Biodiesel Emissions: A State-of-the-Art Review on Health and Environmental Impacts
Abstract
:1. Introduction
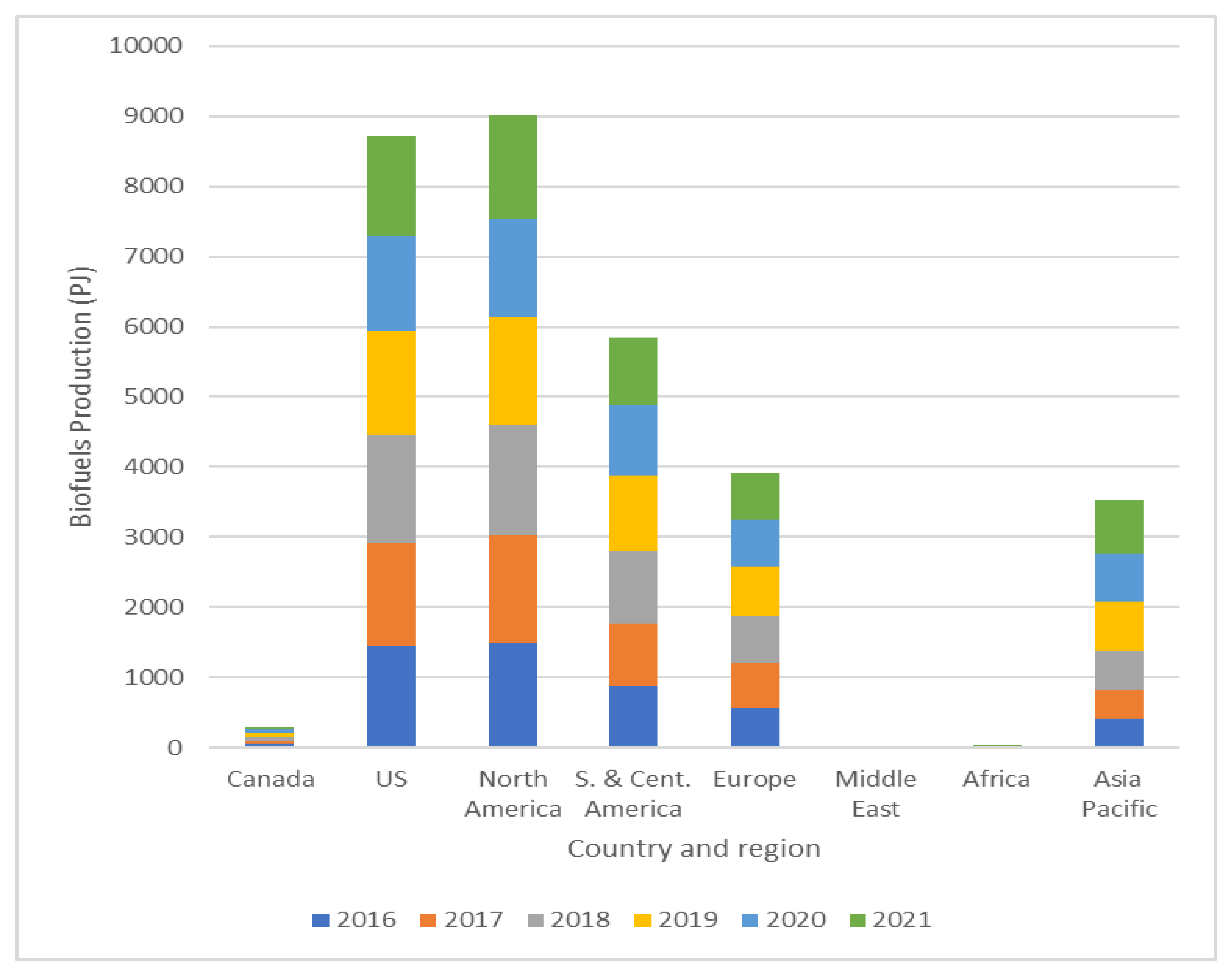
2. Biodiesel as a Renewable Energy Source
2.1. Biodiesel Production
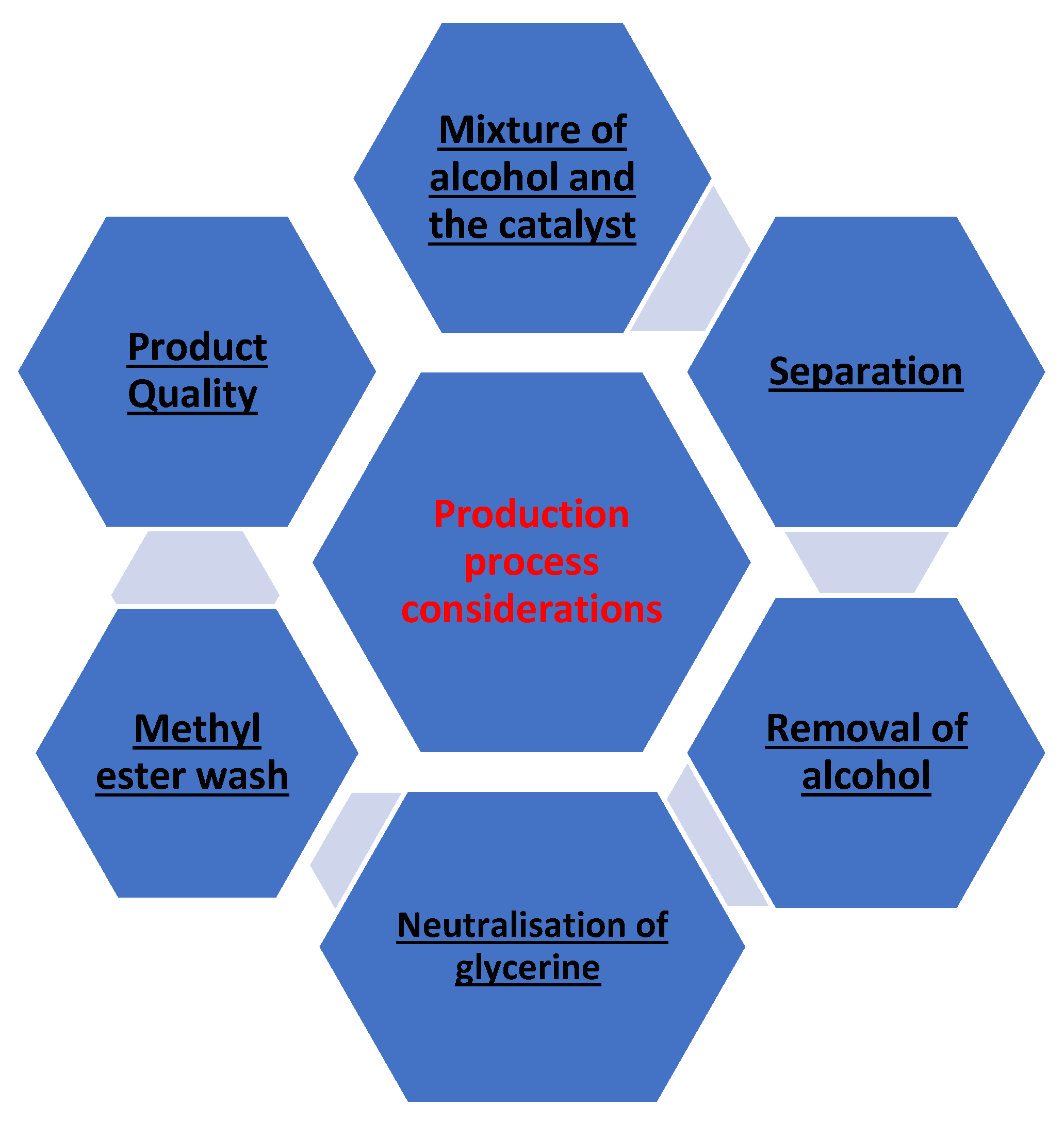
2.2. Production Process Considerations
3. Emission from Biodiesel Production and Combustion
3.1. Biodiesel Gaseous Emissions
3.1.1. Carbon Dioxide
3.1.2. Ozone (O3)
3.1.3. Nitrogen Oxides (NOx)
3.1.4. Carbon Monoxide (CO)
3.1.5. Sulphur Dioxide (SO2)
3.1.6. Hydrocarbon (HC)
3.2. Biodiesel Solid-Phase Emissions
3.2.1. PM Emission
3.2.2. Elemental and Organic Carbon
3.2.3. Trace Metals
4. Impact of the Biodiesel Combustion Process
4.1. Gaseous Emission Effect
4.2. Solid Emission Effect
4.3. Cultivation and Production Impact on Human Health
4.4. Biodiesel Combustion Impact on Human
5. Future of the Biodiesel Landscape
6. Conclusions and Future Research Direction
- An essential resource of alternative fuel would be biodiesel fuel sources, which seem to be both affordable and effective for fuel. However, before people can make the most of these resources, the relevant parties must conduct further research and gain more insight into this topic.
- The challenges in producing oil and the possible side effects on the environment or public health in the long term need to be considered.
- Biodiesel fuel will significantly reduce carbon emissions but increase the ozone in atmospheric air.
- Biodiesel fuel usually emits higher NOx than other regular diesel.
- While biodiesel seems like a suitable investment as a future fuel source, more research is required to ensure that it is a feasible alternative fuel source.
- Biodiesel is an important fuel source and using it the right way will have many benefits for the environment.
- Use of the fuel in the right way will require immense research and analysis to ensure its compatibility with modern-day sources of fuel.
- Research will make it easy to acquire information on the different and essential fuel sources in our lives today, including biodiesel.
- Future research on these fuel sources might include resources such as artificial intelligence, big data, and technical expertise for the best results.
- It influences the protection and conservation of the climate by controlling the emission of harmful chemicals from conventional fuels.
- It enhances the production of renewable fuel for sustainable development and maintaining atmospheric emissions.
- Advanced production of mass quantities of biodiesel as a bio-fuel helps to initiate a shift in the trend towards the utilization of innovative energy production, smart energy generation, and smart energy utilization.
- Respiratory syndromes or issues are highlighted to highlight the harms on human life due to excessive usage of fuels that can be controlled by different means such as reducing the transportation pressure in urban areas, facilitating urbanization, controlling fuel emissions, etc.
- It will add to the apex of low-carbon transport using biodiesel, which encourages further research on alternative sources for biodiesel production.
- The futuristic significance of the present work includes the human health aspects of using biodiesel, which is also causing a few issues that can be minimized using advanced research techniques and technologies.
- It covers the gap in the past literature to elaborate on the significance of vegetable and organic components based on biodiesel that can encourage research on gaining a higher biomass yield per acre to make it more profitable.
- Future research should focus on the full characterization of biodiesel combustion products and the specific mechanisms of interactions of these emissions in connection with the observed inflammatory responses and poor clearance to further understand the negative effects of biodiesel usage on human health.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ogunkunle, O.; Ahmed, N.A. A review of global current scenario of biodiesel adoption and combustion in vehicular diesel engines. Energy Rep. 2019, 5, 1560–1579. [Google Scholar] [CrossRef]
- El Banna, S.; El Deen, O.N. Biodiesel An Alternative Vehicles Fuel; Analytical View. Proceedings of 7th International Conference of Chemical Engineering, Cairo, Egypt, 27–29 December 2004; pp. 495–522. [Google Scholar]
- Sharma, V.; Duraisamy, G. Production and characterization of bio-mix fuel produced from a ternary and quaternary mixture of raw oil feedstock. J. Clean. Prod. 2019, 221, 271–285. [Google Scholar] [CrossRef]
- Baena, L.M.; Calderón, J.A. Effects of palm biodiesel and blends of biodiesel with organic acids on metals. Heliyon 2020, 6, e03735. [Google Scholar] [CrossRef] [PubMed]
- Vasistha, S.; Khanra, A.; Clifford, M.; Rai, M.P. Current advances in microalgae harvesting and lipid extraction processes for improved biodiesel production: A review. Renew. Sustain. Energy Rev. 2021, 137, 110498. [Google Scholar] [CrossRef]
- Krzysztof, B. Biofuels-Status and Perspective; IntechOpen: London, UK, 2015. [Google Scholar]
- Eduardo, J.-L.; Leila Queiroz, Z. Frontiers in Bioenergy and Biofuels; IntechOpen: London, UK, 2017. [Google Scholar]
- Kumar, M.; Sun, Y.; Rathour, R.; Pandey, A.; Thakur, I.S.; Tsang, D.C.W. Algae as potential feedstock for the production of biofuels and value-added products: Opportunities and challenges. Sci. Total Environ. 2020, 716, 137116. [Google Scholar] [CrossRef]
- Sahar; Sadaf, S.; Iqbal, J.; Ullah, I.; Bhatti, H.N.; Nouren, S.; Habib-ur-Rehman; Nisar, J.; Iqbal, M. Biodiesel production from waste cooking oil: An efficient technique to convert waste into biodiesel. Sustain. Cities Soc. 2018, 41, 220–226. [Google Scholar] [CrossRef]
- Abomohra, A.E.-F.; Almutairi, A.W. A close-loop integrated approach for microalgae cultivation and efficient utilization of agar-free seaweed residues for enhanced biofuel recovery. Bioresour. Technol. 2020, 317, 124027. [Google Scholar] [CrossRef]
- Aydin, Z.; Safa, A. Performance and emission characteristics of waste frying oil biodiesel blends as pilot fuel on a dual fuel compression ignition engine. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 15, 273–282. [Google Scholar] [CrossRef]
- Hassan, A.; Rehman, A.U.; Shabbir, N.; Hassan, S.R.; Sadiq, M.T.; Arshad, J. Impact of Inertial Response for the Variable Speed Wind Turbine. In Proceedings of the 2019 International Conference on Engineering and Emerging Technologies (ICEET), Lahore, Pakistan, 21–22 February 2019; pp. 1–6. [Google Scholar]
- Kumar, C.; Rana, K.B.; Tripathi, B. Effect of ternary fuel blends on performance and emission characteristics of stationary VCR diesel engine. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 1–20. [Google Scholar] [CrossRef]
- Liaquat, A.M.; Masjuki, H.H.; Kalam, M.A.; Rizwanul Fattah, I.M. Impact of biodiesel blend on injector deposit formation. Energy 2014, 72, 813–823. [Google Scholar] [CrossRef]
- Yesilyurt, M.K.; Aydin, M. Experimental investigation on the performance, combustion and exhaust emission characteristics of a compression-ignition engine fueled with cottonseed oil biodiesel/diethyl ether/diesel fuel blends. Energy Convers. Manag. 2020, 205, 112355. [Google Scholar] [CrossRef]
- Ghosh, S.; Roy, S. Novel integration of biohydrogen production with fungal biodiesel production process. Bioresour. Technol. 2019, 288, 121603. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.M.A.; Rizwanul Fattah, I.M.; Ong, H.C.; Zamri, M.F.M.A. State-of-the-Art of Strategies to Reduce Exhaust Emissions from Diesel Engine Vehicles. Energies 2021, 14, 1766. [Google Scholar] [CrossRef]
- Hanaki, K.; Portugal-Pereira, J. Chapter 6 The Effect of Biofuel Production on Greenhouse Gas Emission Reductions. In Biofuels and Sustainability: Holistic Perspectives for Policy-Making; Takeuchi, K., Shiroyama, H., Saito, O., Matsuura, M., Eds.; Springer Open: Tokyo, Japan, 2018; pp. 53–71. [Google Scholar]
- Weltschev, M.; Heming, F.; Haufe, M.; Heyer, M. The influence of the age of biodiesel and heating oil with 10% biodiesel on the resistance of sealing materials at different temperatures. Mater. Werkst. 2017, 48, 837–845. [Google Scholar] [CrossRef]
- Chum, H.; Faaij, A.; Moreira, J.; Berndes, G.; Dhamija, P.; Dong, H.; Gabrielle, B.; Eng, A.G.; Lucht, W.; Mapako, M.; et al. Bioenergy. In IPCC Special Report on Renewable Energy Sources and Climate Change Mitigation; Edenhofer, O., Pichs-Madruga, R., Sokona, Y., Seyboth, K., Matschoss, P., Kadner, S., Zwickel, T., Eickemeier, P., Hansen, S., et al., Eds.; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- International Agency for Research on Cancer (IARC). IARC: DIESEL ENGINE EXHAUST CARCINOGENIC. Available online: https://www.iarc.who.int/news-events/iarc-diesel-engine-exhaust-carcinogenic. (accessed on 8 September 2022).
- Guidotti, T.L. Health Risks and Occupation as a Firefighter. 2014. Available online: https://www.dva.gov.au/sites/default/files/guidotti_report.pdf. (accessed on 8 September 2022).
- Estevez, R.; Aguado-Deblas, L.; López-Tenllado, F.J.; Luna, C.; Calero, J.; Romero, A.A.; Bautista, F.M.; Luna, D. Biodiesel Is Dead: Long Life to Advanced Biofuels-A Comprehensive Critical Review. Energies 2022, 15, 3173. [Google Scholar] [CrossRef]
- Naeini, M.A.; Zandieh, M.; Najafi, S.E.; Sajadi, S.M. Analyzing the development of the third-generation biodiesel production from microalgae by a novel hybrid decision-making method: The case of Iran. Energy 2020, 195, 116895. [Google Scholar] [CrossRef]
- Aguilar-Garnica, E.; García-Sandoval, J.P.; Dochain, D. Monitoring of a biodiesel production process via reset observer. J. Process Control 2016, 42, 104–113. [Google Scholar] [CrossRef]
- Purkait, A.; Hazra, D.K. Biodiesel as a carrier for pesticide formulations: A green chemistry approach. Int. J. Pest Manag. 2020, 66, 341–350. [Google Scholar] [CrossRef]
- Banerjee, S.; Chandra Mandal, N. Chapter 13-Fungal Bioagents in the Remediation of Degraded Soils. In Microbial Services in Restoration Ecology; Singh, J.S., Vimal, S.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 191–205. [Google Scholar] [CrossRef]
- Allam, R.J.; Fetvedt, J.E.; Forrest, B.A.; Freed, D.A. The Oxy-Fuel, Supercritical CO2 Allam Cycle: New Cycle Developments to Produce Even Lower-Cost Electricity From Fossil Fuels Without Atmospheric Emissions. In Proceedings of the ASME Turbo Expo 2014: Turbine Technical Conference and Exposition, Düsseldorf, Germany, 16–20 June 2014. [Google Scholar]
- Yadav, S.; Chakrabarti, S.S.; Bose, P. A Systematic Approach in Power Plant Performance Improvement Through Exergy Analysis. Int. J. Energy A Clean Environ. 2021, 22, 1–33. [Google Scholar] [CrossRef]
- Mustapha, W.F.; Kirkerud, J.G.; Bolkesjø, T.F.; Trømborg, E. Large-scale forest-based biofuels production: Impacts on the Nordic energy sector. Energy Convers. Manag. 2019, 187, 93–102. [Google Scholar] [CrossRef]
- Ndiaye, M.; Arhaliass, A.; Legrand, J.; Roelens, G.; Kerihuel, A. Reuse of waste animal fat in biodiesel: Biorefining heavily-degraded contaminant-rich waste animal fat and formulation as diesel fuel additive. Renew Energy 2020, 145, 1073–1079. [Google Scholar] [CrossRef]
- Hazrat, M.A.; Rasul, M.G.; Khan, M.M.K.; Ashwath, N.; Fattah, I.M.R.; Ong, H.C.; Mahlia, T.M.I. Biodiesel production from transesterification of Australian Brassica napus L. oil: Optimisation and reaction kinetic model development. Environ. Dev. Sustain. 2022, 1–26. [Google Scholar] [CrossRef]
- Hariram, V.; John, J.G.; Seralathan, S. Spectrometric analysis of algal biodiesel as a fuel derived through base-catalysed transesterification. Int. J. Ambient Energy 2019, 40, 195–202. [Google Scholar] [CrossRef]
- Aranda, D.A.G.; Santos, R.T.P.; Tapanes, N.C.O.; Ramos, A.L.D.; Antunes, O.A.C. Acid-Catalyzed Homogeneous Esterification Reaction for Biodiesel Production from Palm Fatty Acids. Catal. Lett. 2008, 122, 20–25. [Google Scholar] [CrossRef]
- Loures, C.C.A.; Amaral, M.S.; Da Rós, P.C.M.; Zorn, S.M.F.E.; de Castro, H.F.; Silva, M.B. Simultaneous esterification and transesterification of microbial oil from Chlorella minutissima by acid catalysis route: A comparison between homogeneous and heterogeneous catalysts. Fuel 2018, 211, 261–268. [Google Scholar] [CrossRef]
- Vakros, J. Biochars and Their Use as Transesterification Catalysts for Biodiesel Production: A Short Review. Catalysts 2018, 8, 562. [Google Scholar] [CrossRef]
- Rahman, S.M.A.; Fattah, I.M.R.; Maitra, S.; Mahlia, T.M.I. A ranking scheme for biodiesel underpinned by critical physicochemical properties. Energy Convers. Manag. 2021, 229, 113742. [Google Scholar] [CrossRef]
- Salaheldeen, M.; Mariod, A.A.; Aroua, M.K.; Rahman, S.M.A.; Soudagar, M.E.M.; Fattah, I.M.R. Current State and Perspectives on Transesterification of Triglycerides for Biodiesel Production. Catalysts 2021, 11, 1121. [Google Scholar] [CrossRef]
- Thangaraj, B.; Solomon, P.R.; Muniyandi, B.; Ranganathan, S.; Lin, L. Catalysis in biodiesel production—A review. Clean Energy 2018, 3, 2–23. [Google Scholar] [CrossRef] [Green Version]
- Al-Saadi, A.; Mathan, B.; He, Y. Biodiesel production via simultaneous transesterification and esterification reactions over SrO–ZnO/Al2O3 as a bifunctional catalyst using high acidic waste cooking oil. Chem. Eng. Res. Des. 2020, 162, 238–248. [Google Scholar] [CrossRef]
- Fattah, I.M.R.; Ong, H.C.; Mahlia, T.M.I.; Mofijur, M.; Silitonga, A.S.; Rahman, S.M.A.; Ahmad, A. State of the Art of Catalysts for Biodiesel Production. Front. Energy Res. 2020, 8, 101. [Google Scholar] [CrossRef]
- Jafari, A.; Esmaeilzadeh, F.; Mowla, D.; Sadatshojaei, E.; Heidari, S.; Wood, D.A. New insights to direct conversion of wet microalgae impregnated with ethanol to biodiesel exploiting extraction with supercritical carbon dioxide. Fuel 2021, 285, 119199. [Google Scholar] [CrossRef]
- Madhugiri, N.-R.; Jaya, R.S. Advances in Biofuels and Bioenergy; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Dutton, J.A. The Reaction of Biodiesel: Transesterification. In E-Education; The Pennsylvania State University: State College, PA, USA, 2020. [Google Scholar]
- Changmai, B.; Vanlalveni, C.; Ingle, A.P.; Bhagat, R.; Rokhum, S.L. Widely used catalysts in biodiesel production: A review. RSC Adv. 2020, 10, 41625–41679. [Google Scholar] [CrossRef] [PubMed]
- Vasić, K.; Hojnik Podrepšek, G.; Knez, Ž.; Leitgeb, M. Biodiesel Production Using Solid Acid Catalysts Based on Metal Oxides. Catalysts 2020, 10, 237. [Google Scholar] [CrossRef]
- Vasić, V.M.; Šćiban, M.B.; Kukić, D.V.; Prodanović, J.M.; Maravić, N.R. Sequential micro and ultrafiltration of distillery wastewater. Acta Period. Technol. 2015, 2015, 177–183. [Google Scholar] [CrossRef]
- Pitt, F.D.; Domingos, A.M.; Barros, A.A.C. Purification of residual glycerol recovered from biodiesel production. S. Afr. J. Chem. Eng. 2019, 29, 42–51. [Google Scholar] [CrossRef]
- Gerpen, J.V. Biodiesel processing and production. Fuel Processing Technol. 2005, 86, 1097–1107. [Google Scholar] [CrossRef]
- Baêsso, R.M.; Costa-Felix, R.P.B.; Miloro, P.; Zeqiri, B. Ultrasonic parameter measurement as a means of assessing the quality of biodiesel production. Fuel 2019, 241, 155–163. [Google Scholar] [CrossRef]
- Okumuş, F.; Kökkülünk, G.; Kaya, C.; Aydın, Z. Thermodynamic Assessment of Water Diesel Emulsified Fuel Usage in a Single Cylinder Diesel Engine. Energy Sources Part A Recovery Util. Environ. Eff. 2021, 1–14. [Google Scholar] [CrossRef]
- Konur, O. Biodiesel Fuels Based on Edible and Nonedible Feedstocks, Wastes, and Algae, 1st ed.; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Woźniak-Karczewska, M.; Lisiecki, P.; Białas, W.; Owsianiak, M.; Piotrowska-Cyplik, A.; Wolko, Ł.; Ławniczak, Ł.; Heipieper, H.J.; Gutierrez, T.; Chrzanowski, Ł. Effect of bioaugmentation on long-term biodegradation of diesel/biodiesel blends in soil microcosms. Sci. Total Environ. 2019, 671, 948–958. [Google Scholar] [CrossRef]
- Bitire, S.O.; Jen, T.-C. Performance and emission analysis of a CI engine fueled with parsley biodiesel–diesel blend. Mater. Renew. Sustain. Energy 2022, 11, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Estevez, R.; Aguado-Deblas, L.; Bautista, F.M.; Luna, D.; Luna, C.; Calero, J.; Posadillo, A.; Romero, A.A. Biodiesel at the Crossroads: A Critical Review. Catalysts 2019, 9, 1033. [Google Scholar] [CrossRef]
- Abed, K.A.; Gad, M.S.; Morsi, A.K.E.; Sayed, M.M.; Elyazeed, S.A. Effect of biodiesel fuels on diesel engine emissions. Egypt. J. Pet. 2019, 28, 183–188. [Google Scholar] [CrossRef]
- Veza, I.; Afzal, A.; Mujtaba, M.A.; Tuan Hoang, A.; Balasubramanian, D.; Sekar, M.; Fattah, I.M.R.; Soudagar, M.E.M.; El-Seesy, A.I.; Djamari, D.W.; et al. Review of artificial neural networks for gasoline, diesel and homogeneous charge compression ignition engine. Alex. Eng. J. 2022, 61, 8363–8391. [Google Scholar] [CrossRef]
- Mondal, M.; Goswami, S.; Ghosh, A.; Oinam, G.; Tiwari, O.N.; Das, P.; Gayen, K.; Mandal, M.K.; Halder, G.N. Production of biodiesel from microalgae through biological carbon capture: A review. 3 Biotech 2017, 7, 99. [Google Scholar] [CrossRef]
- Abdollahi Asl, M.; Tahvildari, K.; Bigdeli, T. Eco-friendly synthesis of biodiesel from WCO by using electrolysis technique with graphite electrodes. Fuel 2020, 270, 117582. [Google Scholar] [CrossRef]
- Raud, M.; Kikas, T.; Sippula, O.; Shurpali, N.J. Potentials and challenges in lignocellulosic biofuel production technology. Renew. Sustain. Energy Rev. 2019, 111, 44–56. [Google Scholar] [CrossRef]
- Sánchez Faba, E.M.; Ferrero, G.O.; Dias, J.M.; Eimer, G.A. Alternative Raw Materials to Produce Biodiesel through Alkaline Heterogeneous Catalysis. Catalysts 2019, 9, 690. [Google Scholar] [CrossRef] [Green Version]
- García-Martín, J.F.; Alés-Álvarez, F.J.; López-Barrera, M.d.C.; Martín-Domínguez, I.; Álvarez-Mateos, P. Cetane number prediction of waste cooking oil-derived biodiesel prior to transesterification reaction using near infrared spectroscopy. Fuel 2019, 240, 10–15. [Google Scholar] [CrossRef]
- Serrano, L.M.V.; da Silva, M.G. Study About Nitrogen Oxide Emissions and Fuel Consumption in Diesel Engines Fueled with B20. In Biofuels-State of Development; Biernat, K., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Sharma, A.; Kumar, P. Quantification of air pollution exposure to in-pram babies and mitigation strategies. Environ. Int. 2020, 139, 105671. [Google Scholar] [CrossRef]
- Serrà, A.; Artal, R.; García-Amorós, J.; Gómez, E.; Philippe, L. Circular zero-residue process using microalgae for efficient water decontamination, biofuel production, and carbon dioxide fixation. Chem. Eng. J. 2020, 388, 124278. [Google Scholar] [CrossRef]
- Staehelin, J.; Harris, N.R.P.; Appenzeller, C.; Eberhard, J. Ozone trends: A review. Rev. Geophys. 2001, 39, 231–290. [Google Scholar] [CrossRef]
- Kirk-Davidoff, D.B.; Hintsa, E.J.; Anderson, J.G.; Keith, D.W. The effect of climate change on ozone depletion through changes in stratospheric water vapour. Nature 1999, 402, 399–401. [Google Scholar] [CrossRef]
- Krahl, J.; Baum, K.; Hackbarth, U.; Jeberien, H.E.; Munack, A.; Schütt, C.; Schröder, O.; Walter, N.; Bünger, J.; Müller, M.; et al. Gaseous compounds, ozone precursors, particle number and particle size distributions, and mutagenic effects due to biodiesel. Trans. ASAE 2001, 44, 179. [Google Scholar] [CrossRef]
- Ashworth, K.; Wild, O.; Hewitt, C.N. Impacts of biofuel cultivation on mortality and crop yields. Nat. Clim. Chang. 2013, 3, 492–496. [Google Scholar] [CrossRef]
- Guenther, A.; Hewitt, C.N.; Erickson, D.; Fall, R.; Geron, C.; Graedel, T.; Harley, P.; Klinger, L.; Lerdau, M.; Mckay, W.A.; et al. A global model of natural volatile organic compound emissions. J. Geophys. Res. Atmos. 1995, 100, 8873–8892. [Google Scholar] [CrossRef]
- Hayashi, S.; Yamada, H. NOx emissions in combustion of lean premixed mixtures injected into hot burned gas. Proc. Combust. Inst. 2000, 28, 2443–2449. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Robbins, C. Review of the effects of biodiesel on NOx emissions. Fuel Processing Technol. 2012, 96, 237–249. [Google Scholar] [CrossRef]
- Faulds, J.; Hinz, N.; Coolbaugh, M.; dePolo, C.; Siler, D.; Shevenell, L.; Hammond, W.; Kreemer, C.; Queen, J. Discovering Geothermal Systems in the Great Basin Region: An Integrated Geologic, Geochemical, and Geophysical Approach for Establishing Geothermal Play Fairways. In Proceedings of the 41st Workshop on Geothermal Reservoir Engineering Stanford University, Stanford, CA, USA, 22–24 February 2016. [Google Scholar]
- Sassykova, L.R.; Aubakirov, Y.A.; Sendilvelan, S.; Tashmukhambetova, Z.K.; Faizullaeva, M.F.; Bhaskar, K.; Batyrbayeva, A.A.; Ryskaliyeva, R.G.; Tyussyupova, B.B.; Zhakupova, A.A.; et al. The Main Components of Vehicle Exhaust Gases and Their Effective Catalytic Neutralization. Orient. J. Chem. 2019, 35, 110–127. [Google Scholar] [CrossRef]
- Turns, S.R. Introduction to combustion. Concepts and Applications, 3rd ed.; The McGraw-Hill Companies, Inc.: New York, NY, USA, 2012; p. 754. [Google Scholar]
- Zach, J.J. The Chemistry of Flammable Gas Generation. 2000. Available online: https://www.osti.gov/biblio/805379 (accessed on 12 September 2022).
- Imtenan, S.; Varman, M.; Masjuki, H.H.; Kalam, M.A.; Sajjad, H.; Arbab, M.I.; Rizwanul Fattah, I.M. Impact of low temperature combustion attaining strategies on diesel engine emissions for diesel and biodiesels: A review. Energy Convers. Manag. 2014, 80, 329–356. [Google Scholar] [CrossRef]
- Santhoshkumar, A.; Thangarasu, V.; Anand, R. Chapter 12-Performance, combustion, and emission characteristics of DI diesel engine using mahua biodiesel. In Advanced Biofuels; Azad, A.K., Rasul, M., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 291–327. [Google Scholar] [CrossRef]
- Guo, K.; Guan, Q.; Xu, J.; Tan, W. Mechanism of Preparation of Platform Compounds from Lignocellulosic Biomass Liquefaction Catalyzed by Bronsted Acid: A Review. J. Bioresour. Bioprod. 2019, 4, 202–213. [Google Scholar] [CrossRef]
- Ristovski, Z.D.; Miljevic, B.; Surawski, N.C.; Morawska, L.; Fong, K.M.; Goh, F.; Yang, I.A. Respiratory health effects of diesel particulate matter. Respirology 2012, 17, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Pullen, J.; Saeed, K. Factors affecting biodiesel engine performance and exhaust emissions–Part I: Review. Energy 2014, 72, 1–16. [Google Scholar] [CrossRef]
- Oluwoye, I.; Altarawneh, M.; Gore, J.; Dlugogorski, B.Z. Products of incomplete combustion from biomass reburning. Fuel 2020, 274, 117805. [Google Scholar] [CrossRef]
- United Fire Fighters Union of South Australia. Diesel Particulates. Available online: https://www.ufusa.com.au/diesel-particulates/ (accessed on 4 September 2022).
- Thoai, D.N.; Kumar, A.; Prasertsit, K.; Tongurai, C. Evaluation of Biodiesel Production Process by the Determining of the Total Glycerol Content in Biodiesel. Energy Procedia 2017, 138, 544–551. [Google Scholar] [CrossRef]
- Ghosh, S.; Dutta, D. Performance And Exhaust Emission Analysis Of Direct Injection Diesel Engine Using Pongamia Oil. Int. J. Emerg. Technol. Adv. Eng. 2012, 2, 341–346. [Google Scholar]
- Gromadzińska, J.; Wąsowicz, W. Health risk in road transport workers. Part I. Occupational exposure to chemicals, biomarkers of effect. Int. J. Occup. Med. Environ. Health 2019, 32, 267–280. [Google Scholar] [CrossRef]
- Singh, B.P.; Kumar, K.; Jain, V.K. Source identification and health risk assessment associated with particulate- and gaseous-phase PAHs at residential sites in Delhi, India. Air Qual. Atmos. Health 2021, 14, 1505–1521. [Google Scholar] [CrossRef]
- Belgiorno, G.; Boscolo, A.; Dileo, G.; Numidi, F.; Pesce, F.C.; Vassallo, A.; Ianniello, R.; Beatrice, C.; Di Blasio, G. Experimental Study of Additive-Manufacturing-Enabled Innovative Diesel Combustion Bowl Features for Achieving Ultra-Low Emissions and High Efficiency. SAE Int. J. Adv. Curr. Pract. Mobil. 2020, 3, 672–684. [Google Scholar] [CrossRef]
- Bórawski, P.; Bełdycka-Bórawska, A.; Szymańska, E.J.; Jankowski, K.J.; Dubis, B.; Dunn, J.W. Development of renewable energy sources market and biofuels in The European Union. J. Clean. Prod. 2019, 228, 467–484. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Gupta, T.; Kothari, A. Particulate emissions from biodiesel vs diesel fuelled compression ignition engine. Renew. Sustain. Energy Rev. 2011, 15, 3278–3300. [Google Scholar] [CrossRef]
- Spada, N.J. Comparison of elemental and organic carbon measurements between IMPROVE and CSN before and after method transitions. Atmos. Environ. 2018, 178, 173–180. [Google Scholar] [CrossRef]
- Palash, S.M. Impacts of biodiesel combustion on NOx emissions and their reduction approaches. Renew. Sustain. Energy Rev. 2013, 23, 473–490. [Google Scholar] [CrossRef]
- Bünger, J.; Krahl, J.; Baum, K.; Schröder, O.; Müller, M.; Westphal, G.; Ruhnau, P.; Schulz, T.G.; Hallier, E. Cytotoxic and mutagenic effects, particle size and concentration analysis of diesel engine emissions using biodiesel and petrol diesel as fuel. Arch. Toxicol. 2000, 74, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Haggag, E.S.A.; Abdelsamad, A.A.; Masoud, A.M. Potentiality of uranium extraction from acidic leach liquor by polyacrylamide-acrylic acid titanium silicate composite adsorbent. Int. J. Environ. Anal. Chem. 2020, 100, 204–224. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Zhu, A.-Y.; Liao, X.; Manga, M.; Wang, L.-P. Capillary Effects on Groundwater Response to Earth Tides. Water Resour. Res. 2019, 55, 6886–6895. [Google Scholar] [CrossRef]
- Hall, A.H.; Mathieu, L.; Maibach, H.I. Acute chemical skin injuries in the United States: A review. Crit. Rev. Toxicol. 2018, 48, 540–554. [Google Scholar] [CrossRef]
- Sharma, H.K.; Xu, C.; Qin, W. Biological Pretreatment of Lignocellulosic Biomass for Biofuels and Bioproducts: An Overview. Waste Biomass Valorization 2019, 10, 235–251. [Google Scholar] [CrossRef]
- Singh, N.; Singh, S.; Mall, R.K. Chapter 17-Urban ecology and human health: Implications of urban heat island, air pollution and climate change nexus. In Urban Ecology; Verma, P., Singh, P., Singh, R., Raghubanshi, A.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 317–334. [Google Scholar] [CrossRef]
- Botti, R.F.; Innocentini, M.D.M.; Faleiros, T.A.; Mello, M.F.; Flumignan, D.L.; Santos, L.K.; Franchin, G.; Colombo, P. Biodiesel Processing Using Sodium and Potassium Geopolymer Powders as Heterogeneous Catalysts. Molecules 2020, 25, 2839. [Google Scholar] [CrossRef]
- Azadbakht, M.; Safieddin Ardebili, S.M.; Rahmani, M. Potential for the production of biofuels from agricultural waste, livestock, and slaughterhouse waste in Golestan province, Iran. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Hedayati, M.; Brock, P.M.; Nachimuthu, G.; Schwenke, G. Farm-level strategies to reduce the life cycle greenhouse gas emissions of cotton production: An Australian perspective. J. Clean. Prod. 2019, 212, 974–985. [Google Scholar] [CrossRef]
- Pathak, D.; Whitehead, P.G.; Futter, M.N.; Sinha, R. Water quality assessment and catchment-scale nutrient flux modeling in the Ramganga River Basin in north India: An application of INCA model. Sci. Total Environ. 2018, 631–632, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Osorio-González, C.S.; Gómez-Falcon, N.; Sandoval-Salas, F.; Saini, R.; Brar, S.K.; Ramírez, A.A. Production of Biodiesel from Castor Oil: A Review. Energies 2020, 13, 2467. [Google Scholar] [CrossRef]
- Leikauf, G.D.; Kim, S.H.; Jang, A.S. Mechanisms of ultrafine particle-induced respiratory health effects. Exp. Mol. Med. 2020, 52, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, M.; Wegierek-Ciuk, A.; Brzoska, K.; Wojewodzka, M.; Meczynska-Wielgosz, S.; Gromadzka-Ostrowska, J.; Mruk, R.; Øvrevik, J.; Kruszewski, M.; Lankoff, A. Genotoxic potential of diesel exhaust particles from the combustion of first- and second-generation biodiesel fuels-the FuelHealth project. Environ. Sci. Pollut. Res. 2017, 24, 24223–24234. [Google Scholar] [CrossRef] [PubMed]
- Lankoff, A.; Brzoska, K.; Czarnocka, J.; Kowalska, M.; Lisowska, H.; Mruk, R.; Øvrevik, J.; Wegierek-Ciuk, A.; Zuberek, M.; Kruszewski, M. A comparative analysis of in vitro toxicity of diesel exhaust particles from combustion of 1st- and 2nd-generation biodiesel fuels in relation to their physicochemical properties—the FuelHealth project. Environ. Sci. Pollut. Res. 2017, 24, 19357–19374. [Google Scholar] [CrossRef] [PubMed]
- McConnell, R.; Berhane, K.; Gilliland, F.; London, S.J.; Vora, H.; Avol, E.; Gauderman, W.J.; Margolis, H.G.; Lurmann, F.; Thomas, D.C.; et al. Air pollution and bronchitic symptoms in Southern California children with asthma. Environ. Health Perspect. 1999, 107, 757–760. [Google Scholar] [CrossRef]
- Santos, U.P.; Arbex, M.A.; Braga, A.L.F.; Mizutani, R.F.; Cançado, J.E.D.; Terra-Filho, M.; Chatkin, J.M. Environmental air pollution: Respiratory effects. J. Bras. Pneumol. 2021, 47, e20200267. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, J.; Zhang, H.; Shi, C.; Li, G.; Peng, Z.; Ma, J.; Zhou, Y.; Zhang, L. Short-Term Exposure to Ambient Air Pollution and Asthma Mortality. Am. J. Respir. Crit. Care Med. 2019, 200, 24–32. [Google Scholar] [CrossRef]
- Bowatte, G.; Erbas, B.; Lodge, C.J.; Knibbs, L.D.; Gurrin, L.C.; Marks, G.B.; Thomas, P.S.; Johns, D.P.; Giles, G.G.; Hui, J.; et al. Traffic-related air pollution exposure over a 5-year period is associated with increased risk of asthma and poor lung function in middle age. Eur. Respir. J. 2017, 50, 1602357. [Google Scholar] [CrossRef]
- Duan, Y.; Pandey, A.; Zhang, Z.; Awasthi, M.K.; Bhatia, S.K.; Taherzadeh, M.J. Organic solid waste biorefinery: Sustainable strategy for emerging circular bioeconomy in China. Ind. Crops Prod. 2020, 153, 112568. [Google Scholar] [CrossRef]
- Kang, C.M.; Jang, A.S.; Ahn, M.H.; Shin, J.A.; Kim, J.H.; Choi, Y.S.; Rhim, T.Y.; Park, C.S. Interleukin-25 and interleukin-13 production by alveolar macrophages in response to particles. Am. J. Respir. Cell Mol. Biol 2005, 33, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Jeswani, H.K.; Chilvers, A.; Azapagic, A. Environmental sustainability of biofuels: A review. Proc. R. Soc. A 2020, 476, 20200351. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.; Metheny, E.; Sergent, S.R. Hydrocarbon Toxicity. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499883/ (accessed on 16 September 2022).
- Nauss, K. Diesel Exhaust: A Critical Analysis of Emissions, Exposure, and Health Effects. Available online: https://dieselnet.com/papers/9710nauss.html (accessed on 8 September 2022).
- Yanamala, N.; Hatfield, M.K.; Farcas, M.T.; Schwegler-Berry, D.; Hummer, J.A.; Shurin, M.R.; Birch, M.E.; Gutkin, D.W.; Kisin, E.; Kagan, V.E.; et al. Biodiesel versus diesel exposure: Enhanced pulmonary inflammation, oxidative stress, and differential morphological changes in the mouse lung. Toxicol. Appl. Pharmacol. 2013, 272, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.K.; Shetti, N.P.; Reddy, K.R.; Kwon, E.E.; Nadagouda, M.N.; Aminabhavi, T.M. Biomass utilization and production of biofuels from carbon neutral materials. Environ. Pollut. 2021, 276, 116731. [Google Scholar] [CrossRef]
- Skogstad, G.; Wilder, M. Strangers at the gate: The role of multidimensional ideas, policy anomalies and institutional gatekeepers in biofuel policy developments in the USA and European Union. Policy Sci. 2019, 52, 343–366. [Google Scholar] [CrossRef]
- Gupta, P.K. Fate, Transport, and Bioremediation of Biodiesel and Blended Biodiesel in Subsurface Environment: A Review. J. Environ. Eng. 2020, 146, 03119001. [Google Scholar] [CrossRef]
- Pikula, K.S.; Zakharenko, A.M.; Chaika, V.V.; Stratidakis, A.K.; Kokkinakis, M.; Waissi, G.; Rakitskii, V.N.; Sarigiannis, D.A.; Hayes, A.W.; Coleman, M.D.; et al. Toxicity bioassay of waste cooking oil-based biodiesel on marine microalgae. Toxicol. Rep. 2019, 6, 111–117. [Google Scholar] [CrossRef]
- Manigandan, S.; Gunasekar, P.; Devipriya, J.; Nithya, S. Emission and injection characteristics of corn biodiesel blends in diesel engine. Fuel 2019, 235, 723–735. [Google Scholar] [CrossRef]
- Antar, M.; Lyu, D.; Nazari, M.; Shah, A.; Zhou, X.; Smith, D.L. Biomass for a sustainable bioeconomy: An overview of world biomass production and utilization. Renew. Sustain. Energy Rev. 2021, 139, 110691. [Google Scholar] [CrossRef]
- Simon, H.; Fallmann, J.; Kropp, T.; Tost, H.; Bruse, M. Urban Trees and Their Impact on Local Ozone Concentration—A Microclimate Modeling Study. Atmosphere 2019, 10, 154. [Google Scholar] [CrossRef]
- Godri Pollitt, K.J.; Chhan, D.; Rais, K.; Pan, K.; Wallace, J.S. Biodiesel fuels: A greener diesel? A review from a health perspective. Sci. Total Environ. 2019, 688, 1036–1055. [Google Scholar] [CrossRef] [PubMed]
- Mullins, B.J.; Kicic, A.; Ling, K.-M.; Mead-Hunter, R.; Larcombe, A.N. Biodiesel exhaust–induced cytotoxicity and proinflammatory mediator production in human airway epithelial cells. Environ. Toxicol. 2016, 31, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Obula Reddy, C.; Reddy, Y.S.; Subhadra, M.; Rajagopal, K. Effect of long–term storage on the fatty-acid profile of biodiesel and its impact on key ultrasonic properties of biodiesels and blends. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 1–20. [Google Scholar] [CrossRef]
- Chozhavendhan, S.; Karthiga Devi, G.; Bharathiraja, B.; Praveen Kumar, R.; Elavazhagan, S. 9-Assessment of crude glycerol utilization for sustainable development of biorefineries. In Refining Biomass Residues for Sustainable Energy and Bioproducts; Kumar, R.P., Gnansounou, E., Raman, J.K., Baskar, G., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 195–212. [Google Scholar] [CrossRef]
- Hasan, M.H.; Mahlia, T.M.; Mofijur, M.; Rizwanul Fattah, I.M.; Handayani, F.; Ong, H.C.; Silitonga, A.S. A Comprehensive Review on the Recent Development of Ammonia as a Renewable Energy Carrier. Energies 2021, 14, 3732. [Google Scholar] [CrossRef]
- Hassan, A.B.; Ayodeji, O.V. Benefits and challenges of biodiesel production in West Africa. Niger. J. Technol. 2019, 38, 621–627. [Google Scholar] [CrossRef] [Green Version]



| Application | Explanation | Application | References |
| Automobiles | Mining, Hybrid electric | Biodiesel may be used as fuel for automobiles and farm machinery, off-road work of construction, and mining. Biodiesel is also applied within hybrid electric automobiles | [24,25] |
| Agricultural adjuvants |
| They can be used as a carrier for pesticides and fertilizers in agricultural sprays because of their non-toxic and biodegradable properties | [26,27] |
| Boiler fuel | Alternative for boiler use. | With the increasing price of natural gas, biodiesel is a better alternative, with slight modifications required for boiler use | [28,29] |
| Generation of power |
| Generators that run on biodiesel provide standby power at the time of power shortage. Additionally, the enhanced lubricity of such generators can potentially minimize engine deterioration | [30] |
| Process Name | Things to Note | References |
|---|---|---|
| A mixture of alcohol and the catalyst | Careful about alcohol excess and monitor level of water and fatty acids. | [46] |
| Separation | Gravity and centrifugal force | [42] |
| Removal of alcohol | No water should be accumulated in recovered alcohol | [51] |
| Neutralization of glycerine | Purity of glycerine | [48] |
| Methyl ester wash | Color and viscosity | [3] |
| Product Quality | Analyzed through advanced analytical techniques | [52] |
| Name | Comparison with Other Fuels | Emission | References |
|---|---|---|---|
| Ozone | Less than other diesel fuels | Gas | [56] |
| Sulfur | Very less, only in impure biodiesel | Oxides and sulphates | [53] |
| Carbon Monoxide | 48% less | Unburned Carbon monoxide | [62] |
| Hydrocarbons | 66% less | All series | [63] |
| Particulate | 46% less | Inhaling particulate | [62] |
| Carbon dioxide | 78% less | CO2 | [62] |
| Nitrogen Oxides | 10% | NO2 | [64] |
| Systems Affected | Diseases | References |
|---|---|---|
| CVS (Cardiovascular System) | Cardiovascular Diseases, Increased Heart Rate, Decreased Blood Pressure. | [102] |
| Hematological Disorder | Blue Baby Syndrome, Met Hemoglobinemia | [101] |
| Infections | Skin and Eye Infection | [101] |
| Skin effect | Skin to turn bluish | [101] |
| Others | Increased Risk of Cancer, Inflammatory Diseases, Type II Diabetes, Vomiting and Cramps | [101] |
| Properties | Biodiesel | Diesel | References |
|---|---|---|---|
| Environmental Impact | Less | More | [56] |
| Emission of Particulate Matter | Less | More | [99] |
| PAH (Cancer Causing) | Decreases to 80% | Increased | [99] |
| Sulphur Emission | More Efficient | Less Efficient | [44] |
| CO (Carbon Monoxide) | Decreases to 66% | Increases | [103] |
| Hydrocarbons | Decreases to 47% | Increases | [103] |
| Other Particulate Matter | Decreases to 45% | Increases | [56] |
| Air Quality | More Improved | Less Improved | [104] |
| Sulphates, Carbon Metal oxides, Metal Ash, Toxic Compounds, Carbonyl, Reactive Oxygen Groups. | Decreased | Increased to Wide Range | [96] |
| Lungs Cancer, Mortality, Respiratory Problems | Reduced Rate | High Rate | [46] |
| Stimulus | Structure Damaged | Ref. |
|---|---|---|
| Reactive Oxygen Species | Damages DNA | [56] |
| Oily liquid | Irregular Heart Rhythm Damage to Kidney | [100] |
| Biocidal Fumes | Destroys Walls of Breathing System Which Causes Chemical Pneumonia | [111] |
| Stimuli destroying surfactants, Airway Epithelium, Alveolar Septa, Pulmonary Capillaries | Pneumonia, CNS effects (Slurred Speech, Headache Disorientation, Dizziness, Syncope, Hallucination, Violent Behavior), Skin Irritation, Mucous Membrane Irritation, Asthma, Cancer | [113] |
| Fumes | Asthma, cancer, destroy surfactant, airway epithelium, alveolar septae, and pulmonary capillaries | [113] |
| Combustion | Cell death | [104] |
| Country | Current Target | Targets for the Future |
|---|---|---|
| Argentina | B7, E5 | B10 by 2019 |
| Brazil | B2, E22–23 | B5 by 2022 |
| Canada | B2, E5 | * |
| USA | Biodiesel: 1.0 billion gallons; 0.91% Advanced biofuels: 2.01 billion gallons; 1.22% Cellulosic biofuels: 3.46–12.9 million gallons; 0.001–0.010% Total renewable fuels: 15.3 billion gallons; 9.22% 7.7 billion US gallons (approximately 29 billion liters) of renewable fuel be blended with gasoline by 2012 | 36.5 billion gallons of biofuels by 2022 21.9 billion gallons from lignocellulosic biofuels |
| Costa Rica | B20, E7 | * |
| EU Stats | 5.77% renewable transport fuel | 11% renewable transport fuel by 2020 |
| China | N/A | E10 by 2020 |
| India | E5 | 21% biofuels by 2020 |
| Japan | N/A | 11.5% biofuels by 2030 |
| Australia | Queensland: E5 New South Wales: E10 | * |
| Factors | Effect | References |
|---|---|---|
| Increased ethanol | Increase in ozone, isoprene up to 39 % increase in injury, Asthma, and Lung Cancer. | [40] |
| Soil and water pollution | Former and local harvesters are affected. | [40] |
| Air pollution | Cardiorespiratory diseases, National Impact | [127] |
| Burning effect | National and regional effect | [127] |
| Combustion | activation of cell signaling and the release of proinflammatory mediators | [125] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljaafari, A.; Fattah, I.M.R.; Jahirul, M.I.; Gu, Y.; Mahlia, T.M.I.; Islam, M.A.; Islam, M.S. Biodiesel Emissions: A State-of-the-Art Review on Health and Environmental Impacts. Energies 2022, 15, 6854. https://doi.org/10.3390/en15186854
Aljaafari A, Fattah IMR, Jahirul MI, Gu Y, Mahlia TMI, Islam MA, Islam MS. Biodiesel Emissions: A State-of-the-Art Review on Health and Environmental Impacts. Energies. 2022; 15(18):6854. https://doi.org/10.3390/en15186854
Chicago/Turabian StyleAljaafari, Abdulelah, I. M. R. Fattah, M. I. Jahirul, Yuantong Gu, T. M. I. Mahlia, Md. Ariful Islam, and Mohammad S. Islam. 2022. "Biodiesel Emissions: A State-of-the-Art Review on Health and Environmental Impacts" Energies 15, no. 18: 6854. https://doi.org/10.3390/en15186854
APA StyleAljaafari, A., Fattah, I. M. R., Jahirul, M. I., Gu, Y., Mahlia, T. M. I., Islam, M. A., & Islam, M. S. (2022). Biodiesel Emissions: A State-of-the-Art Review on Health and Environmental Impacts. Energies, 15(18), 6854. https://doi.org/10.3390/en15186854











