address these issues, which might take hard particles. Nanoparticles improve toughness, but due to their small size, they also produce a smaller rise in viscosity when the filler amount is increased [14,15].
Nonetheless, silica (SiO2) nanoparticles in polymer matrices have grown among the other nanofillers given the high specific surface area, better bonding capability with the polymer matrices, low cost, and strong mechanical features [16,17]. Due to their capacity to withstand thermal and mechanical stress, silica/epoxy composites are among the most frequently utilized in construction in the electronics, aerospace, and automotive sectors. The inclusion of silica nanoparticles has not impacted the transition temperature of glass and epoxy resin yield stress. The glass transition and yield stress stayed constant with a decrease or increase in the particle size of nanosilica. The inclusion of nanosilica substantially influenced fracture toughness, modulus, and thermal expansion coefficient, as predicted [18,19].
M. Conradi et al. [20] explored the mechanical characteristics of epoxy composites loaded with a 0.5% volume fraction of 130 nm and 30 nm sphere-shaped silica particles. It was discovered that the elasticity modulus increased by 20% and fracture toughness improved by 30%. The size of the silica has a significant impact on the composites’ impact energy. In comparison to pure epoxy, the impact energy of silica/epoxy composites seems to be 30% higher for the 130 nm and 60% higher for the 30 nm [20]. Anisha Christy et al. [21] explored the mechanical characteristics of epoxy/SiO2 with nanosized polymer matrix composites, such as tensile strengths and impact strengths. Using an ultrasonication technique, various weight percentages of nano-SiO2 were added to an epoxy matrix, ranging from 0 wt% to 3 wt%. They found that adding a great weight percentage of nanosilica particles in polymer matrix nanocomposites significantly improves their dynamical, mechanical, and thermal characteristics [21]. Peerapan Dittanet et al. [22] researched using a sol–gel method to create an epoxy composite reinforced with SiO2 particles. The silica nanoparticles were 23 nm, 74 nm, and 170 nm in size. The volume rates varied between 0 and 30% nano-SiO2 particles; with the growth of SiO2 particles, yield stress and Young’s modulus increased. The coefficient of heated development (CTE) decreased as SiO2 concentration increased [22]. The mechanical and wear characteristics of epoxy and polymer matrix composite-based nanosilica were investigated by Zhang et al. [23]. The amount of silica in a composite increases its mechanical characteristics. When silica was introduced as reinforcement in the epoxy matrix, the flexural modulus and tensile strength increased by approximately 30% [23].
Silica has been used as a filler in composites, ceramics engineering, chromatography, rubber, thermal insulators, drug delivery systems, catalysts, and electronic components for many years. Silica seems to be the most common mineral in the Earth’s shell, yet despite its abundance, it is usually synthesized to be used in technology, and silica is considered among the most versatile inorganic chemical compounds [24,25]. Today, all major concerns are energy conservation, natural resource protection, and substituted components such as industrial waste. As a result, alternative sources must be found to reduce manufacturing production energy and costs and provide a positive image for the environment (due to waste reduction) [26,27]. Therefore, the importance of the research is related to reducing the issue of landfills and CO2 resulting from waste deposits and reducing the industrial waste by recycling. This research aims to look at the impacts of nanoparticles from waste materials (fly ash and glass), particularly great silica contents and the added filler, on the features of composite-based epoxy. The findings of epoxy with fly ash and glass addition have been compared with nanosilica addition samples.
2. Materials and Methods
2.1. Materials
Different specimens were synthesized using commercially available epoxy resin (Sikadur-52, Sika Australia Pty Limited, Bundall, Australia) with a density of approximately 1.1 kg/L and hardener as a matrix material. As reinforcements, three powders were utilized: nanosilica, nanoglass, and nano–fly ash. The nanosilica (particle size 70 nm) utilized in this experiment was from Fluka Company, Buchs, Switzerland. Local sources provided shattered fly ash (industrial power plant) and glass (local waste). In the engineering laboratory at Babylon University in Iraq, fly ash and glass nanopowders were created using planetary ball mill type mechanical techniques. Two hundred grams of powder was put into the container, which contained 6 st. balls with a diameter of 15 mm, and was milled for 12 h at 600 rpm. A 90 Plus laser particle size analyzer was used to determine the particle size of the prepared nanoglass waste and nano–fly ash powders. Chemical analysis of fly ash and glass powders was performed using an XRF microprobe analyzer, as shown in Table 1. The particle size of glass and fly ash powder is shown in Table 2.

Table 1.
The chemical compositions of glass and fly ash powder.

Table 2.
The particle size of glass and fly ash powder.
2.2. Method
To achieve a homogeneous mixture, the filler material was progressively added to epoxy resin (part A) while vigorously swirling with a magnetic stirrer (SH-2 type). The hardener (component B) was then added depending on the glue manufacturer’s recommended mix ratio (2:1). Nano–fly ash, glass, and silica were added in various amounts (0, 5, 10, 15, and 20 wt%) to three sets of specimens.
2.3. Tests
Fourier transform infrared (FTIR) spectroscopy was utilized to analyze the chemical composition of the produced specimens (pure, 15% nanosilica, 15% nanoglass, 15% nano–fly ash) (IRAffinity-1 device kind). Moreover, DSC analysis was performed for the same sample above to determine the fillers’ impact on the glass transition temperatures (Tg).
All epoxy specimens were tested for microhardness and viscosity. A computerized micro Vickers hardness tester was used to determine the microhardness (TH-717 model). All of the produced specimens were subjected to microhardness testing. The test was performed utilizing a square base diamond pyramid and a load of 0.24 N for 20 s on a Digital Micro Vickers Hardness Tester (TH-717 model). Microhardness was calculated as the average of three measurements for each specimen. The viscosity was measured using Rheocalc V3.3 Build 49-1 tester/USA. The viscosity test was performed at 26 C.
3. Results and Discussion
3.1. Results of Fourier Transform Infrared Analysis
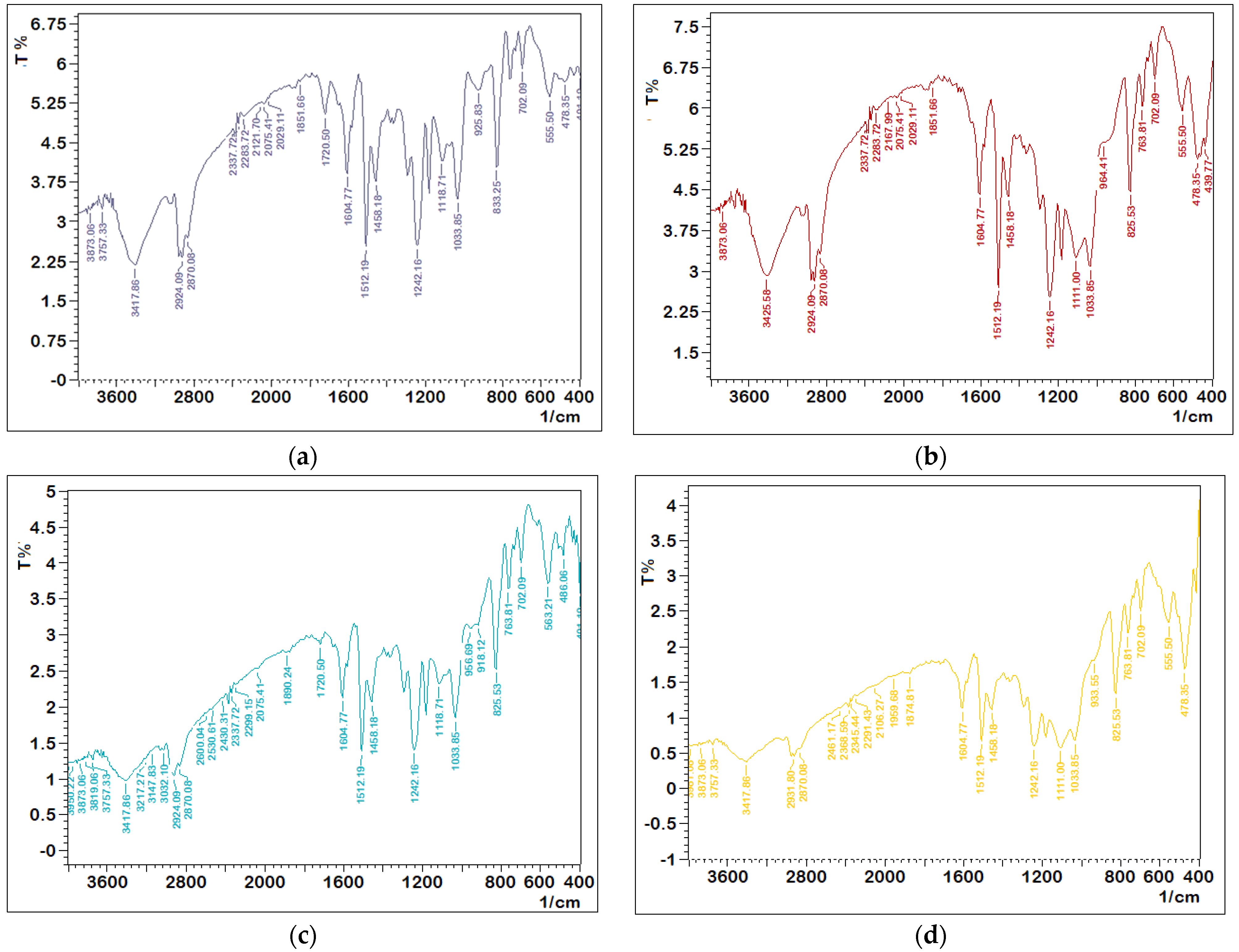
The epoxy/nanopowder composite structure has been investigated using Fourier transform infrared analysis, as demonstrated in Figure 1. The molecular structure of pure epoxy was confirmed by the reflection bonding of stretching and contraction in the epoxy circle phases, which can be seen at approximately 1242 cm−1 and 833 cm−1 in the FTIR analysis. The aromatic ring peaks were found at 1512 and 1604 cm−1. The stretching vibration of O-H is associated with the wide band (3100–3600 cm−1). The peaks in the range 2800–2990 cm−1 are associated with epoxy C-H vibrations [28,29]. All of these energy gap findings verified the neat epoxy’s composition.
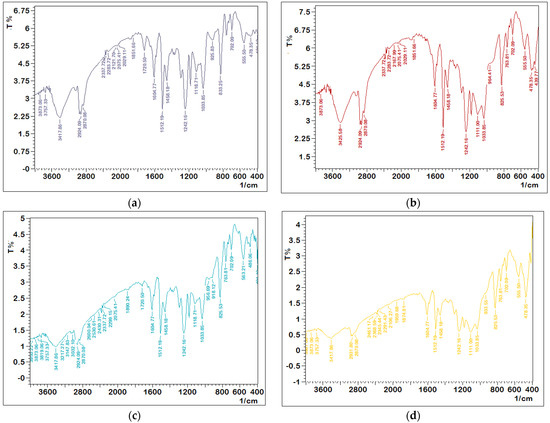
Figure 1.
FTIR analysis: (a) neat epoxy, (b) 15% nanosilica, (c) 15% nanoglass, and (d) 15% nano–fly ash.
Figure 1b–d demonstrate the FTIR transmittance spectra of an epoxy/nanopowder combination with a weight proportion of 15% nanosilica, 15% nanoglass, and 15% nano–fly ash to examine the silica bands in epoxy. The study’s findings revealed that the bulk of the peak transmission could be traced back to the original epoxy. The development of new band peaks of silica may also be observed. The stretching, symmetric, and asymmetric vibrations of the siloxane set (Si-O-Si) in the silica also confirmed the network of SiO2 transmission bands at 825 cm−1 and 1111 cm−1 [30]. The Si-O-H band in silica was responsible for another new band peak at 3757 cm−1. Once nanosilica was treated with epoxy, the epoxide set would react with the SiO2 silanol set via H-bonding. The OH group stretching vibrations of epoxy resin were shifted to 3417 cm−1 for silica-reinforced epoxy [30,31], as seen from the clean epoxy resin spectra and its composites of fly ash, glass, and silica. The quantity of transmission rose with the addition of nanosilica powder and was reduced with nano–fly ash and glass powder, as shown in the figures. The interaction of radiation with the powder and the presence of absorption and dispersion causes a rise or reduction in radiation transmission.
3.2. Results of Glass Transition Temperatures
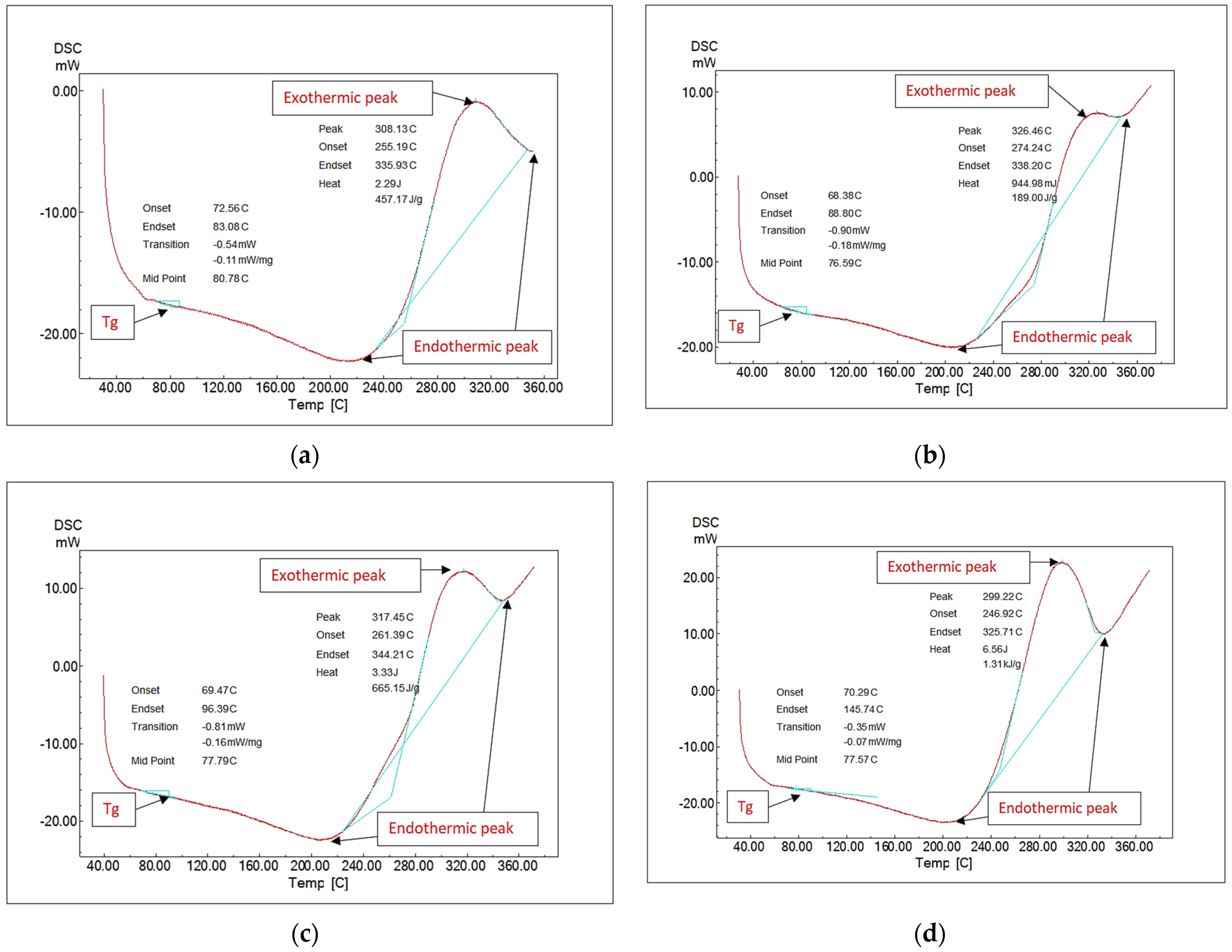
Polymer materials with flexible backbone show lower Tg, whereas polymer materials whose molecular structure is stiff, rigid, and inflexible show a higher Tg. Glass transition temperature helps determine various flexible and rigid applications for a material. The formulations’ glass transition temperature (Tg) has been determined using differential scanning calorimetry (DSC). The Tg of the network epoxy has been determined at 80.78 degrees centigrade, as illustrated in Figure 2. The addition of 15% nanosilica had minimal impact on the epoxy’s glass transition temperature; the glass transition temperature observed was 76.59 degrees centigrade. This verified that the silica–thermal epoxy’s characteristics seemed similar to those of pure epoxy [18,32,33]. According to studies, reactions between polymer chains and the considerably charging nanoparticle surface result in the development of a polymer nanolayer near the nanoparticle surface, and the glass transition temperatures are determined by this interfacial nanolayer (Tg). This nanoparticle–polymer interaction may be attractive, repulsive, or neutral, and Tg could increase, decrease, or stay constant based on these characteristics [34]. The slight reduction in Tg caused by nanoparticle modification of epoxy may substantially impact the changing free volumes between polymer chains. The chains’ movability rises and the Tg decreases as the nanoparticles expand the free volumes, for example, by pushing the chains apart. An additional explanation is that the nanoparticles affect the adhesive’s curing processes and prevent the polymer chains from cross-linking [35]. The addition of 15% nanoglass and 15% nano–fly ash had the same impact as adding nanosilica; the observed glass transition temperatures seem to have been 77.79 and 77.57 degrees centigrade, respectively, closely matching the nanosilica addition finding. Nano–fly ash, silica, and glass all have the same impact on the Tg of epoxy, as shown in Figure 2.
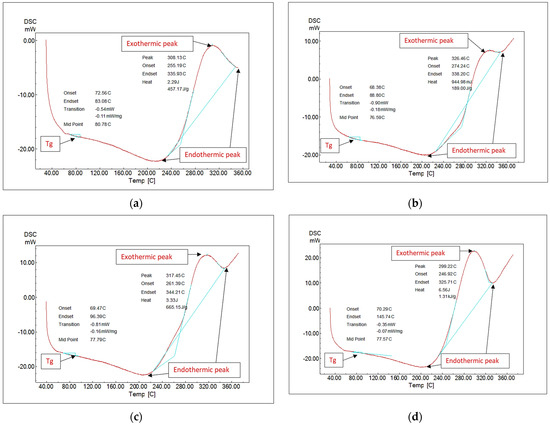
Figure 2.
Differential scanning calorimetry (DSC) analysis: (a) neat epoxy, (b) 15% nanosilica, (c) 15% nanoglass, and (d) 15% nano–fly ash.
3.3. Results of Viscosity
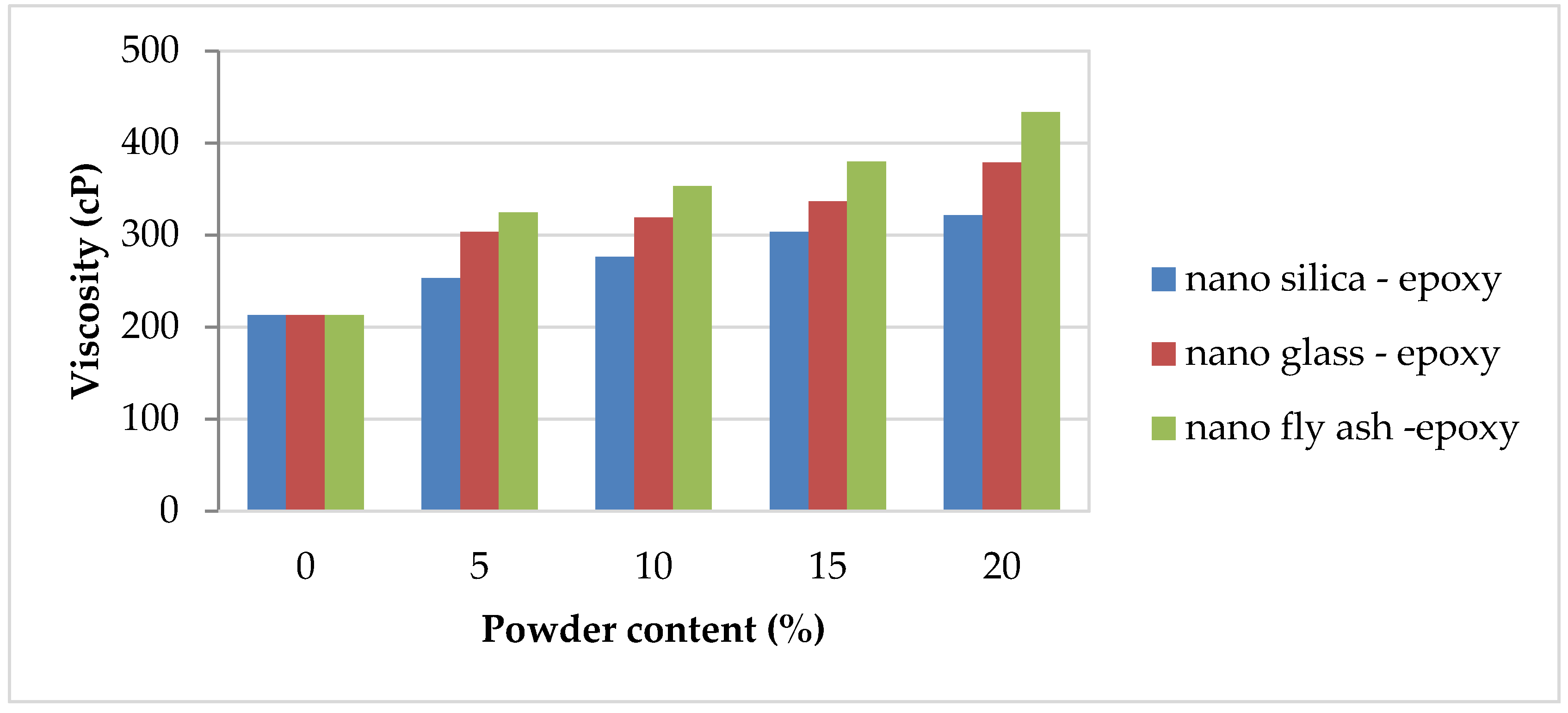
The critical element of workability properties is the complex knowledge of how additions influence the rheological properties of the polymer. The viscosity was determined for pure epoxy and nano–fly ash/epoxy, nanoglass/epoxy, and nanosilica/epoxy added in various amounts (0, 5, 10, 15, and 20 wt%) to three sets of specimens. Figure 3 depicts the viscosity of nanopowder-filled epoxy matrix composites. Increasing the powder amount from 0 to 20 cause a significant increase in the viscosity value from 212 cP to 433 cP when using of nano–fly ash epoxy, which records the highest viscosity values in comparison with other selected powders, while nanosilica epoxy records the lowest viscosity values in all selected powder ratios compared with other powders.
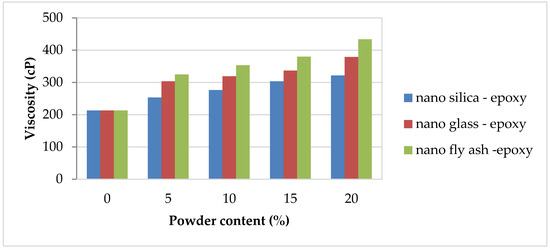
Figure 3.
Viscosity magnitudes of the nanopowder-filled epoxy.
Because of the high surface area of nanosilica, the initial viscosity rises with increasing nanosilica concentration, preventing the resin combination from being stirred at room temperature [36,37]. The reduction in cross-linking density of the resultant polymers is the cause of increased viscosity. More minor nanosilica phases provide more surface area for interphase interactions, resulting in improved interphase strengths [38]. Nanoglass and nano–fly ash powder have the same impact as nanosilica; the viscosity of the epoxy notably increases with nanoglass and nano–fly ash additions.
The high viscosity magnitudes with nanoglass and fly ash additions can be explained because the nanopowder was obtained by mechanical technique, and therefore the surface of the grains was irregular and deformed. Because of the agglomeration of a colloidal dispersion of the nanosized particles in the resin, the nanofilled epoxy resin exhibits a relatively high viscosity as the nano–fly ash and glass contents increase [3]. The resin blend viscosity would not be significantly altered below these nanofiller loadings. However, the comparatively high quantities of nanopowder increase the resin’s viscosity, making it more difficult to process [18].
3.4. Results of Hardness
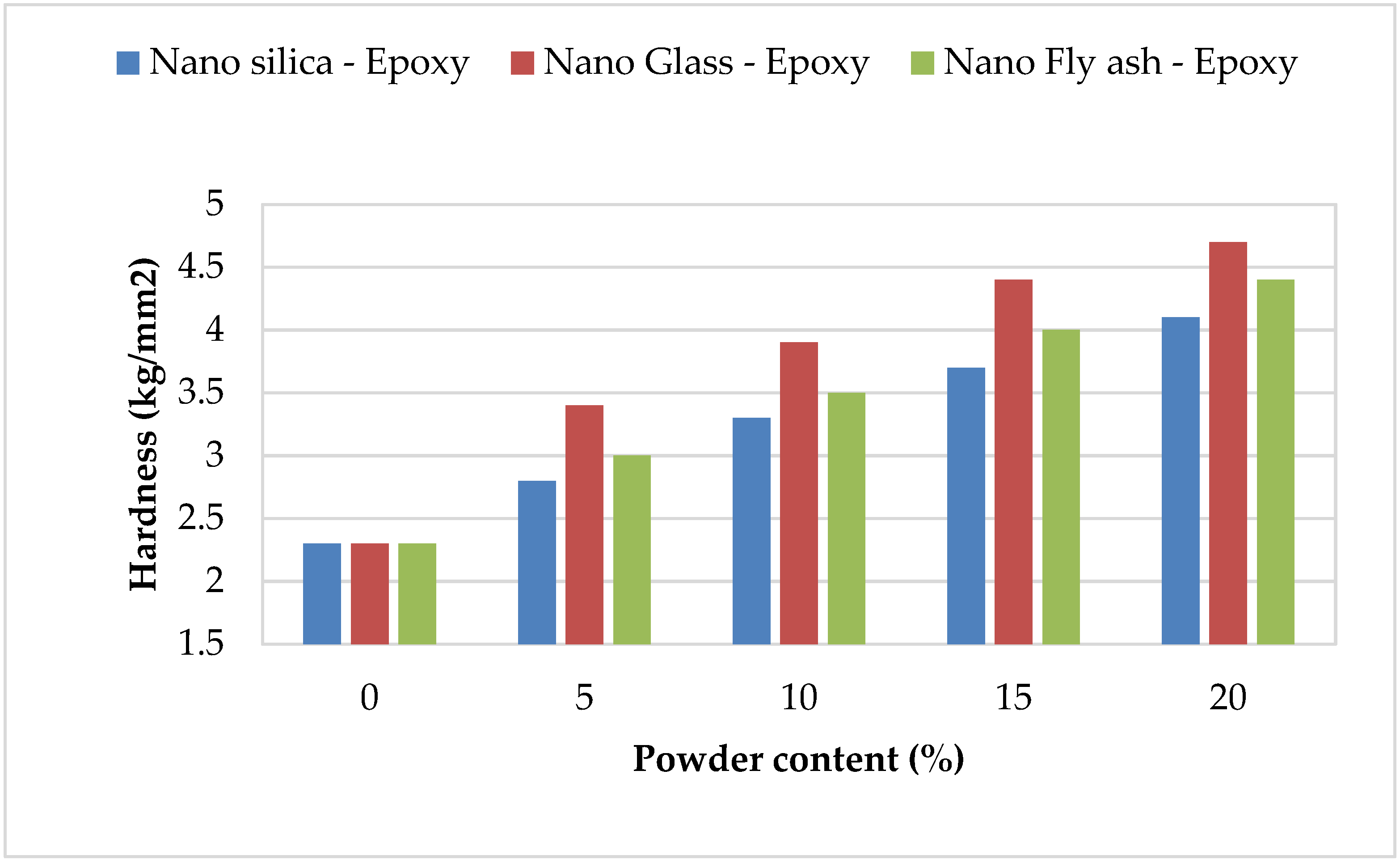
The hardness of epoxy matrix composites filled with nanopowder was already investigated. The hardness was determined for pure epoxy and nano–fly ash/epoxy, nanoglass/epoxy, and nanosilica/epoxy added in various amounts (0, 5, 10, 15, and 20 wt%) to three sets of specimens. The Vickers hardness testing was performed on the specimens with a load of 0.24 N for 20 ms. Figure 4 depicts the change in hardness as a function of the number of fillers in the epoxy. In most instances, strengthening nanosilica, glass, and fly ash in epoxy polymer improves microhardness. In addition, increasing the powder amount from 0 to 20 led to the hardness value doubling for most of the powders; at the same time using nanoglass epoxy records the highest hardness value in comparison with other selected powders that returned to a high specific area for glass powder, while nanosilica epoxy recorded the lowest improvement in hardness values in all selected ratios in comparison with other powders. The strong H-bonding between the OH sets in the parent matrices and the silanol (Si-OH) sets on the SiO2 surface, which restricts chain mobility and enhances cross-linking, was responsible for this enhancement [39]. The hardness magnitudes rose when the nanopowder filler had been expanded, which correlates to the uniform dispersion of the nanofiller and the high adhesion between nanopowder and epoxy. The hardness magnitudes of the nanoglass/epoxy composite were likewise greater than even those of the nano–fly ash/epoxy and nanosilica/epoxy composites, which is attributable to the glass powder’s hardness and the strengths of the connection between the epoxy and powder.
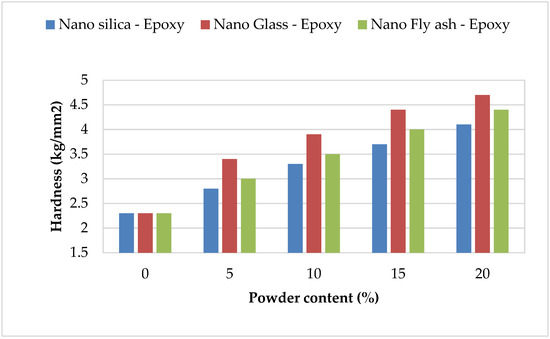
Figure 4.
Hardness magnitudes of the nanopowder-filled epoxy.
4. Conclusions
In the epoxy matrix, nanowaste products with a high silica concentration have been utilized as reinforcement material. The nano–fly ash/epoxy and glass/epoxy studies yielded findings similar to those in the nanosilica/epoxy composites research.
- It could be observed clearly that the nanosized silica, glass, and fly ash have the same impact on the Tg of epoxy. The slight reduction in Tg after the improvement of the epoxy by nanoparticles can significantly change free volumes between the polymer chains. Another explanation is that nanosized particles affect the adhesive’s curing processes and prevent the polymer chains from cross-linking.
- The hardness magnitudes improve when the nanopowder filler rises, which correlates to the uniform distribution of nanosized fillers and the high adhesion between epoxy and nanopowder. The hardness magnitudes of the nanoglass/epoxy composites seemed to be likewise greater than those of the nanosilica/epoxy and fly ash/epoxy composites, which are attributable to the hardness of the nanosized glass powders as well as the strengths of the connection between the epoxy and powder.
- The viscosity continued to increase with the addition of nanosilica, fly ash, and glass. Because of the agglomeration of a colloidal suspension of the nanosized particles in the resins, the nanofilled epoxy resins seem to have a relatively high viscosity as the nano–fly ash and glass levels rise.
Author Contributions
Conceptualization, A.J.S., Z.F.J., R.J.G. and F.A.K.; methodology, A.J.S., Z.F.J., R.J.G. and F.A.K.; software, A.J.S., Z.F.J., R.J.G. and F.A.K.; validation, A.J.S., Z.F.J., R.J.G. and F.A.K.; formal analysis, A.J.S., Z.F.J., R.J.G. and F.A.K.; investigation, A.J.S., Z.F.J., R.J.G. and F.A.K.; resources, A.J.S., Z.F.J., R.J.G., F.A.K. and Z.A.-k.; data curation, A.J.S., Z.F.J., R.J.G., F.A.K. and Z.A.-k.; writing—original draft preparation, A.J.S., Z.F.J., R.J.G., F.A.K. and Z.A.-k.; writing—review and editing, A.J.S., Z.F.J., R.J.G., F.A.K. and Z.A.-k.; visualization, A.J.S. and Z.A.-k.; supervision, A.J.S.; project administration, A.J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank Al Furat Al Awsat Technical University, University of Babylon, and Al-Mustaqbal University College for providing technical support for this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yadav, P.S.; Purohit, R.; Kothari, A. Study of Friction and Wear Behaviour of Epoxy/Nano SiO2 Based Polymer Matrix Composites-a Review. Mater. Today Proc. 2019, 18, 5530–5539. [Google Scholar] [CrossRef]
- Egbo, M.K. A Fundamental Review on Composite Materials and Some of Their Applications in Biomedical Engineering. J. King Saud Univ. Sci. 2021, 33, 557–568. [Google Scholar] [CrossRef]
- Tzetzis, D.; Tsongas, K.; Mansour, G. Determination of the Mechanical Properties of Epoxy Silica Nanocomposites through FEA-Supported Evaluation of Ball Indentation Test Results. Mater. Res. 2017, 20, 1571–1578. [Google Scholar] [CrossRef]
- He, E.; Wang, S.; Li, Y.; Wang, Q. Enhanced Tribological Properties of Polymer Composites by Incorporation of Nano-SiO2 Particles: A Molecular Dynamics Simulation Study. Comput. Mater. Sci. 2017, 134, 93–99. [Google Scholar] [CrossRef]
- Uddin, M.F.; Sun, C.T. Strength of Unidirectional Glass/Epoxy Composite with Silica Nanoparticle-Enhanced Matrix. Compos. Sci. Technol. 2008, 68, 1637–1643. [Google Scholar] [CrossRef]
- Dorigato, A.; Pegoretti, A.; Penati, A. Linear Low-Density Polyethylene/Silica Micro-and Nanocomposites: Dynamic Rheological Measurements and Modelling. Express Polym. Lett. 2010, 4, 115–129. [Google Scholar] [CrossRef]
- Tomić, M.; Dunjić, B.; Nikolić, M.S.; Stanković, N.; Pavlović, V.B.; Bajat, J.; Djonlagić, J. Polyamidoamine as a Clay Modifier and Curing Agent in Preparation of Epoxy Nanocomposites. Prog. Org. Coat. 2019, 131, 311–321. [Google Scholar] [CrossRef]
- Mostovoy, A.S.; Yakovlev, A.V. Reinforcement of Epoxy Composites with Graphite-Graphene Structures. Sci. Rep. 2019, 9, 16246. [Google Scholar] [CrossRef] [PubMed]
- Cañavate, J.; Colom, X.; Pages, P.; Carrasco, F. Study of the Curing Process of an Epoxy Resin by FTIR Spectroscopy. Polym. Plast. Technol. Eng. 2000, 39, 937–943. [Google Scholar] [CrossRef]
- Njuguna, J.; Pielichowski, K.; Alcock, J.R. Epoxy-based Fibre Reinforced Nanocomposites. Adv. Eng. Mater. 2007, 9, 835–847. [Google Scholar] [CrossRef]
- Deng, S.; Ye, L.; Friedrich, K. Fracture Behaviours of Epoxy Nanocomposites with Nano-Silica at Low and Elevated Temperatures. J. Mater. Sci. 2007, 42, 2766–2774. [Google Scholar] [CrossRef]
- Jin, F.-L.; Li, X.; Park, S.-J. Synthesis and Application of Epoxy Resins: A Review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Pradhan, S.; Pandey, P.; Mohanty, S.; Nayak, S.K. Insight on the Chemistry of Epoxy and Its Curing for Coating Applications: A Detailed Investigation and Future Perspectives. Polym. Plast. Technol. Eng. 2016, 55, 862–877. [Google Scholar] [CrossRef]
- Liang, Y.L.; Pearson, R.A. Toughening Mechanisms in Epoxy–Silica Nanocomposites (ESNs). Polymer 2009, 50, 4895–4905. [Google Scholar] [CrossRef]
- Kang, Y.; Chen, X.; Song, S.; Yu, L.; Zhang, P. Friction and Wear Behavior of Nanosilica-Filled Epoxy Resin Composite Coatings. Appl. Surf. Sci. 2012, 258, 6384–6390. [Google Scholar] [CrossRef]
- Tsai, J.-L.; Hsiao, H.; Cheng, Y.-L. Investigating Mechanical Behaviors of Silica Nanoparticle Reinforced Composites. J. Compos. Mater. 2010, 44, 505–524. [Google Scholar] [CrossRef]
- Ilangovan, S.; Kumaran, S.S.; Vasudevan, A.; Naresh, K. Effect of Silica Nanoparticles on Mechanical and Thermal Properties of Neat Epoxy and Filament Wounded E-Glass/Epoxy and Basalt/Epoxy Composite Tubes. Mater. Res. Express 2019, 6, 0850e2. [Google Scholar] [CrossRef]
- Johnsen, B.B.; Kinloch, A.J.; Mohammed, R.D.; Taylor, A.C.; Sprenger, S. Toughening Mechanisms of Nanoparticle-Modified Epoxy Polymers. Polymer 2007, 48, 530–541. [Google Scholar] [CrossRef]
- Hsieh, T.H.; Kinloch, A.J.; Masania, K.; Sohn Lee, J.; Taylor, A.C.; Sprenger, S. The Toughness of Epoxy Polymers and Fibre Composites Modified with Rubber Microparticles and Silica Nanoparticles. J. Mater. Sci. 2010, 45, 1193–1210. [Google Scholar] [CrossRef]
- Conradi, M.; Zorko, M.; Kocijan, A.; Verpoest, I. Mechanical Properties of Epoxy Composites Reinforced with a Low Volume Fraction of Nanosilica Fillers. Mater. Chem. Phys. 2013, 137, 910–915. [Google Scholar] [CrossRef]
- Christy, A.; Purohit, R.; Rana, R.S.; Singh, S.K.; Rana, S. Development and Analysis of Epoxy/Nano SiO2 Polymer Matrix Composite Fabricated by Ultrasonic Vibration Assisted Processing. Mater. Today Proc. 2017, 4, 2748–2754. [Google Scholar] [CrossRef]
- Dittanet, P.; Pearson, R.A. Effect of Silica Nanoparticle Size on Toughening Mechanisms of Filled Epoxy. Polymer 2012, 53, 1890–1905. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Z.; Friedrich, K.; Eger, C. Property Improvements of in Situ Epoxy Nanocomposites with Reduced Interparticle Distance at High Nanosilica Content. Acta Mater. 2006, 54, 1833–1842. [Google Scholar] [CrossRef]
- Permatasari, N.; Sucahya, T.N.; Nandiyanto, A.B.D. Agricultural Wastes as a Source of Silica Material. Indones J. Sci. Technol. 2016, 1, 82–106. [Google Scholar] [CrossRef]
- Guo, Q.; Zhu, P.; Li, G.; Wen, J.; Wang, T.; Lu, D.D.; Sun, R.; Wong, C. Study on the Effects of Interfacial Interaction on the Rheological and Thermal Performance of Silica Nanoparticles Reinforced Epoxy Nanocomposites. Compos. Part B Eng. 2017, 116, 388–397. [Google Scholar] [CrossRef]
- Jawad, Z.F.; Ghayyib, R.J.; Salman, A.J. Microstructural and Compressive Strength Analysis for Cement Mortar with Industrial Waste Materials. Civ. Eng. J. 2020, 6, 1007–1016. [Google Scholar] [CrossRef]
- Ghayyib, R.J.; Salman, A.J.; Jawad, Z.F.; Al-Khafaji, Z.S. Effect of Silica-Based Wastes on Wear Rate and Hardness Properties of Epoxy Composites as a Construction Material. In Key Engineering Materials; Trans Tech Publications: Zurich, Switzerland, 2021; Volume 895, pp. 31–40. [Google Scholar]
- Ali, F.; Ali, N.; Altaf, M.; Said, A.; Shah, S.S.; Bilal, M. Epoxy Polyamide Composites Reinforced with Silica Nanorods: Fabrication, Thermal and Morphological Investigations. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3869–3877. [Google Scholar] [CrossRef]
- Alam, M.A.; Samad, U.A.; Anis, A.; Alam, M.; Ubaidullah, M.; Al-Zahrani, S.M. Effects of SiO2 and ZnO Nanoparticles on Epoxy Coatings and Its Performance Investigation Using Thermal and Nanoindentation Technique. Polymers 2021, 13, 1490. [Google Scholar] [CrossRef]
- Joni, I.M.; Nulhakim, L.; Vanitha, M.; Panatarani, C. Characteristics of Crystalline Silica (SiO2) Particles Prepared by Simple Solution Method Using Sodium Silicate (Na2SiO3) Precursor. J. Phys. Conf. Ser. 2018, 1080, 12006. [Google Scholar] [CrossRef]
- Soontaranon, S.; Limphirat, W.; Pratapa, S. XRD, WAXS, FTIR, and XANES Studies of Silica-Zirconia Systems. Ceram. Int. 2019, 45, 15660–15670. [Google Scholar]
- Kinloch, A.J.; Masania, K.; Taylor, A.C.; Sprenger, S.; Egan, D. The Fracture of Glass-Fibre-Reinforced Epoxy Composites Using Nanoparticle-Modified Matrices. J. Mater. Sci. 2008, 43, 1151–1154. [Google Scholar] [CrossRef]
- Choi, Y.-M.; Hwangbo, S.; Lee, T.G.; Ham, Y.-B. Effect of Particle Size on the Mechanical Properties of TiO2–Epoxy Nanocomposites. Materials 2021, 14, 2866. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.A.; Nguyen, T.V.; Thai, H.; Shi, X. Effect of Nanoparticles on the Thermal and Mechanical Properties of Epoxy Coatings. J. Nanosci. Nanotechnol. 2016, 16, 9874–9881. [Google Scholar] [CrossRef]
- Mach, P.; Geczy, A.; Polanský, R.; Bušek, D. Glass Transition Temperature of Nanoparticle-Enhanced and Environmentally Stressed Conductive Adhesive Materials for Electronics Assembly. J. Mater. Sci. Mater. Electron. 2019, 30, 4895–4907. [Google Scholar] [CrossRef]
- Luo, H.; Ding, J.; Huang, Z.; Yang, T. Investigation of Properties of Nano-Silica Modified Epoxy Resin Films and Composites Using RFI Technology. Compos. Part B Eng. 2018, 155, 288–298. [Google Scholar] [CrossRef]
- Liu, S.; Fan, X.; He, C. Improving the Fracture Toughness of Epoxy with Nanosilica-Rubber Core-Shell Nanoparticles. Compos. Sci. Technol. 2016, 125, 132–140. [Google Scholar] [CrossRef]
- Afzal, A.; Siddiqi, H.M.; Saeed, S.; Ahmad, Z. Exploring Resin Viscosity Effects in Solventless Processing of Nano-SiO2/Epoxy Polymer Hybrids. RSC Adv. 2013, 3, 3885–3892. [Google Scholar] [CrossRef]
- Majdi, H.S.; Shubbar, A.A.; Nasr, M.S.; Al-Khafaji, Z.S.; Jafer, H.; Abdulredha, M.; Al Masoodi, Z.; Sadique, M.; Hashim, K. Experimental Data on Compressive Strength and Ultrasonic Pulse Velocity Properties of Sustainable Mortar Made with High Content of GGBFS and CKD Combinations. Data Br. 2020, 31, 105961. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).