Phase Change Slurries for Cooling and Storage: An Overview of Research Trends and Gaps
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Literature Trends: Number of Publications, Top Journals, Authors, and Institutions
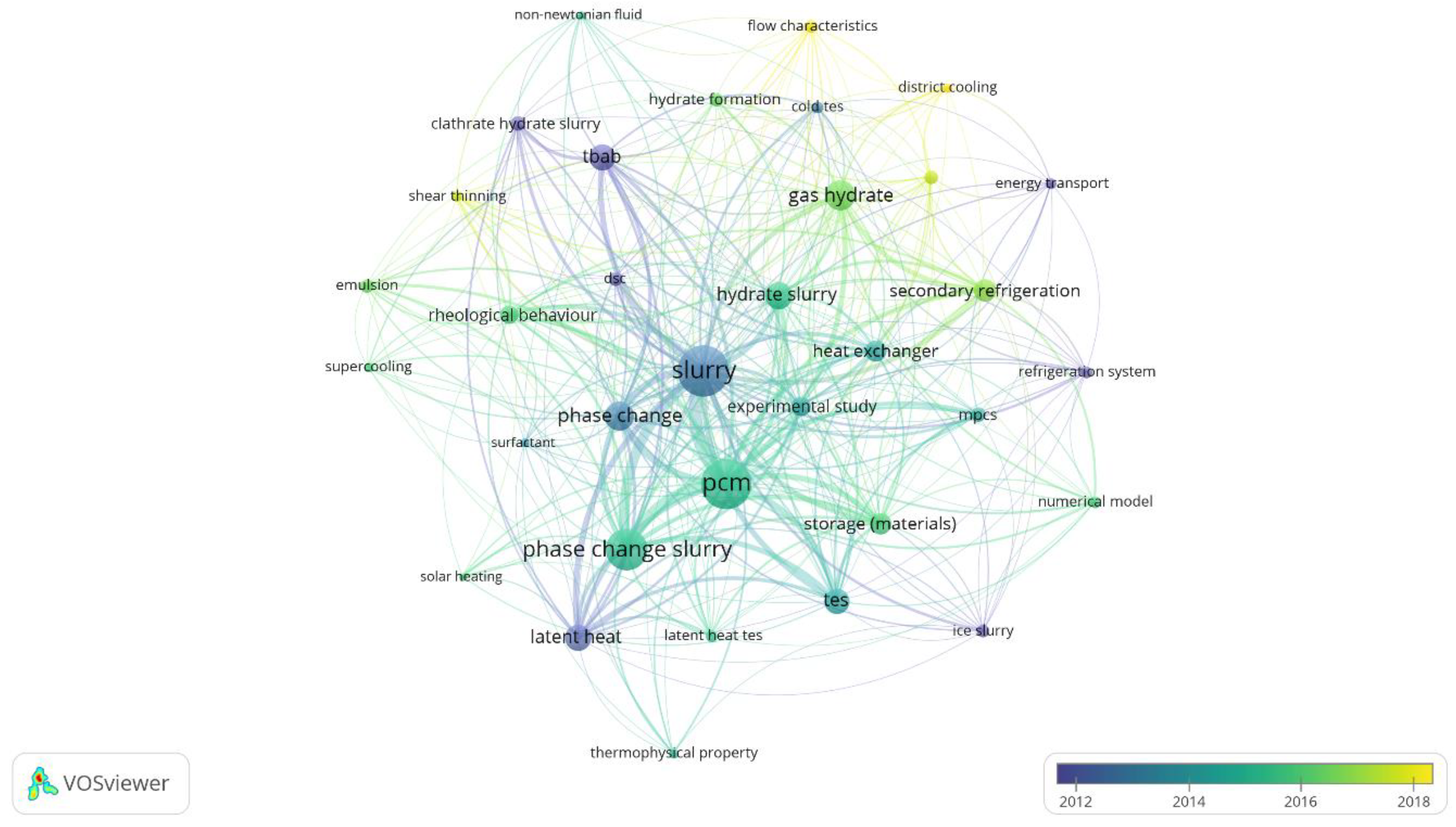
3.2. Keywords Analysis
3.2.1. Keywords Related to Gas Hydrates
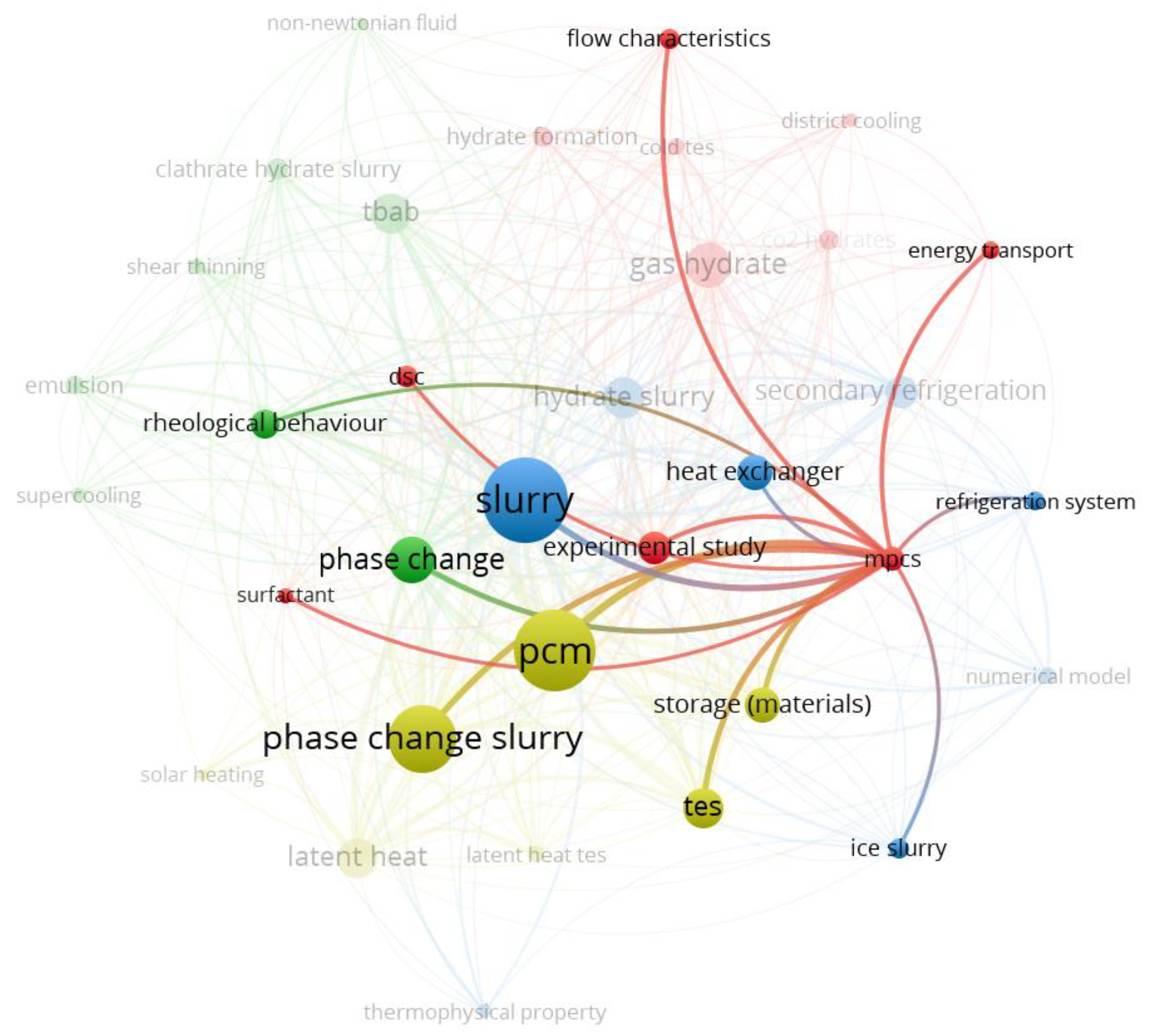
3.2.2. Keywords Related to Microencapsulated Phase Change Slurries
3.2.3. Keywords Related to Hydrate Slurries for Refrigeration
3.2.4. Keywords Related to Phase Change Slurries for Latent Thermal Energy Storage
3.2.5. Overlay Visualization of Keywords
4. Discussion
5. Conclusions
- The first paper on PCS was published in 1976. However, research on the topic started to gain interest in 2005 and the number of publications per year raised until 2019. The COVID-19 pandemic situation could affect the number of studies published in 2020 and 2021 due to lab closures in most countries.
- Most of the documents on PCS were articles published in Q1 journals, with Europe and China leading in the number of papers published. Most of the studies from Europe were performed at Universite Paris-Saclay in France.
- Most of the gas hydrates-based PCS research was carried out from the application angle and at a system level, while the fundamental understanding of the fast formation and long-term stability of the PCS, as well as the related compatibility and flow behaviors, are less investigated, although its importance is fully recognized.
- Encapsulated PCS were proposed as heat transfer fluids for heat exchangers at different temperatures employing different designs: helically coiled tube heat exchangers, circular tubes, or mini/microchannel heat sinks.
- An interesting application and potential future use for encapsulated PCS is as a cooling medium to reduce the warming from the back of the photovoltaic modules.
- The main base fluid for cooling applications with EPCMS was water, however, these materials can be applied in passive systems, i.e., cement slurries.
- Special attention should be paid to the viscosity increasing and the final low latent heat of encapsulated PCS since they could be the main challenges in their applications. Consequently, the determination and characterization of their rheological properties are crucial.
- Since the specific heat of PCS tends to decrease after the encapsulation, an improvement in thermal conductivity will be required to achieve a final material meeting all requirements of the desired application.
- As well, numerical simulations of the thermal and hydraulic performance of heat exchangers with encapsulated PCS represent a significant step for optimal experimental applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghasemi, K.; Tasnim, S.; Mahmud, S. PCM, nano/microencapsulation and slurries: A review of fundamentals, categories, fabrication, numerical models and applications. Sustain. Energy Technol. Assess. 2022, 52, 102084. [Google Scholar] [CrossRef]
- Vorbeck, L.; Gschwander, S.; Thiel, P.; Lüdemann, B.; Schossig, P. Pilot application of phase change slurry in a 5 m3 storage. Appl. Energy 2013, 109, 538–543. [Google Scholar] [CrossRef]
- Milián, Y.E.; Gutiérrez, A.; Grágeda, M.; Ushak, S. A review on encapsulation techniques for inorganic phase change materials and the influence on their thermophysical properties. Renew. Sustain. Energy Rev. 2017, 73, 983–999. [Google Scholar] [CrossRef]
- Mohammadi, O.; Shafii, M.B.; Rezaee Shirin-Abadi, A.; Heydarian, R.; Ahmadi, M.H. The impacts of utilizing nano-encapsulated PCM along with RGO nanosheets in a pulsating heat pipe, a comparative study. Int. J. Energy Res. 2021, 45, 19481–19499. [Google Scholar] [CrossRef]
- Jurkowska, M.; Szczygieł, I. Review on properties of microencapsulated phase change materials slurries (mPCMS). Appl. Therm. Eng. 2016, 98, 365–373. [Google Scholar] [CrossRef]
- Yu, Q.; Tchuenbou-Magaia, F.; Al-Duri, B.; Zhang, Z.; Ding, Y.; Li, Y. Thermo-mechanical analysis of microcapsules containing phase change materials for cold storage. Appl. Energy 2018, 211, 1190–1202. [Google Scholar] [CrossRef]
- Sun, Q.; Kang, Y.T. Review on CO2 hydrate formation/dissociation and its cold energy application. Renew. Sustain. Energy Rev. 2016, 62, 478–494. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Chàfer, M.; Mata, É. Comparative analysis of web of science and scopus on the energy efficiency and climate impact of buildings. Energies 2020, 13, 409. [Google Scholar] [CrossRef]
- Roebelen, G.J., Jr. A fusible heat sink concept for extravehicular activity/EVA/thermal control. In Proceedings of the Intersociety Conference on Environmental Systems, 1976; Available online: https://ntrs.nasa.gov/citations/19770036653 (accessed on 1 September 2022).
- Kasza, K.E.; Chen, M.M. Improvement of the performance of solar energy or waste heat utilization systems by using phase-change slurry as an enhanced heat-transfer storage fluid. J. Sol. Energy Eng. 1985, 107, 229–236. [Google Scholar] [CrossRef]
- Darbouret, M.; Cournil, M.; Herri, J.-M. Rheological study of TBAB hydrate slurries as secondary two-phase refrigerants. Int. J. Refrig. 2005, 28, 663–671. [Google Scholar] [CrossRef]
- Delahaye, A.; Fournaison, L.; Marinhas, S.; Chatti, I.; Petitet, J.-P.; Dalmazzone, D.; Fürst, W. Effect of THF on equilibrium pressure and dissociation enthalpy of CO2 hydrates applied to secondary refrigeration. Ind. Eng. Chem. Res. 2006, 45, 391–397. [Google Scholar] [CrossRef]
- Delahaye, A.; Fournaison, L.; Marinhas, S.; Martínez, M.C. Rheological study of CO2 hydrate slurry in a dynamic loop applied to secondary refrigeration. Chem. Eng. Sci. 2008, 63, 3551–3559. [Google Scholar] [CrossRef]
- Dufour, T.; Hoang, H.M.; Oignet, J.; Osswald, V.; Clain, P.; Fournaison, L.; Delahaye, A. Impact of pressure on the dynamic behavior of CO2 hydrate slurry in a stirred tank reactor applied to cold thermal energy storage. Appl. Energy 2017, 204, 641–652. [Google Scholar] [CrossRef]
- Mayoufi, N.; Dalmazzone, D.; Delahaye, A.; Clain, P.; Fournaison, L.; Fürst, W. Experimental data on phase behavior of simple tetrabutylphosphonium bromide (TBPB) and mixed CO2+ TBPB semiclathrate hydrates. J. Chem. Eng. Data 2011, 56, 2987–2993. [Google Scholar] [CrossRef]
- Clain, P.; Delahaye, A.; Fournaison, L.; Mayoufi, N.; Dalmazzone, D.; Fürst, W. Rheological properties of tetra-n-butylphosphonium bromide hydrate slurry flow. Chem. Eng. J. 2012, 193–194, 112–122. [Google Scholar] [CrossRef]
- Lin, W.; Dalmazzone, D.; Fürst, W.; Delahaye, A.; Fournaison, L.; Clain, P. Thermodynamic properties of semiclathrate hydrates formed from the TBAB+ TBPB+ water and CO2+ TBAB+ TBPB+ water systems. Fluid Phase Equilib. 2014, 372, 63–68. [Google Scholar] [CrossRef]
- Shi, X.J.; Zhang, P. A comparative study of different methods for the generation of tetra-n-butyl ammonium bromide clathrate hydrate slurry in a cold storage air-conditioning system. Appl. Energy 2013, 112, 1393–1402. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, Z.W.; Bai, Z.Y.; Ye, J. Rheological and energy transport characteristics of a phase change material slurry. Energy 2016, 106, 63–72. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, Z.W. An overview of fundamental studies and applications of phase change material slurries to secondary loop refrigeration and air conditioning systems. Renew. Sustain. Energy Rev. 2012, 16, 5021–5058. [Google Scholar] [CrossRef]
- Makogon, Y.F. Hydrates of Hydrocarbons; PennWell Publishing Co.: Tulsa, OK, USA, 1997; ISBN 0878147187 9780878147182. [Google Scholar]
- Omran, A.; Nesterenko, N.; Valtchev, V. Ab initio mechanistic insights into the stability, diffusion and storage capacity of sI clathrate hydrate containing hydrogen. Int. J. Hydrog. Energy 2022, 47, 8419–8433. [Google Scholar] [CrossRef]
- Dendy Sloan, E., Jr.; Carolyn, A.; Koh, C.A.K. Clathrate Hydrates of Natural Gases; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Boufares, A.; Provost, E.; Dalmazzone, D.; Osswald, V.; Clain, P.; Delahaye, A.; Fournaison, L. Kinetic study of CO2 hydrates crystallization: Characterization using FTIR/ATR spectroscopy and contribution modeling of equilibrium/non-equilibrium phase-behavior. Chem. Eng. Sci. 2018, 192, 371–379. [Google Scholar] [CrossRef]
- Mohammadi, A.; Jodat, A. Investigation of the kinetics of TBAB+ carbon dioxide semiclathrate hydrate in presence of tween 80 as a cold storage material. J. Mol. Liq. 2019, 293, 111433. [Google Scholar] [CrossRef]
- Matsumoto, K.; Murase, M.; Ehara, K.; Sakamoto, J.; Ueda, J. Investigation on adhesion force of TBAB hydrate to cooling copper surface Analyse de la force d ’ adhérence de l ’ hydrate de TBAB sur une surface en cuivre refroidissante. Int. J. Refrig. 2017, 78, 121–127. [Google Scholar] [CrossRef]
- Voronov, V.P.; Gorodetskii, E.E.; Podnek, V.E.; Grigoriev, B.A. Properties of equilibrium carbon dioxide hydrate in porous medium. Chem. Phys. 2016, 476, 61–68. [Google Scholar] [CrossRef]
- Wang, X.; Dennis, M.; Hou, L. Clathrate hydrate technology for cold storage in air conditioning systems. Renew. Sustain. Energy Rev. 2014, 36, 34–51. [Google Scholar] [CrossRef]
- Karanjkar, P.U.; Ahuja, A.; Zylyftari, G.; Lee, J.W.; F Morris, J. Rheology of cyclopentane hydrate slurry in a model oil-continuous emulsion. Rheol. Acta 2016, 55, 235–243. [Google Scholar] [CrossRef]
- Griffiths, P.W.; Eames, P.C. Performance of chilled ceiling panels using phase change material slurries as the heat transport medium. Appl. Therm. Eng. 2007, 27, 1756–1760. [Google Scholar] [CrossRef]
- Ogawa, T.; Ito, T.; Watanabe, K.; Tahara, K.; Hiraoka, R.; Ochiai, J.; Ohmura, R.; Mori, Y.H. Development of a novel hydrate-based refrigeration system: A preliminary overview. Appl. Therm. Eng. 2006, 26, 2157–2167. [Google Scholar] [CrossRef]
- Xie, N.; Tan, C.; Yang, S.; Liu, Z. Conceptual design and analysis of a novel CO2 hydrate-based refrigeration system with cold energy storage. ACS Sustain. Chem. Eng. 2018, 7, 1502–1511. [Google Scholar] [CrossRef]
- Choi, S.; Park, J.; Kang, Y.T. Experimental investigation on CO2 hydrate formation/dissociation for cold thermal energy harvest and transportation applications. Appl. Energy 2019, 242, 1358–1368. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.H.; Kang, Y.T. Characteristics of CO2 hydrate formation/dissociation in H2O+ THF aqueous solution and estimation of CO2 emission reduction by district cooling application. Energy 2017, 120, 362–373. [Google Scholar] [CrossRef]
- Shao, J.; Darkwa, J.; Kokogiannakis, G. Review of phase change emulsions (PCMEs) and their applications in HVAC systems. Energy Build. 2015, 94, 200–217. [Google Scholar] [CrossRef]
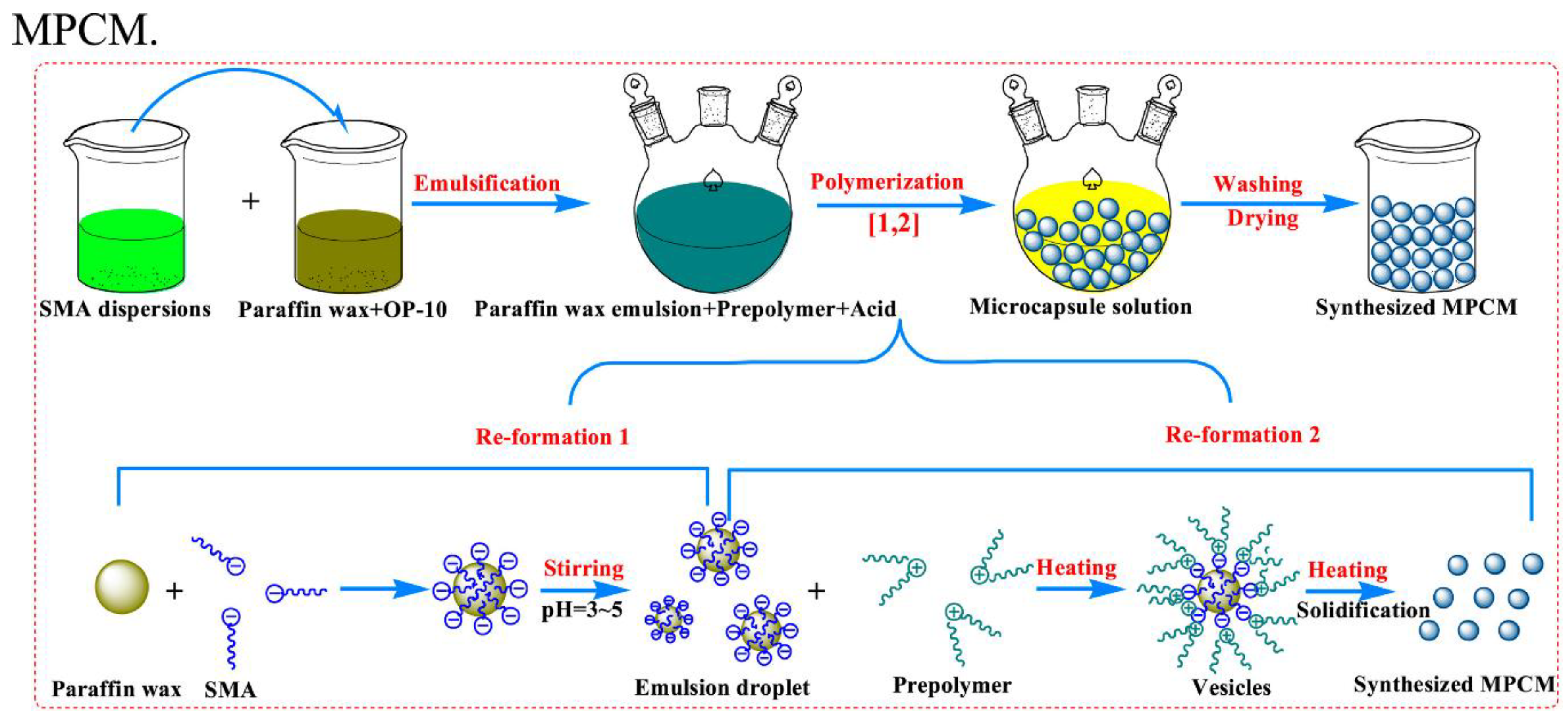
- Giro-Paloma, J.; Martínez, M.; Cabeza, L.F.; Fernández, A.I. Types, methods, techniques, and applications for microencapsulated phase change materials (MPCM): A review. Renew. Sustain. Energy Rev. 2016, 53, 1059–1075. [Google Scholar] [CrossRef]
- Chai, L.; Shaukat, R.; Wang, L.; Wang, H.S. A review on heat transfer and hydrodynamic characteristics of nano/microencapsulated phase change slurry (N/MPCS) in mini/microchannel heat sinks. Appl. Therm. Eng. 2018, 135, 334–349. [Google Scholar] [CrossRef]
- Liu, C.; Rao, Z.; Zhao, J.; Huo, Y.; Li, Y. Review on nanoencapsulated phase change materials: Preparation, characterization and heat transfer enhancement. Nano Energy 2015, 13, 814–826. [Google Scholar] [CrossRef]
- Ghoghaei, M.S.; Mahmoudian, A.; Mohammadi, O.; Shafii, M.B.; Jafari Mosleh, H.; Zandieh, M.; Ahmadi, M.H. A review on the applications of micro-/nano-encapsulated phase change material slurry in heat transfer and thermal storage systems. J. Therm. Anal. Calorim. 2021, 145, 245–268. [Google Scholar] [CrossRef]
- Zhang, G.H.; Zhao, C.Y. Thermal property investigation of aqueous suspensions of microencapsulated phase change material and carbon nanotubes as a novel heat transfer fluid. Renew. Energy 2013, 60, 433–438. [Google Scholar] [CrossRef]
- Wu, W.; Bostanci, H.; Chow, L.C.; Hong, Y.; Wang, C.M.; Su, M.; Kizito, J.P. Heat transfer enhancement of PAO in microchannel heat exchanger using nano-encapsulated phase change indium particles. Int. J. Heat Mass Transf. 2013, 58, 348–355. [Google Scholar] [CrossRef]
- Kong, M.; Alvarado, J.L.; Terrell Jr, W.; Thies, C. Performance characteristics of microencapsulated phase change material slurry in a helically coiled tube. Int. J. Heat Mass Transf. 2016, 101, 901–914. [Google Scholar] [CrossRef]
- Languri, E.M.; Rokni, H.B.; Alvarado, J.; Takabi, B.; Kong, M. Heat transfer analysis of microencapsulated phase change material slurry flow in heated helical coils: A numerical and analytical study. Int. J. Heat Mass Transf. 2018, 118, 872–878. [Google Scholar] [CrossRef]
- Ran, F.; Xu, C.; Chen, Y.; Cong, R.; Fang, G. Numerical flow characteristics of microencapsulated phase change slurry flowing in a helically coiled tube for thermal energy storage. Energy 2021, 223, 120128. [Google Scholar] [CrossRef]
- Hasan, M.I. Numerical investigation of counter flow microchannel heat exchanger with MEPCM suspension. Appl. Therm. Eng. 2011, 31, 1068–1075. [Google Scholar] [CrossRef]
- Ran, F.; Chen, Y.; Cong, R.; Fang, G. Flow and heat transfer characteristics of microencapsulated phase change slurry in thermal energy systems: A review. Renew. Sustain. Energy Rev. 2020, 134, 110101. [Google Scholar] [CrossRef]
- Yang, L.; Liu, S.; Zheng, H. A comprehensive review of hydrodynamic mechanisms and heat transfer characteristics for microencapsulated phase change slurry (MPCS) in circular tube. Renew. Sustain. Energy Rev. 2019, 114, 109312. [Google Scholar] [CrossRef]
- Ali, S.; Mustafa, M. Barriers facing Micro-encapsulated Phase Change Materials Slurry (MPCMS) in Photovoltaic Thermal (PV/T) application. Energy Rep. 2020, 6, 565–570. [Google Scholar] [CrossRef]
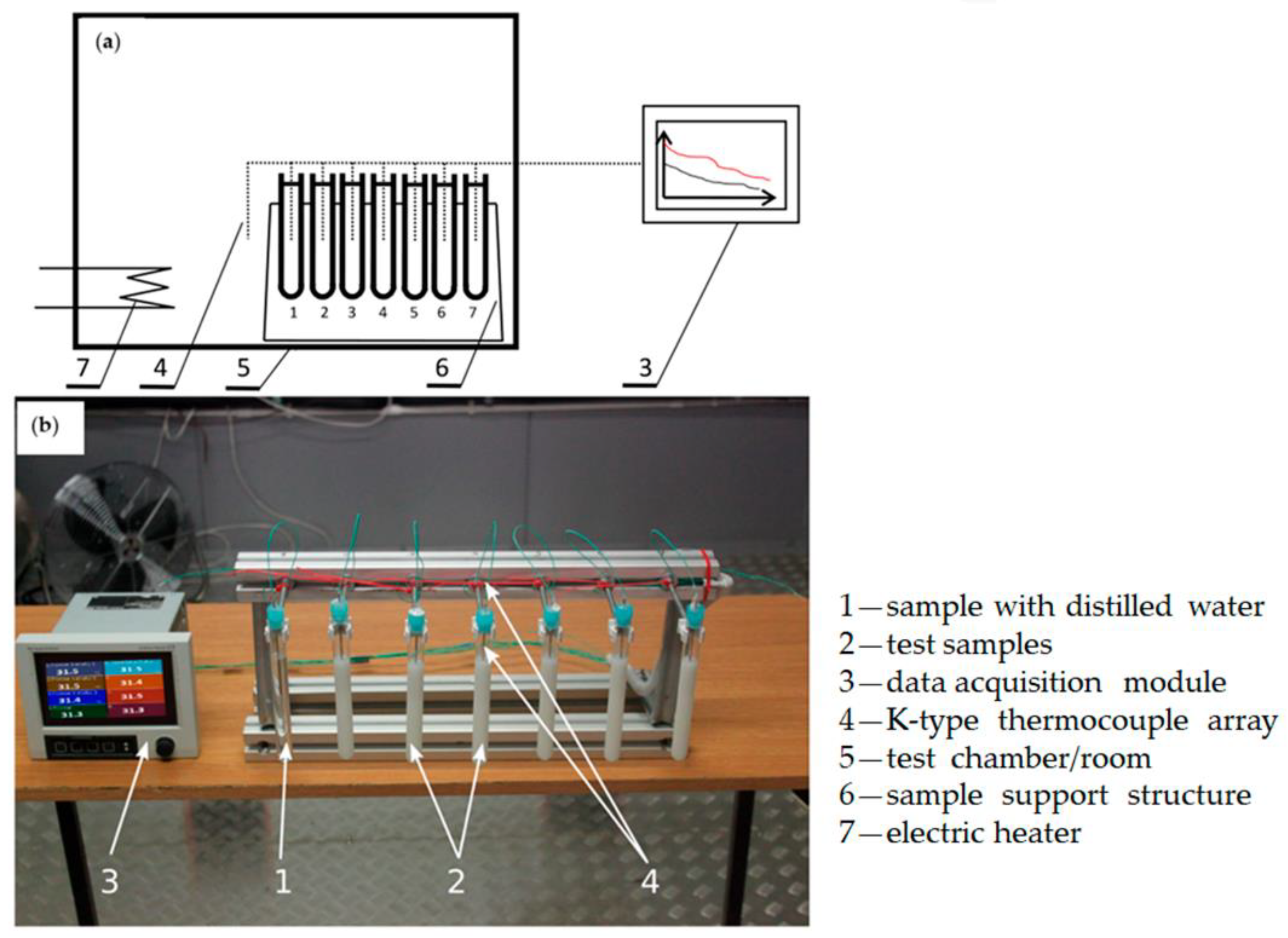
- Dutkowski, K.; Kruzel, M.; Zajaczkowski, B. Determining the heat of fusion and specific heat of microencapsulated phase change material slurry by thermal delay method. Energies 2021, 14, 179. [Google Scholar] [CrossRef]
- Huo, J.; Peng, Z.; Xu, K.; Feng, Q.; Xu, D. Novel micro-encapsulated phase change materials with low melting point slurry: Characterization and cementing application. Energy 2019, 186, 115920. [Google Scholar] [CrossRef]
- Kim, H.; Zheng, J.; Yin, Z.; Kumar, S.; Tee, J.; Seo, Y.; Linga, P. An electrical resistivity-based method for measuring semi-clathrate hydrate formation kinetics: Application for cold storage and transport. Appl. Energy 2022, 308, 118397. [Google Scholar] [CrossRef]
- Youssef, Z.; Fournaison, L.; Delahaye, A.; Pons, M. Management of vapor release in secondary refrigeration processes based on hydrates involving CO2 as guest molecule. Int. J. Refrig. 2019, 98, 202–210. [Google Scholar] [CrossRef]
- Clain, P.; Ndoye, F.T.; Delahaye, A.; Fournaison, L.; Lin, W.; Dalmazzone, D. Particle size distribution of TBPB hydrates by focused beam reflectance measurement (FBRM) for secondary refrigeration application. Int. J. Refrig. 2015, 50, 19–31. [Google Scholar] [CrossRef]
- Ma, Z.W.; Zhang, P.; Wang, R.Z.; Furui, S.; Xi, G.N. Forced flow and convective melting heat transfer of clathrate hydrate slurry in tubes. Int. J. Heat Mass Transf. 2010, 53, 3745–3757. [Google Scholar] [CrossRef]
- Bbosa, B.; DelleCase, E.; Volk, M.; Ozbayoglu, E. Development of a mixer-viscometer for studying rheological behavior of settling and non-settling slurries. J. Pet. Explor. Prod. Technol. 2017, 7, 511–520. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, W.; Lang, C.; Liu, R.; Song, Y.; Li, Y. Viscosity investigation on metastable hydrate suspension in oil-dominated systems. Chem. Eng. Sci. 2021, 238, 116608. [Google Scholar] [CrossRef]
- Moradpour, H.; Chapoy, A.; Tohidi, B. Bimodal model for predicting the emulsion-hydrate mixture viscosity in high water cut systems. Fuel 2011, 90, 3343–3351. [Google Scholar] [CrossRef]
- Rehman, Z.; Seong, K.; Lee, S.; Song, M.H. Experimental study on the rheological behavior of tetrafluoroethane (R-134a) hydrate slurry. Chem. Eng. Commun. 2018, 205, 822–832. [Google Scholar] [CrossRef]
- Delahaye, A.; Fournaison, L.; Jerbi, S.; Mayoufi, N. Rheological properties of CO2 hydrate slurry flow in the presence of additives. Ind. Eng. Chem. Res. 2011, 50, 8344–8353. [Google Scholar] [CrossRef]
- Clain, P.; Boufares, A.; Benmesbah, F.; Zennoune, A.; Osswald, V.; Hoang, H.M.; Dalmazzone, D.; Delahaye, A.; Fournaison, L. Improvement of CO2 hydrate slurry transportation in flow loop: Effect of anti-agglomerant additive on rheological properties. In Proceedings of the Refrigeration Science and Technology, Montreal, QC, Canada, 24–30 August 2019; Volume 2019-August, pp. 1627–1634. [Google Scholar]
- Sandoval, G.A.B.; Ozorio, M.C.; Naccache, M.F.; De Souza Mendes, P.R.; Sum, A.K.; Valim, L.; Teixeira, A. A Rheological Study of Parameters That Influence the Formation of Cyclopentane Hydrates. Energy Fuels 2021, 35, 18467–18477. [Google Scholar] [CrossRef]
- Youssef, Z.; Delahaye, A.; Huang, L.; Trinquet, F.; Fournaison, L.; Pollerberg, C.; Doetsch, C. State of the art on phase change material slurries. Energy Convers. Manag. 2013, 65, 120–132. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, P. A review of thermo-fluidic performance and application of shellless phase change slurry: Part 1—Preparations, properties and applications. Energy 2019, 189, 116246. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, P. A review of thermo-fluidic performance and application of shellless phase change slurry: Part 2—Flow and heat transfer characteristics. Energy 2020, 192, 116602. [Google Scholar] [CrossRef]
- Wang, Z.; Li, F.; Fan, T.; Xiong, W.; Yang, B. Research on the application of gas hydrate in cool storage air conditioning. Procedia Eng. 2015, 121, 1118–1125. [Google Scholar] [CrossRef]
- Sun, Q.; Kim, S.; Kang, Y.T. Study on dissociation characteristics of CO2 hydrate with THF for cooling application. Appl. Energy 2017, 190, 249–256. [Google Scholar] [CrossRef]
- Prah, B.; Lee, W.J.; Yun, R. In-tube convective heat transfer characteristics of a CO2-hydrate mixture. J. Mech. Sci. Technol. 2017, 31, 4035–4042. [Google Scholar] [CrossRef]
- Oignet, J.; Hoang, H.M.; Osswald, V.; Delahaye, A.; Fournaison, L.; Haberschill, P. Experimental study of convective heat transfer coefficients of CO2 hydrate slurries in a secondary refrigeration loop. Appl. Therm. Eng. 2017, 118, 630–637. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Lang, X.; Fan, S. Performance analysis of hydrate-based refrigeration system. Energy Convers. Manag. 2017, 146, 43–51. [Google Scholar] [CrossRef]
- Matsuura, R.; Watanabe, K.; Yamauchi, Y.; Sato, H.; Chen, L.-J.; Ohmura, R. Thermodynamic analysis of hydrate-based refrigeration cycle. Energy 2021, 220, 119652. [Google Scholar] [CrossRef]
- Hua, N.; Lu, T.; Yang, L.; Mckeown, A.; Yu, Z.; Xu, B.; Sciacovelli, A.; Ding, Y.; Li, Y. Thermodynamic analysis and economic assessment of a carbon dioxide hydrate-based vapor compression refrigeration system using load shifting controls in summer. Energy Convers. Manag. 2022, 251, 114901. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, X.; Peng, Z.; Zheng, Y.; Huo, J.; Liu, H. Preparation of low hydration heat cement slurry with micro-encapsulated thermal control material. Energy 2019, 187, 116000. [Google Scholar] [CrossRef]
- Doshi, S.; Kashyap, G.; Tiwari, N. Thermo-hydraulic and entropy generation investigation of nano-encapsulated phase change material (NEPCM) slurry in hybrid wavy microchannel. Int. J. Numer. Methods Heat Fluid Flow 2022, 32, 3161–3190. [Google Scholar] [CrossRef]
- Ashagre, T.B.; Rakshit, D. Study on flow and heat transfer characteristics of Encapsulated Phase Change Material (EPCM) slurry in Double-Pipe Heat Exchanger. J. Energy Storage 2022, 46, 103931. [Google Scholar] [CrossRef]
- Zhang, J.J.; Yang, C.H.; Jin, Z.G.; Ma, S.X.; Zhang, J.S.; Pang, X.M. Experimental study of jet impingement heat transfer with microencapsulated phase change material slurry. Appl. Therm. Eng. 2021, 188, 116588. [Google Scholar] [CrossRef]
- Mohaghegh, M.R.; Tasnim, S.H.; Aliabadi, A.A.; Mahmud, S. Jet Impingement Cooling Enhanced with Nano-Encapsulated PCM. Energies 2022, 15, 1034. [Google Scholar] [CrossRef]
- Galazutdinova, Y.; Al-Hallaj, S.; Grágeda, M.; Ushak, S. Development of the inorganic composite phase change materials for passive thermal management of Li-ion batteries: Material characterization. Int. J. Energy Res. 2020, 44, 2011–2022. [Google Scholar] [CrossRef]
- Bai, F.; Chen, M.; Song, W.; Yu, Q.; Li, Y.; Feng, Z.; Ding, Y. Investigation of thermal management for lithium-ion pouch battery module based on phase change slurry and mini channel cooling plate. Energy 2019, 167, 561–574. [Google Scholar] [CrossRef]
- Teggar, M.; Arıcı, M.; Mert, M.S.; Mousavi Ajarostaghi, S.S.; Niyas, H.; Tunçbilek, E.; Ismail, K.A.R.; Younsi, Z.; Benhouia, A.T.; Mezaache, E.H. A comprehensive review of micro/nano enhanced phase change materials. J. Therm. Anal. Calorim. 2022, 147, 3989–4016. [Google Scholar] [CrossRef]
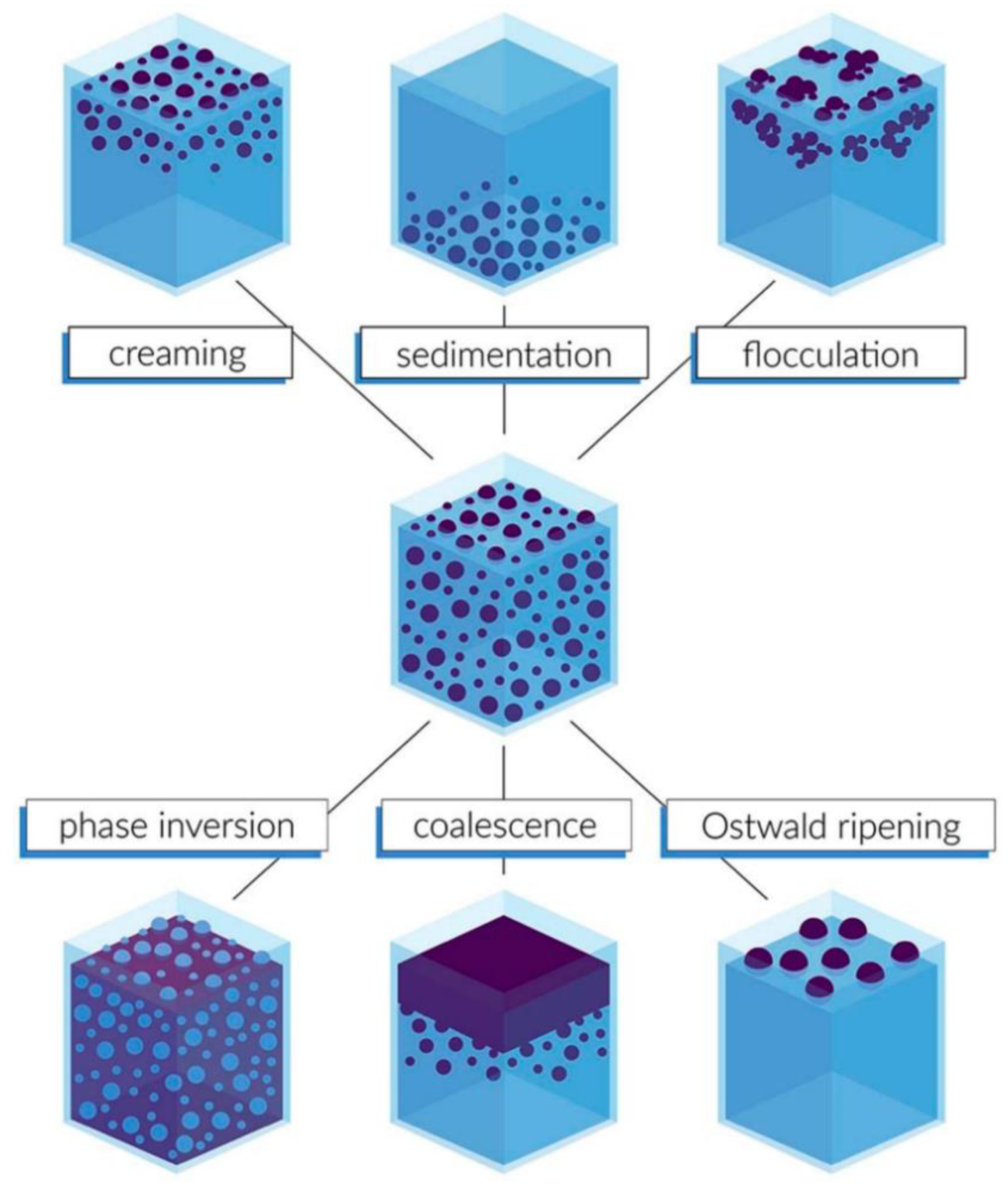
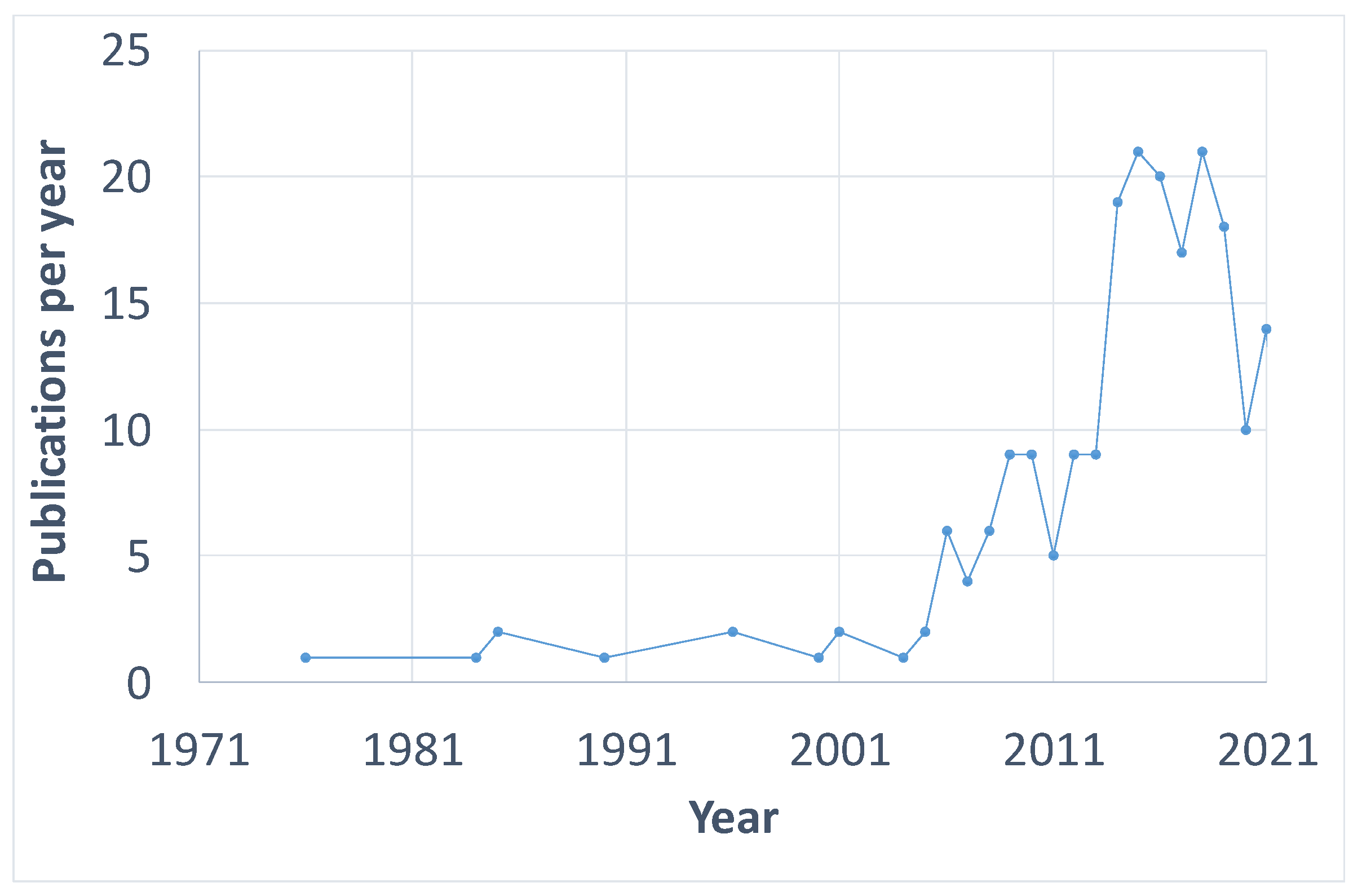


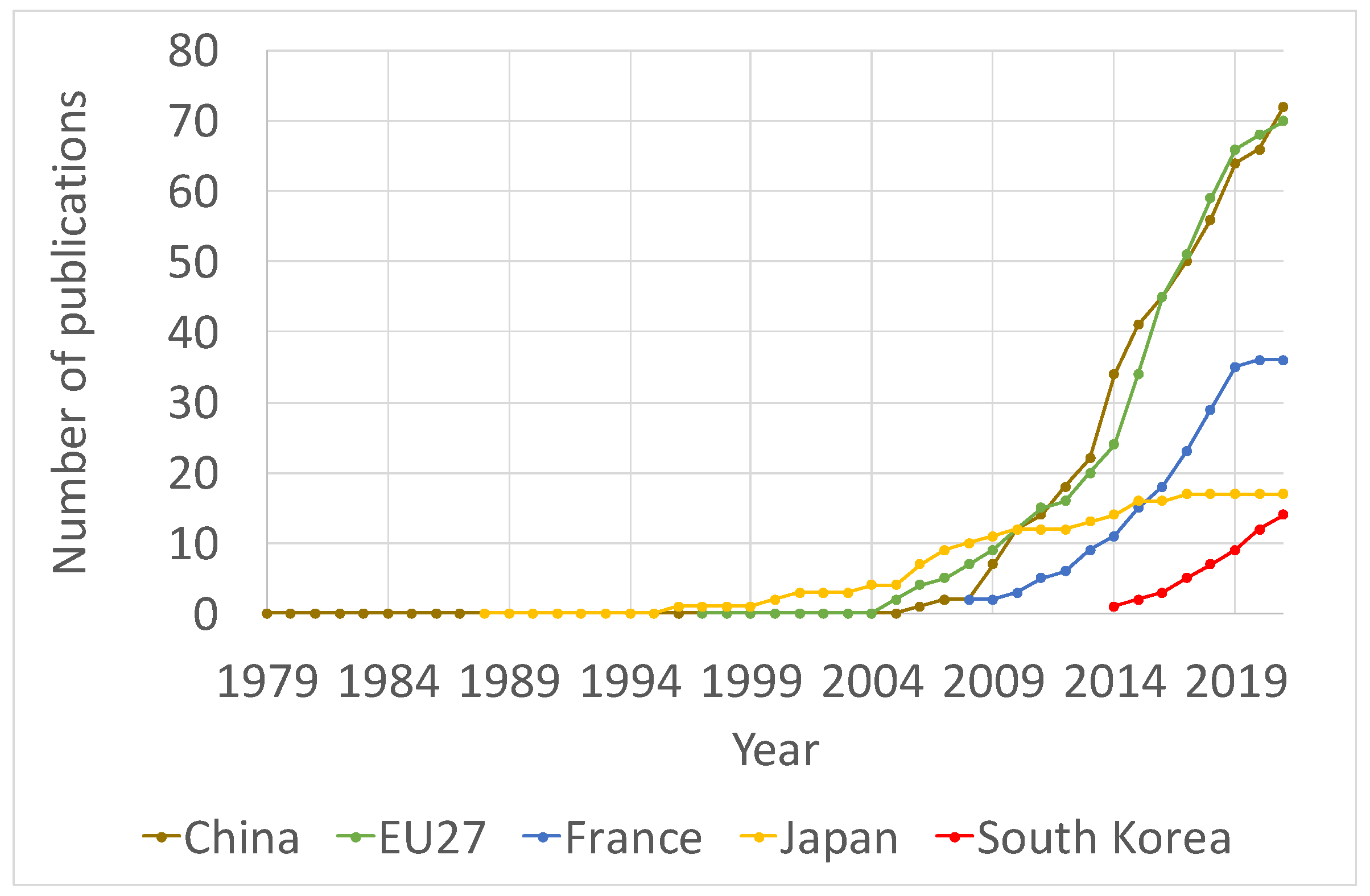
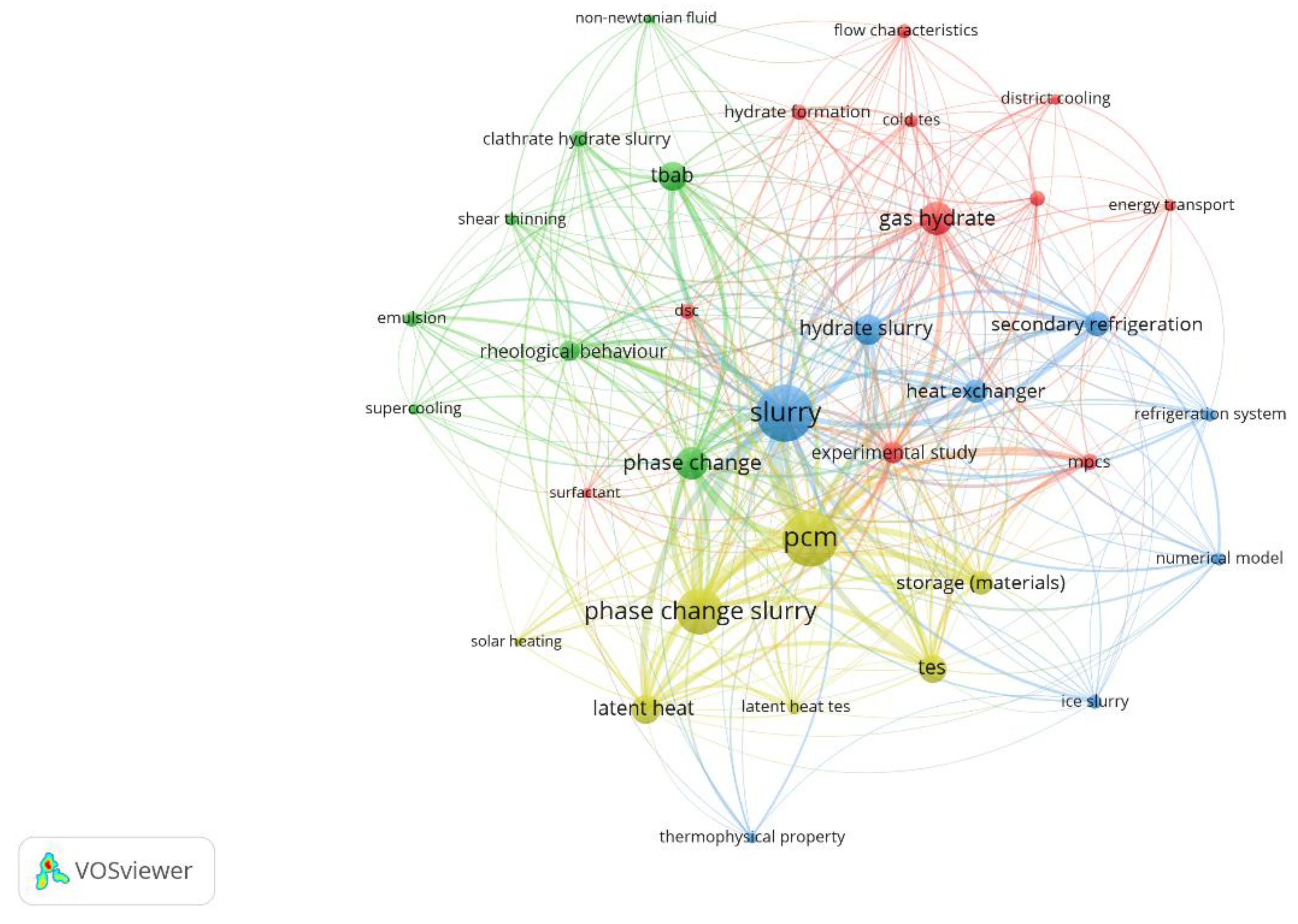
| Author | Institution | Country | Number of Publications in this Query | Total Number of Publications | H-Index |
|---|---|---|---|---|---|
| L. Fournaison | Universite Paris-Saclay | France | 35 | 80 | 27 |
| A. Delahaye | Universite Paris-Saclay | France | 34 | 80 | 26 |
| P. Zhang | Shanghai Jiao Tong University | China | 26 | 300 | 40 |
| H.M. Hoang | Universite Paris-Saclay | France | 14 | 60 | 18 |
| P. Clain, | Pôle Universitaire Léonard De Vinci | France | 13 | 19 | 8 |
| D. Dalmazzone | ENSTA Paris | France | 11 | 53 | 25 |
| V. Osswald | Universite Paris-Saclay | France | 11 | 15 | 6 |
| Z.W. Ma | Durham University | UK | 10 | 49 | 20 |
| Z.P. Feng | University of Chinese Academy of Sciences | China | 9 | 100 | 18 |
| J. Oignet. | Génie des Procédés Frigorifiques pour la Sécurité alimentaire et l’Environnement, | France | 9 | 9 | 5 |
| X.J. Shi | Shanghai Jiao Tong University | China | 9 | 16 | 10 |
| Density (kg/m3) | Specific Heat (kJ/kg·K) | Thermal Conductivity (W/m·K) | |
| TBAB solution | |||
| ω = 0.10 | 1010.22 | 4.03 | 0.521 |
| ω = 0.20 | 1019.86 | - | 0.472 |
| ω = 0.30 | 1030.82 | - | 0.418 |
| ω = 0.40 | 1040.98 | - | 0.351 |
| TBAB-CHS | |||
| ω = 0.10 | 1009.83 | 3.97 | 0.502 |
| ω = 0.20 | 1009.67 | 3.98 | 0.507 |
| ω = 0.30 | 1009.51 | 3.99 | 0.523 |
| ω = 0.40 | 1009.34 | 3.99 | 0.577 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borri, E.; Hua, N.; Sciacovelli, A.; Wu, D.; Ding, Y.; Li, Y.; Brancato, V.; Zhang, Y.; Frazzica, A.; Li, W.; et al. Phase Change Slurries for Cooling and Storage: An Overview of Research Trends and Gaps. Energies 2022, 15, 6873. https://doi.org/10.3390/en15196873
Borri E, Hua N, Sciacovelli A, Wu D, Ding Y, Li Y, Brancato V, Zhang Y, Frazzica A, Li W, et al. Phase Change Slurries for Cooling and Storage: An Overview of Research Trends and Gaps. Energies. 2022; 15(19):6873. https://doi.org/10.3390/en15196873
Chicago/Turabian StyleBorri, Emiliano, Nan Hua, Adriano Sciacovelli, Dawei Wu, Yulong Ding, Yongliang Li, Vincenza Brancato, Yannan Zhang, Andrea Frazzica, Wenguang Li, and et al. 2022. "Phase Change Slurries for Cooling and Storage: An Overview of Research Trends and Gaps" Energies 15, no. 19: 6873. https://doi.org/10.3390/en15196873









