Study on Detonation Characteristics of Ammonium Nitrate-Polyhydroxy Alcohol Mixtures
Abstract
:1. Introduction
2. Experimental Section
2.1. The Characteristics of Applied Raw Materials and the Methodology of Sample Preparation
2.2. Research Methodology
2.2.1. Detonation Velocity Measurement
2.2.2. Measurement of Air Blast Wave’s Overpressure
2.3. Research Results
2.3.1. Results of Detonation Velocity Measurements
2.3.2. The Results of Measurements of the Air Blast Wave Overpressure
3. Analysis of Test Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marshall, A. Dictionary of Explosives; P. Blakiston’s Sons & Co.: Philadelphia, PA, USA, 1920; pp. 5–6. [Google Scholar]
- Maranda, A. Przemysłowe Materiały Wybuchowe; Publisher Military University of Technology: Warsaw, Poland, 2010; pp. 129–131. [Google Scholar]
- Maranda, A. Study of Effect of Organic Fuel on Detonation Characteristics of Ammonium Nitrate Compositions. Combust. Explos. Shock Waves 1991, 27, 88–93. [Google Scholar] [CrossRef]
- Maranda, A. Study of Detonation of Aluminium Sensitized Slurry Explosives Containing Organic Fuels. Propellants Explos. Pyrotech. 1991, 16, 266–272. [Google Scholar] [CrossRef]
- Tan, L.; Xia, L.H.; Wu, Q.J.; Xu, S.; Liu, D.B. Effect of urea on the detonation performance and thermal stability of ammonium nitrate. J. Loss Prev. Proc. Ind. 2015, 38, 168–175. [Google Scholar] [CrossRef]
- Maranda, A.; Szymański, R. Badanie średnicy krytycznej i prędkości detonacji mieszanin azotanu(V) amonu z wybranymi substancjami organicznymi. CHEMIK 2013, 67, 13–18. [Google Scholar] [CrossRef]
- Miyake, A.; Kobayashi, H.; Echigoya, H.; Kubota, S.; Wada, Y.; Ogata, Y.; Arai, H.; Ogawa, T. Detonation characteristics of ammonium nitrate and activated carbon mixtures. J. Loss Prev. Proc. Ind. 2007, 20, 354–358. [Google Scholar] [CrossRef]
- Resende, S.A.; Silva, V.C.; De Lima, H.M. Study of non-conventional fuels for explosives mixes. Rev. Esc. Minas 2014, 67, 297–302. [Google Scholar] [CrossRef]
- Stanković, S.; Škrlec, V.; Dobrilović, M.; Bohanek, V. Velocity of detonation of AN blasting agent with addition of hay and recycled rubber. In Proceedings of the 21st Seminar New Trends in Research of Energetic Materials, Pardubice, Czech Republic, 18–20 April 2018; Volume II, pp. 1042–1050. [Google Scholar]
- Ali, A.; Ali, M.F.; Javed, T.; Abidi, S.H.; Syed, Q.; Zulfiqar, U.; Alotaibi, S.S.; Siuta, D.; Adamski, R.; Wolny, P. Mitigating Ammonia and Greenhouse Gaseous Emission from Arable Land by Co-application of Zeolite and Biochar. Front. Plant Sci. 2022, 13, 950944. [Google Scholar] [CrossRef] [PubMed]
- Maranda, A. Research on the Process of Detonation of Explosive Mixtures of the Fuel Type Containing Aluminium Powder. Propellants Explos. Pyrotech. 1990, 15, 161–165. [Google Scholar] [CrossRef]
- Zygmunt, B. Detonation Parameters of Mixtures Containing Ammonium Nitrate and Aluminum. Cent. Eur. J. Energ. Mat. 2009, 6, 57–66. [Google Scholar]
- Paszula, J.; Trzciński, W.; Sprzątczak, K. Detonation Performance of Aluminium—Ammonium Nitrate Explosives. Cent. Eur. J. Energ. Mater. 2008, 5, 3–11. [Google Scholar]
- Sitkiewicz-Wołodko, R.; Maranda, A.; Paszula, J.M. Detonation Parameters by Addition of Ground of Ammonium Nitrate(V) and Aluminium Powder. Cent. Eur. J. Energ. Mater. 2019, 16, 122–134. [Google Scholar] [CrossRef]
- Sanchidrián, J.A.; Castedo, R.; López, L.M.; Segarra, P.; Santos, A.P. Determination of the JWL Constans for ANFO and emulsion explosives from cylinder test data. Cent. Eur. J. Energ. Mater. 2015, 12, 177–194. [Google Scholar]
- Žganec, S.; Bohanek, V.; Dobrilović, M. Influence of primer on the velocity detonation of ANFO and heavy ANFO blends. Cent. Eur. J. Energ. Mat. 2016, 13, 695–705. [Google Scholar] [CrossRef]
- Biessikirski, A.; Wądrzyk, M.; Janus, R.; Biegańska, J.; Jodłowski, G.; Kuterasiński, Ł. Badania ciekłych składników palnych w materiałach wybuchowych opartych na azotanie amonu. Przemysł Chem. 2018, 97, 457–462. [Google Scholar]
- Biessikirski, A.; Kuterasiński, Ł. Właściwości morfologiczne materiałów wybuchowych ANFO otrzymanych z użyciem ciekłych substancji palnych. Przemysł Chem. 2018, 97, 587–590. [Google Scholar] [CrossRef]
- Biessikirski, A. Analiza właściwości morfologicznych materiałów wybuchowych ANFO wytworzonych na bazie różnych typów azotanu(V) oraz ich mieszanin. Przemysł Chem. 2018, 97, 1689–1692. [Google Scholar] [CrossRef]
- Biessikirski, A.; Kuterasiński, Ł. Badanie właściwości strukturalnych i morfologicznych materiałów wybuchowych otrzymanych przez dodatek alkoholi do saletry amonowej. Przemysł Chem. 2018, 97, 1718–1721. [Google Scholar]
- Araos, M.; Onederra, I. Preliminary detonation study of dry, wet and aluminized ANFO using high-speed video. Cent. Eur. J. Energ. Mater. 2019, 16, 228–244. [Google Scholar] [CrossRef]
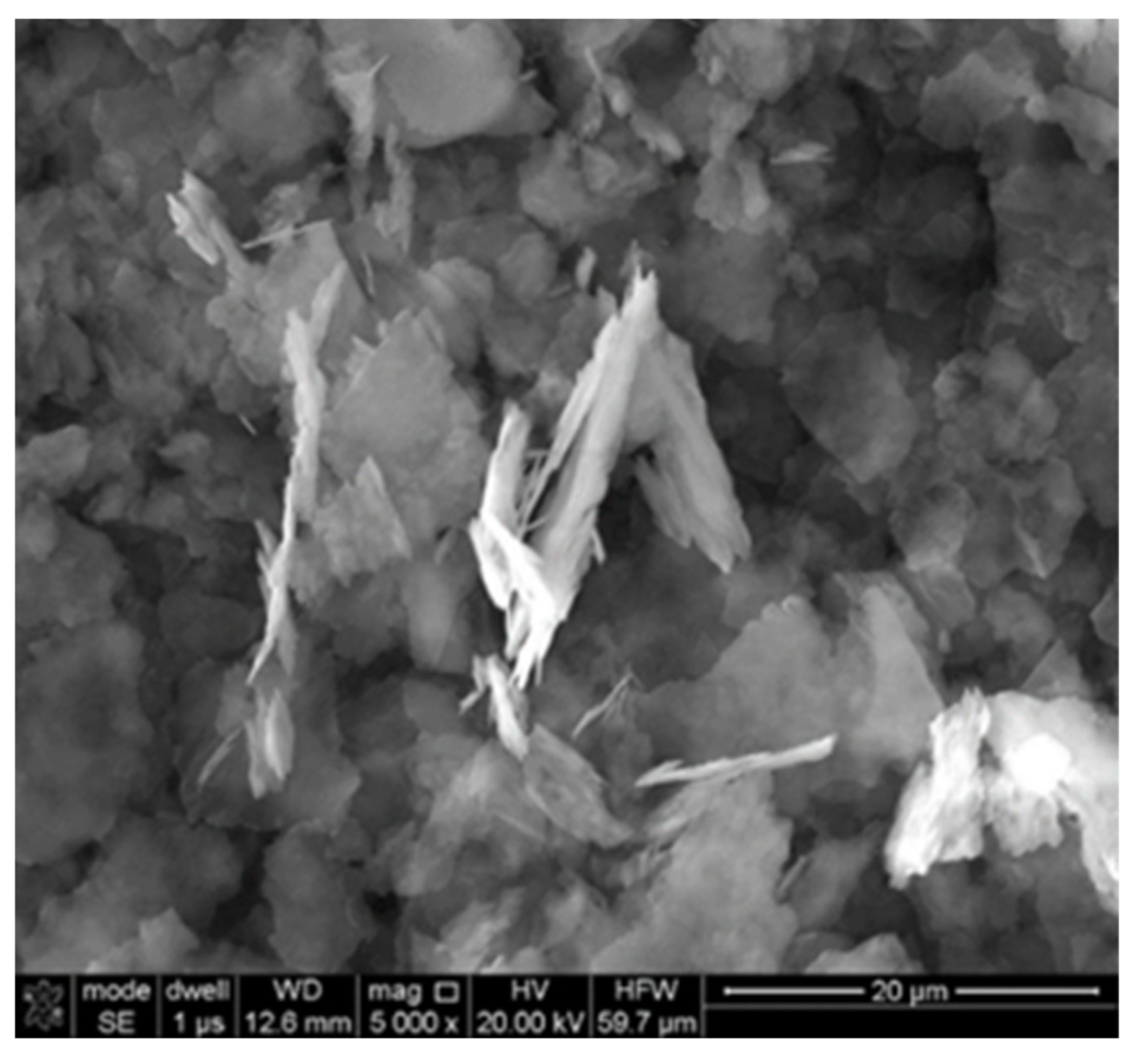

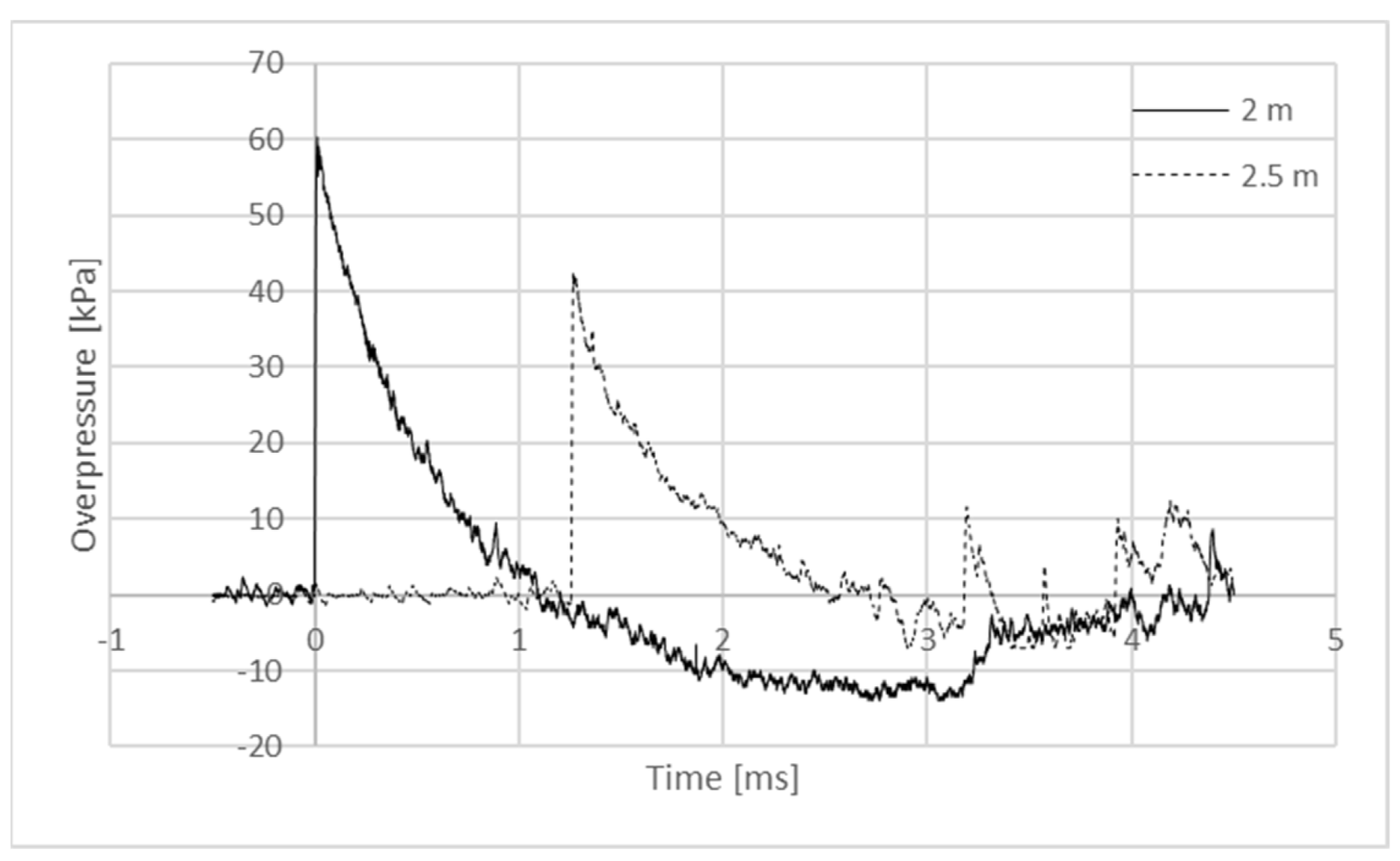

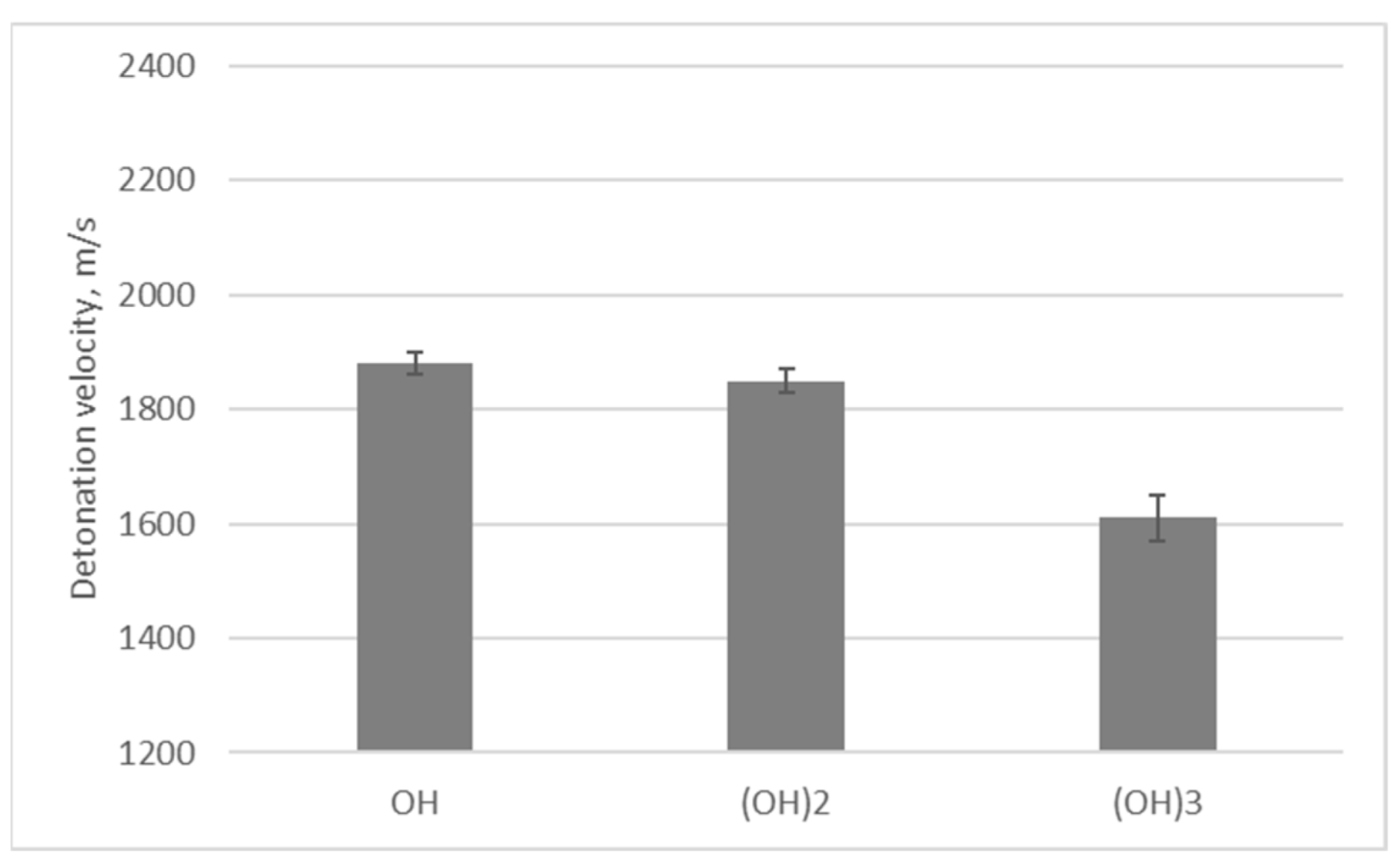
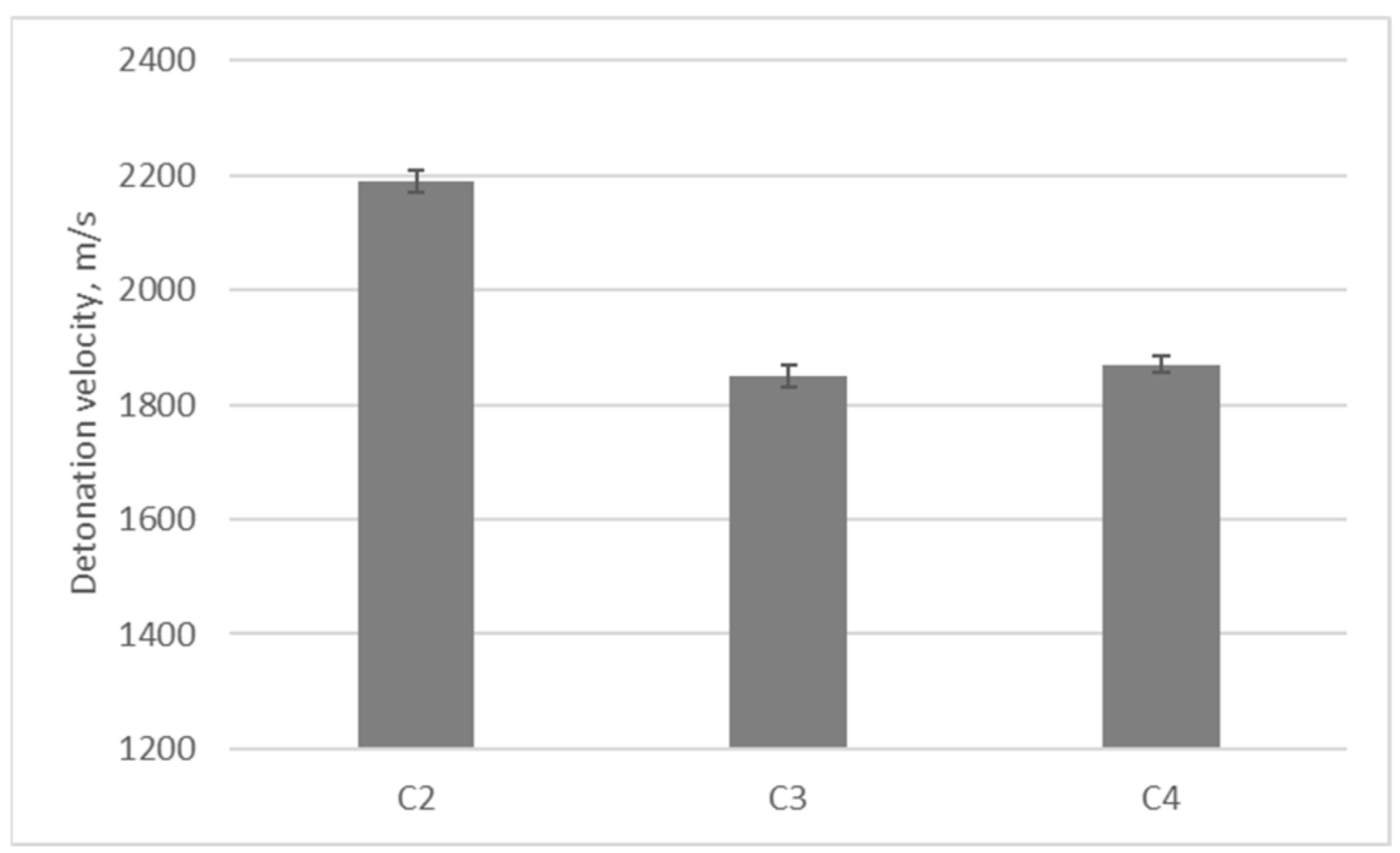

| Oxygen Fuel | Oxygen Balance [%] | Dynamic Viscosity [mPa·s] in 25 °C * | Flashpoint [°C] * |
|---|---|---|---|
| Ethanol | −208.70 | 1.074 | 17 |
| Ethan-1,2-diol | −129.03 | 16.01 | 111 |
| Propan-2-ol | −240.00 | 2.038 | 12 |
| Propan-1,3-diol | −168.42 | 43.4 | 345 |
| Propan-1,2,3-triol | −121.74 | 954 | 177 |
| Butan-1,4-diol | −195.56 | 84.9 (20 °C) | 121 |
| Fuel oil | −348.24 | 150 | >55 (closed-cup) |
| Organic Fuel | Content [%] |
|---|---|
| Ethanol | 8.75 |
| Ethan-1,2-diol | 13.42 |
| Propan-2-ol | 7.69 |
| Propan-1,3-diol | 10.61 |
| Propan-1,2,3-triol | 14.11 |
| Butan-1,4-diol | 9.28 |
| Fuel oil | 5.80 |
| Explosive | Organic Fuel | Oxygen Balance [%] |
|---|---|---|
| ANFO | Fuel oil | −2.09 |
| ANPT | Propan-1,2,3-triol | 11.49 |
| ANPD | Propan-1,3-diol | 8.69 |
| ANPO | Propan-2-ol | 4.40 |
| ANE | Ethanol | 6.28 |
| ANBD | Butan-1,4-diol | 7.07 |
| ANED | Ethan-1,2-diol | 11.06 |
| Explosive Composition | Organic Fuel | Aluminium Content [%] | Oxygen Balance [%] |
|---|---|---|---|
| ANFO10 | Fuel oil | 10 | −10.77 |
| ANED5 | Ethan-1,2-diol | 5 | 6.06 |
| ANED10 | Ethan-1,2-diol | 10 | 1.06 |
| ANED15 | Ethan-1,2-diol | 15 | −3.93 |
| ED Alcohol Content [% by Mass] | Density [g/cm3] | Detonation Velocity [m/s] |
|---|---|---|
| 6 | 0.72 | 2190 ± 20 |
| 9 | 0.77 | 1560 ± 10 |
| 13 | 0.81 | No detonation |
| Explosive Composition | Organic Fuel | Density [g/cm3] | Detonation Velocity [m/s] |
|---|---|---|---|
| ANFO | Fuel oil | 0.70 | 1600 ± 40 |
| ANPT | Propan-1,2,3-triol | 0.73 | 1610 ± 40 |
| ANPD | Propan-1,3-diol | 0.70 | 1850 ± 30 |
| ANPO | Propan-2-ol | 0.68 | 1880 ± 20 |
| ANE | Ethanol | 0.68 | 2040 ± 10 |
| ANBD | Butan-1,4-diol | 0.71 | 1870 ± 10 |
| ANED | Ethan-1,2-diol | 0.72 | 2190 ± 20 |
| Explosive Composition | Aluminium Content [%] | Density [g/cm3] | Detonation Velocity [m/s] |
|---|---|---|---|
| ANFO10 | 10 | 0.69 | 2320 ± 20 |
| ANED5 | 5 | 0.69 | 2250 ± 20 |
| ANED10 | 10 | 0.70 | 2420 ± 20 |
| ANED15 | 15 | 0.71 | 2260 ± 20 |
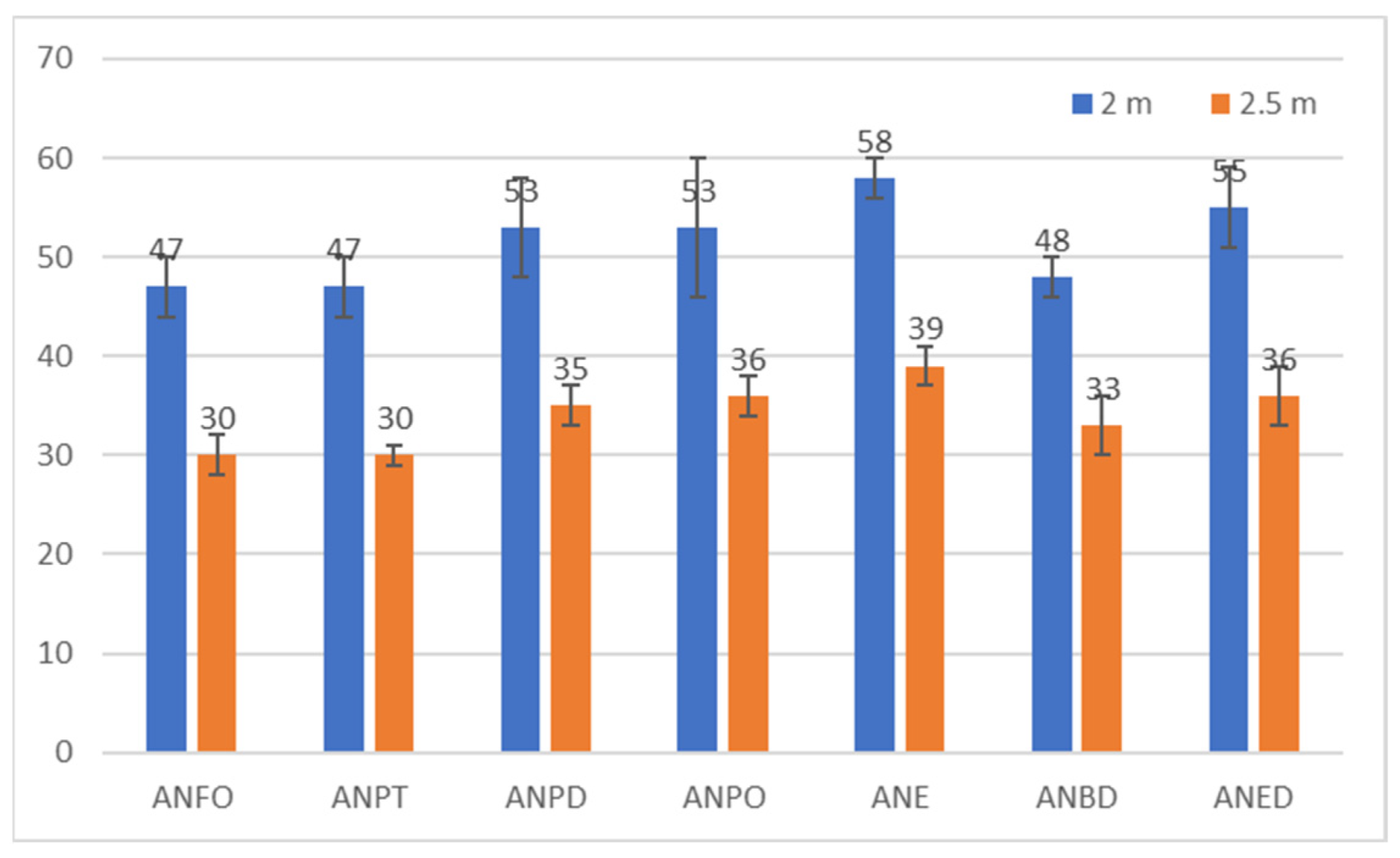
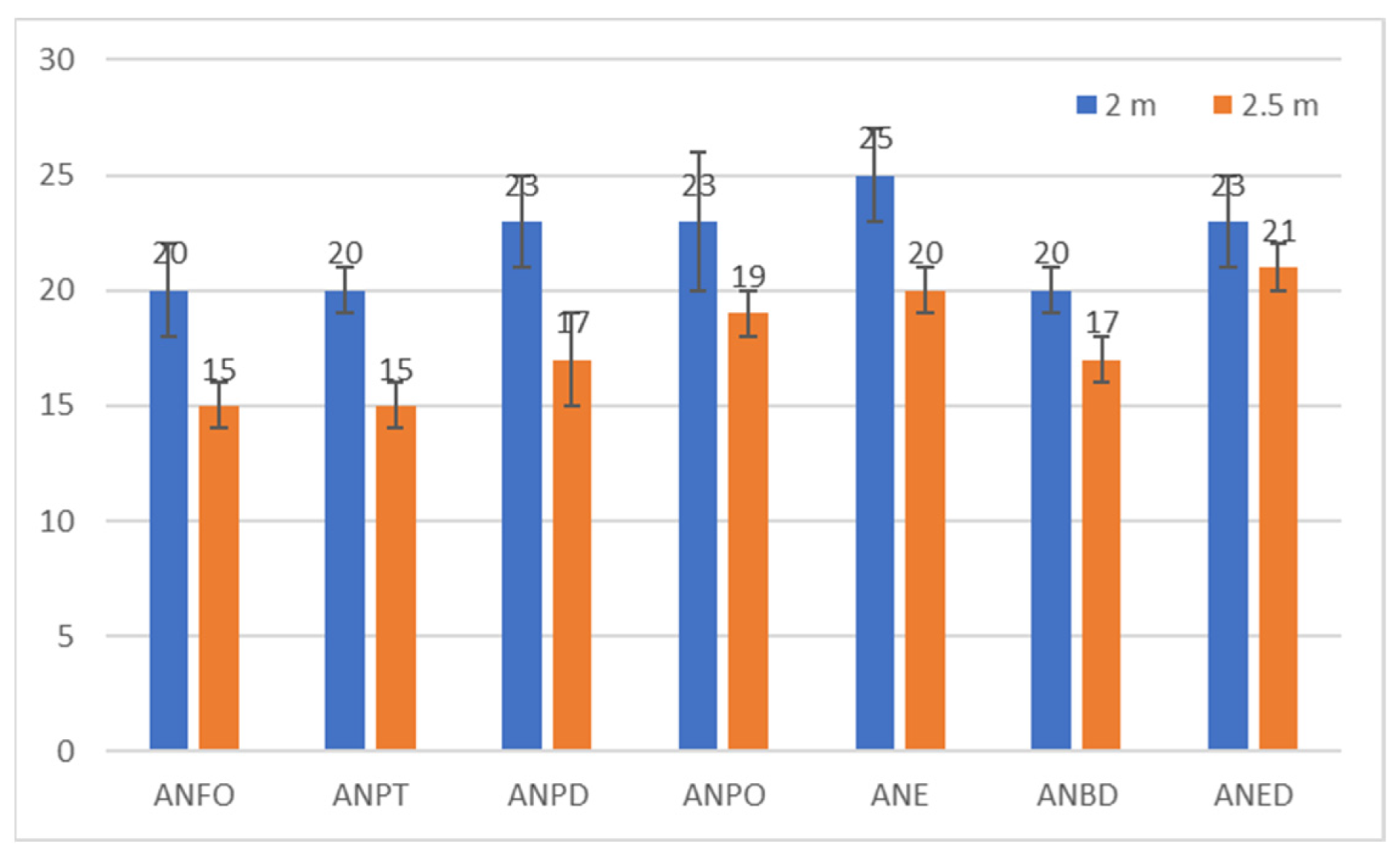
| Explosive Composition | Overpressure at 2 m [kPa] | Specific Impulse at 2 m [Pa·s] | Overpressure at 2.5 m [kPa] | Specific Impulse at 2.5 m [Pa·s] |
|---|---|---|---|---|
| ANFO | 47 ± 3 | 20 ± 2 | 30 ± 2 | 15 ± 1 |
| ANPT | 47 ± 3 | 20 ± 1 | 30 ± 1 | 15 ± 1 |
| ANPD | 53 ± 5 | 23 ± 2 | 35 ± 2 | 17 ± 2 |
| ANPO | 53 ± 7 | 23 ± 3 | 36 ± 2 | 19 ± 1 |
| ANE | 58 ± 2 | 25 ± 2 | 39 ± 2 | 20 ± 1 |
| ANBD | 48 ± 2 | 20 ± 1 | 33 ± 3 | 17 ± 1 |
| ANED | 55 ± 4 | 23 ± 2 | 36 ± 3 | 21 ± 1 |
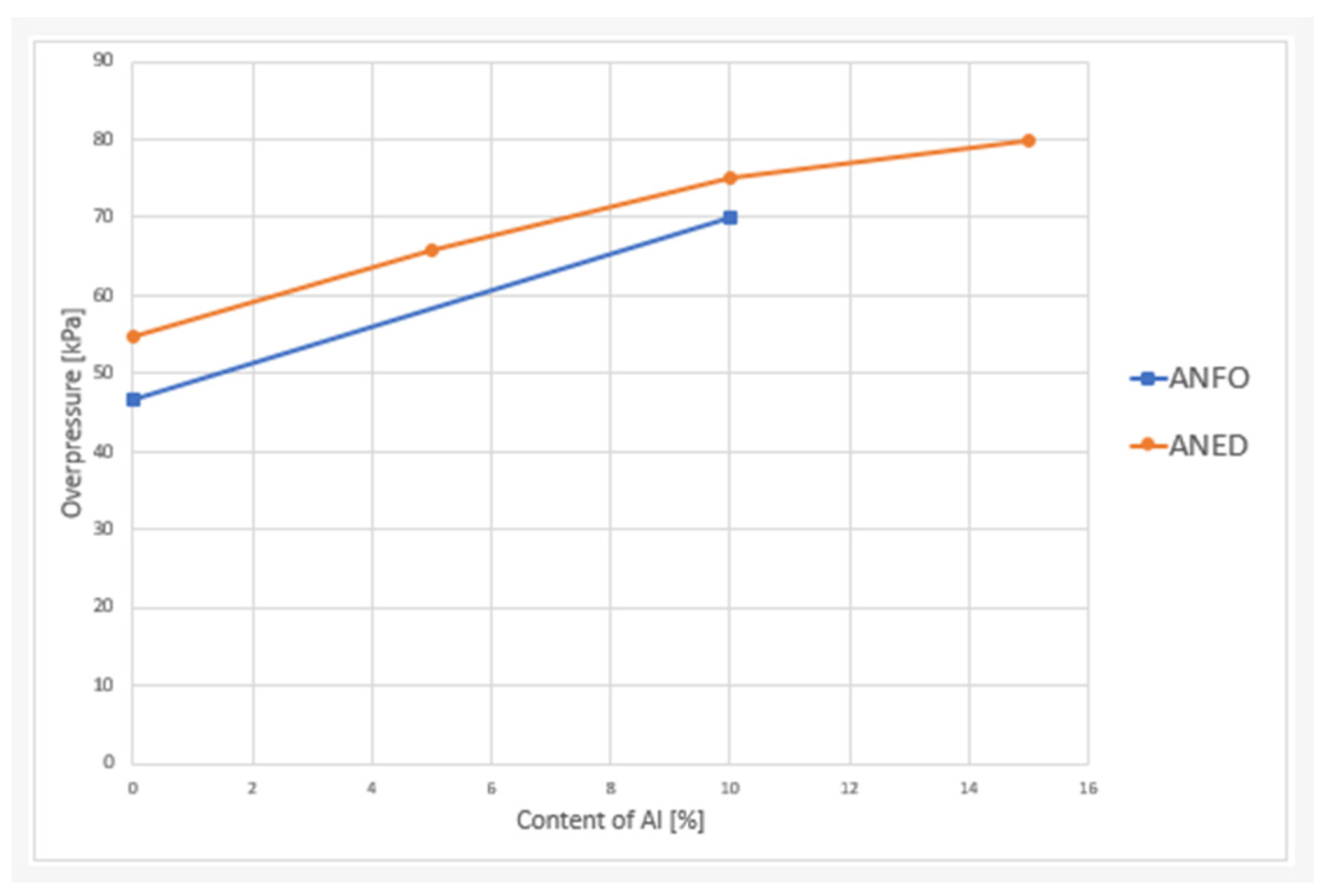
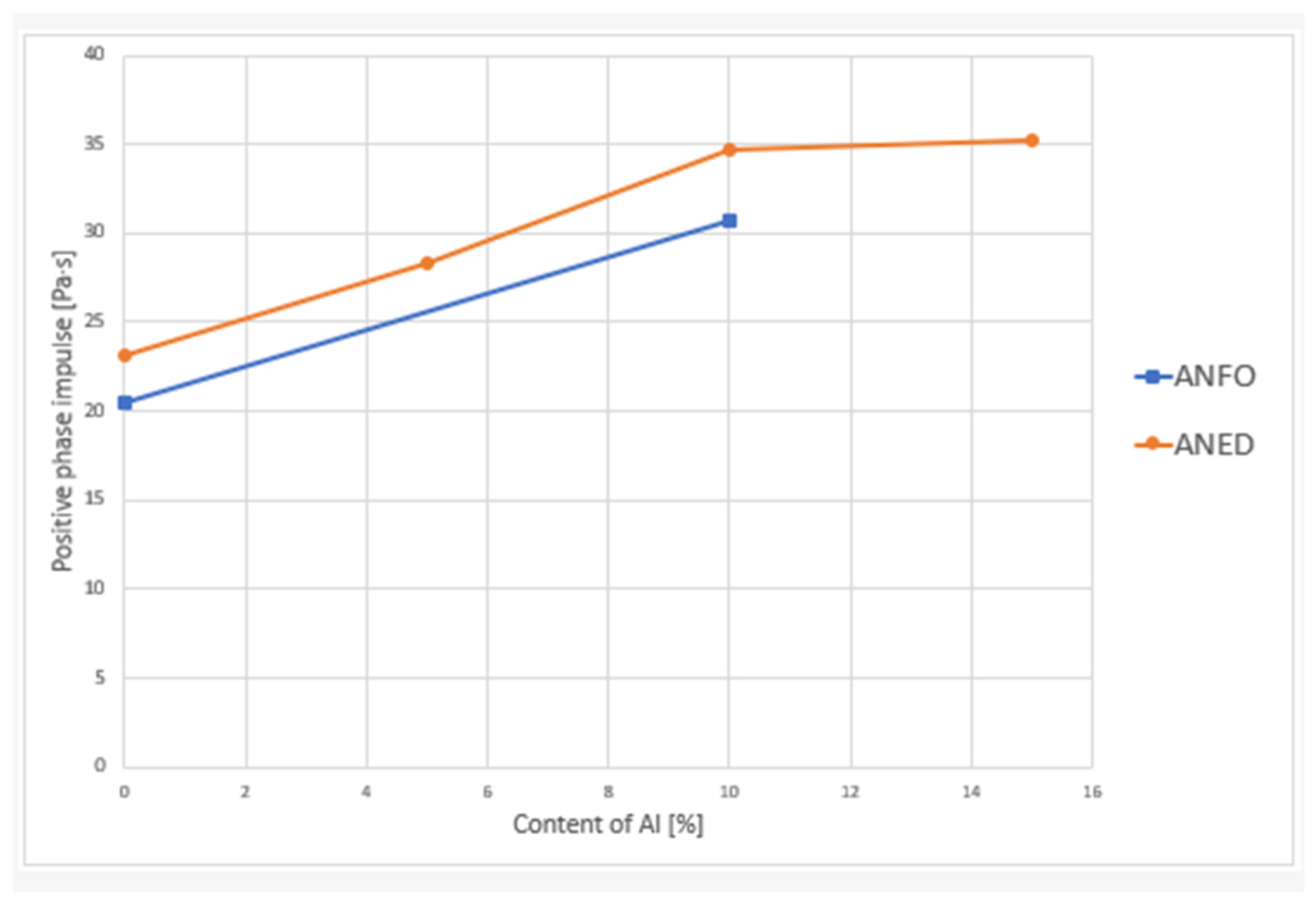
| Explosive Composition | Alf Content [%] | Overpressure at 2 m [kPa] | Specific Impulse at 2 m [Pa·s] | Overpressure at 2.5 m [kPa] | Specific Impulse at 2.5 m [Pa·s] |
|---|---|---|---|---|---|
| ANFO10 | 10 | 70 ± 5 | 31 ± 3 | 48 ± 1 | 25 ± 1 |
| ANED5 | 5 | 66 ± 4 | 28 ± 1 | 39 ± 3 | 20 ± 2 |
| ANED10 | 10 | 75 ± 4 | 35 ± 3 | 45 ± 4 | 25 ± 3 |
| ANED15 | 15 | 80 ± 1 | 35 ± 3 | 55 ± 3 | 32 ± 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paszula, J.; Kępisty, A.; Maranda, A.; Kukfisz, B. Study on Detonation Characteristics of Ammonium Nitrate-Polyhydroxy Alcohol Mixtures. Energies 2022, 15, 6843. https://doi.org/10.3390/en15186843
Paszula J, Kępisty A, Maranda A, Kukfisz B. Study on Detonation Characteristics of Ammonium Nitrate-Polyhydroxy Alcohol Mixtures. Energies. 2022; 15(18):6843. https://doi.org/10.3390/en15186843
Chicago/Turabian StylePaszula, Józef, Alan Kępisty, Andrzej Maranda, and Bożena Kukfisz. 2022. "Study on Detonation Characteristics of Ammonium Nitrate-Polyhydroxy Alcohol Mixtures" Energies 15, no. 18: 6843. https://doi.org/10.3390/en15186843






