Study on CO2–Water Co-Injection Miscible Characteristics in Low-Permeability Near-Critical Volatile Oil Reservoir
Abstract
:1. Introduction
2. Experimental Section
2.1. Experimental Sample
2.2. Experimental Device
2.3. Experimental Procedure
2.3.1. Phase Behavior Characteristic Experiment
- (1)
- The steps of phase equilibrium experiments for single-phase near-critical volatile oil consisted of:
- (2)
- The steps of two-phase equilibrium experiment for CO2-near-critical volatile oil consisted of the following:
- (3)
- The steps of three-phase equilibrium experiment for CO2-near-critical volatile oil-formation water consisted of the following:
2.3.2. Miscible Characteristics Experiment
- (1)
- Slim-tube experiment for CO2 injection on near-critical volatile oil consisted of the following:
- (2)
- Slim-tube experiment for CO2–water co-injection on near-critical volatile oil. The experimental procedure of near-critical volatile oil CO2–water co-injection refers to the experimental procedure of near-critical volatile oil CO2 injection slim-tube, except that the CO2 injected in ③ became CO2–water co-injection.
3. Simulation
3.1. Simulation Study on Phase Behavior Characteristics of CO2 Injection
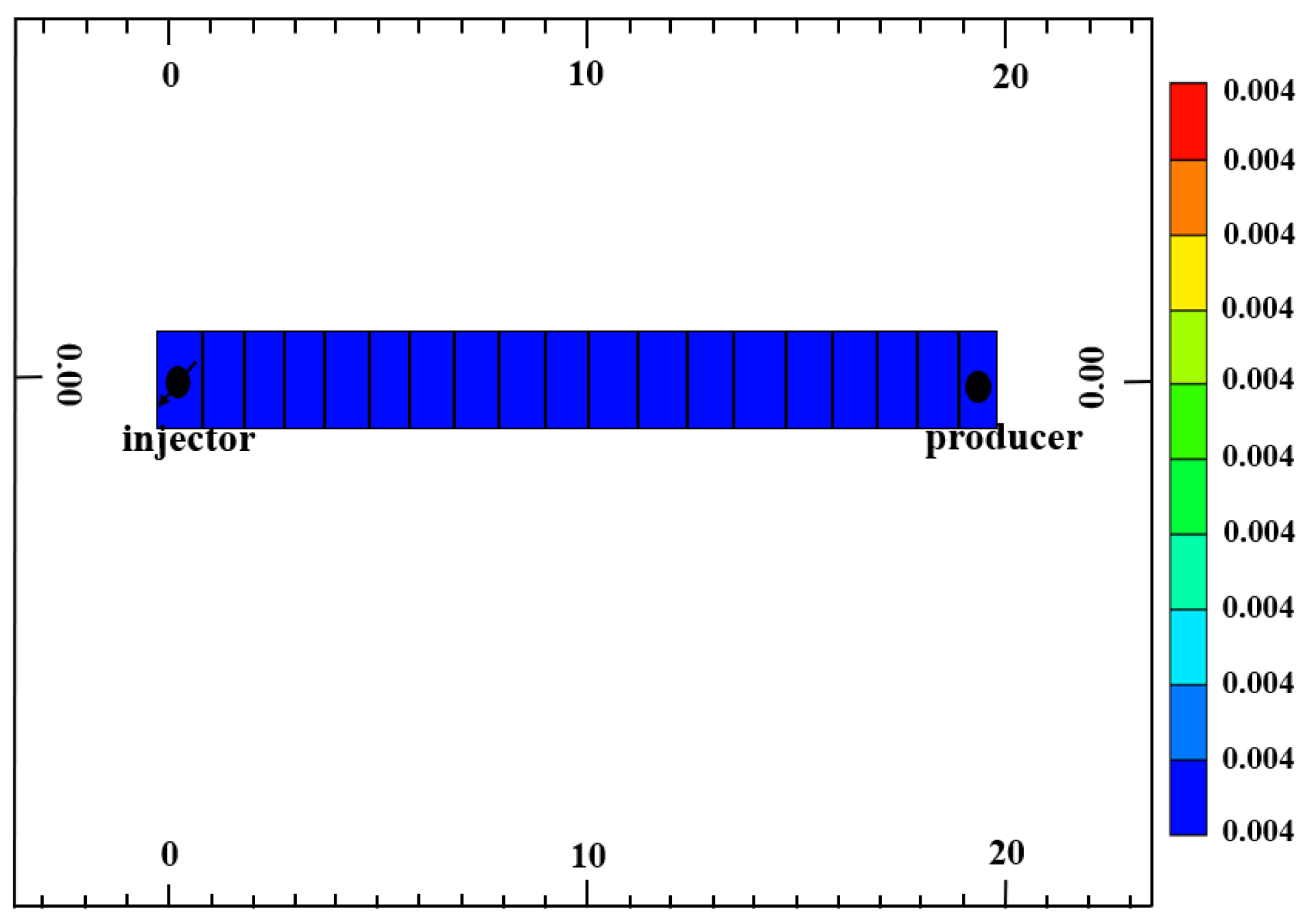
3.2. Pure CO2 Injection and CO2–Water Co-Injection Slim-Tube Simulation Study
4. Result and Analysis
4.1. Study of Complex Phase Behavior Characteristics of CO2 Injection
4.1.1. Result Analysis for Single-Phase Behavior Characterization on Near-Critical Volatile Oil
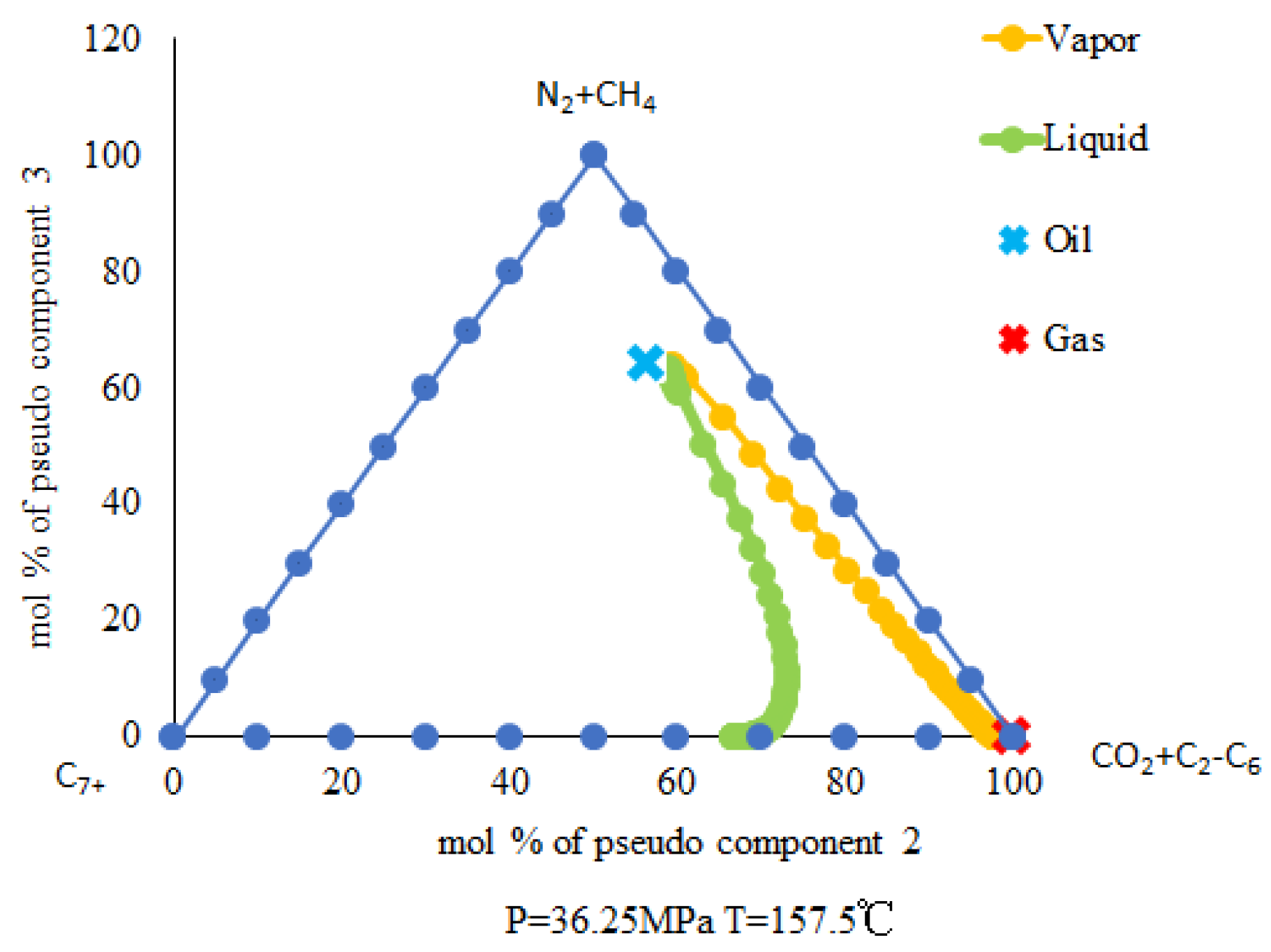
4.1.2. The Two-Phase Behavior Characteristic Result Analysis for CO2-Near-Critical Volatile Oil
4.1.3. The Three-Phase Behavior Characteristic Result Analysis for CO2-Near-Critical Volatile Oil-Formation Water
4.2. Study on the Miscible Characteristics for CO2 Injection and CO2–Water Co-Injection
4.2.1. Slim-Tube Experiment Result Analysis with Pure CO2 Injection and CO2–Water Co-Injection
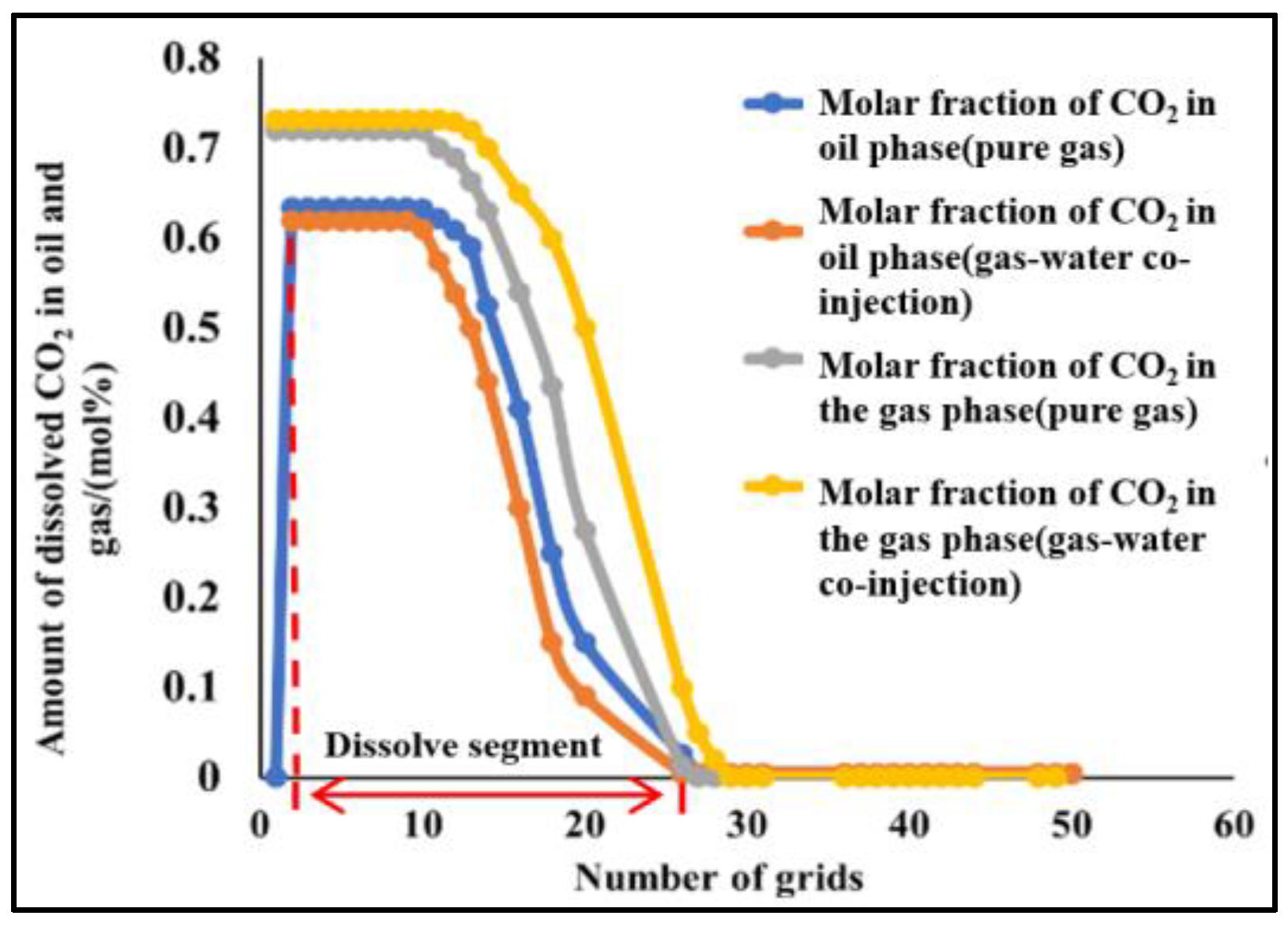
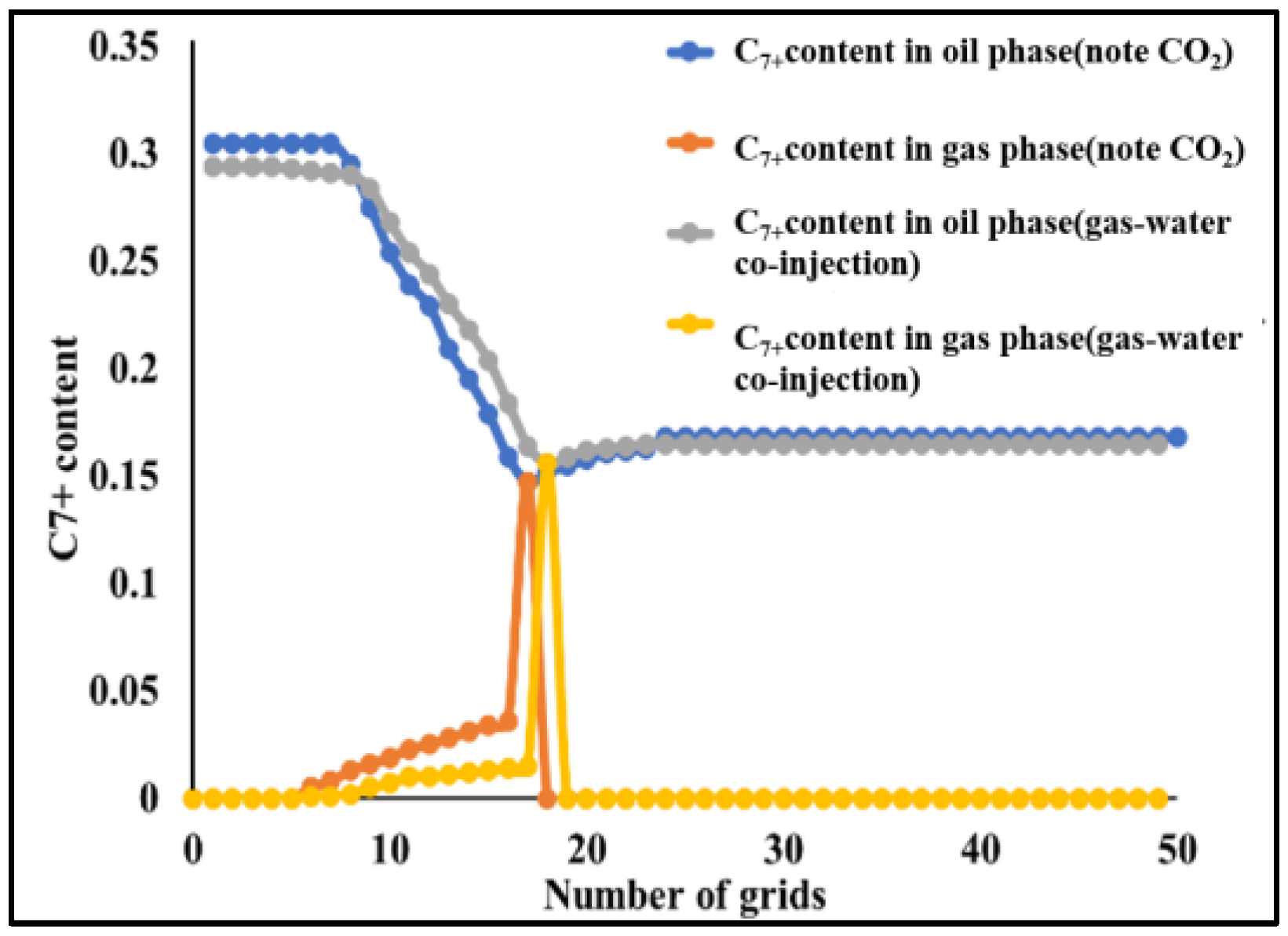
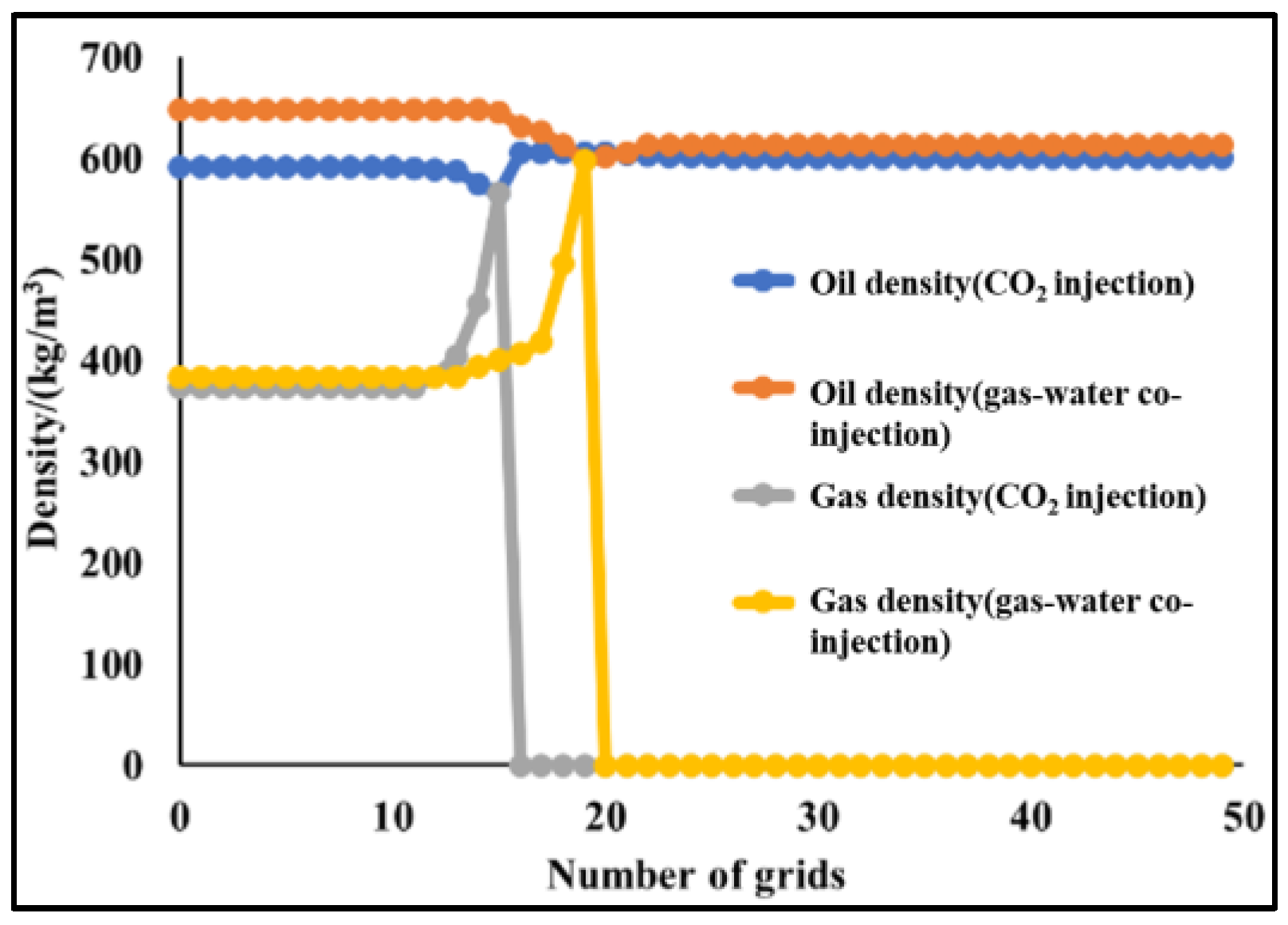
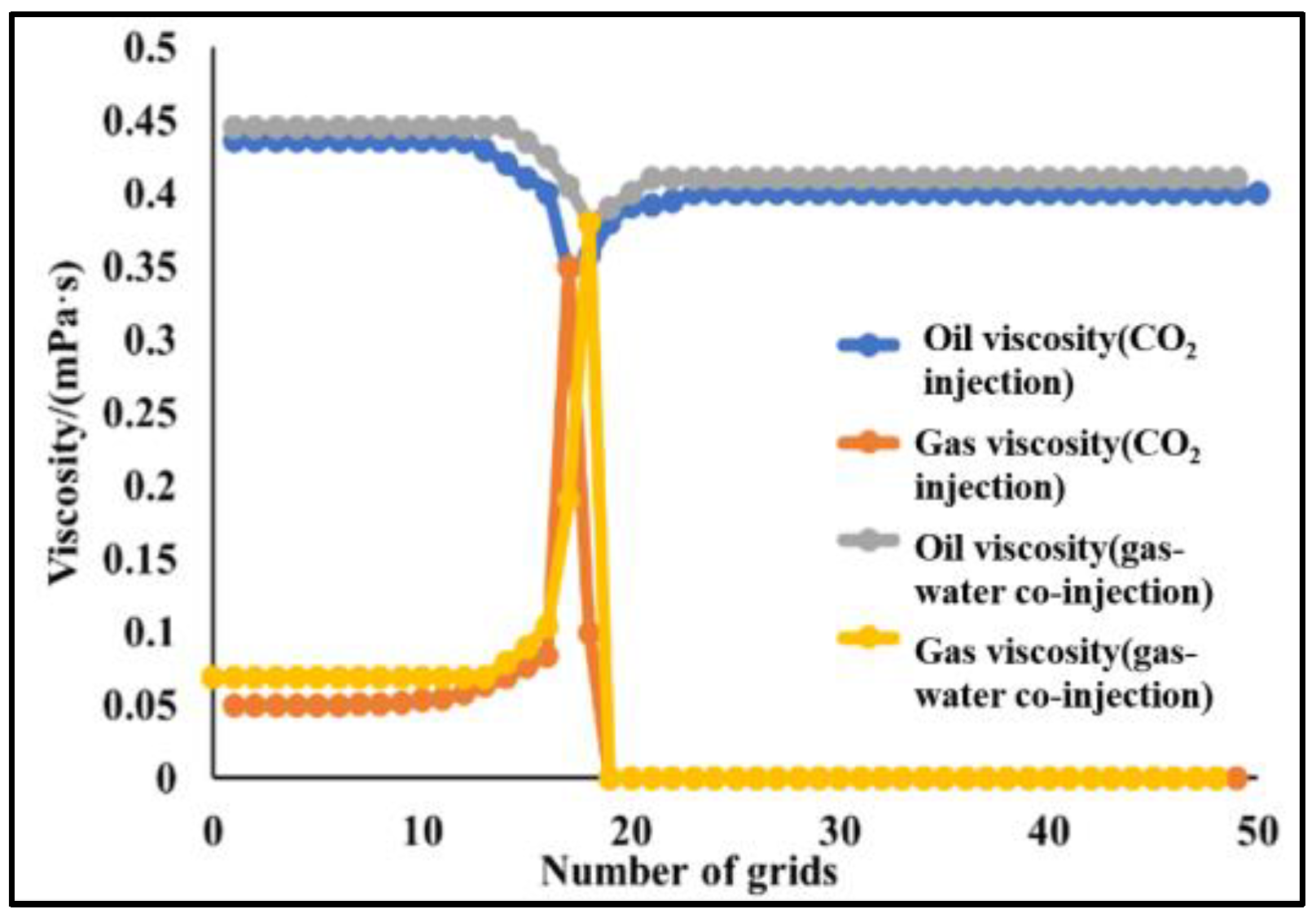
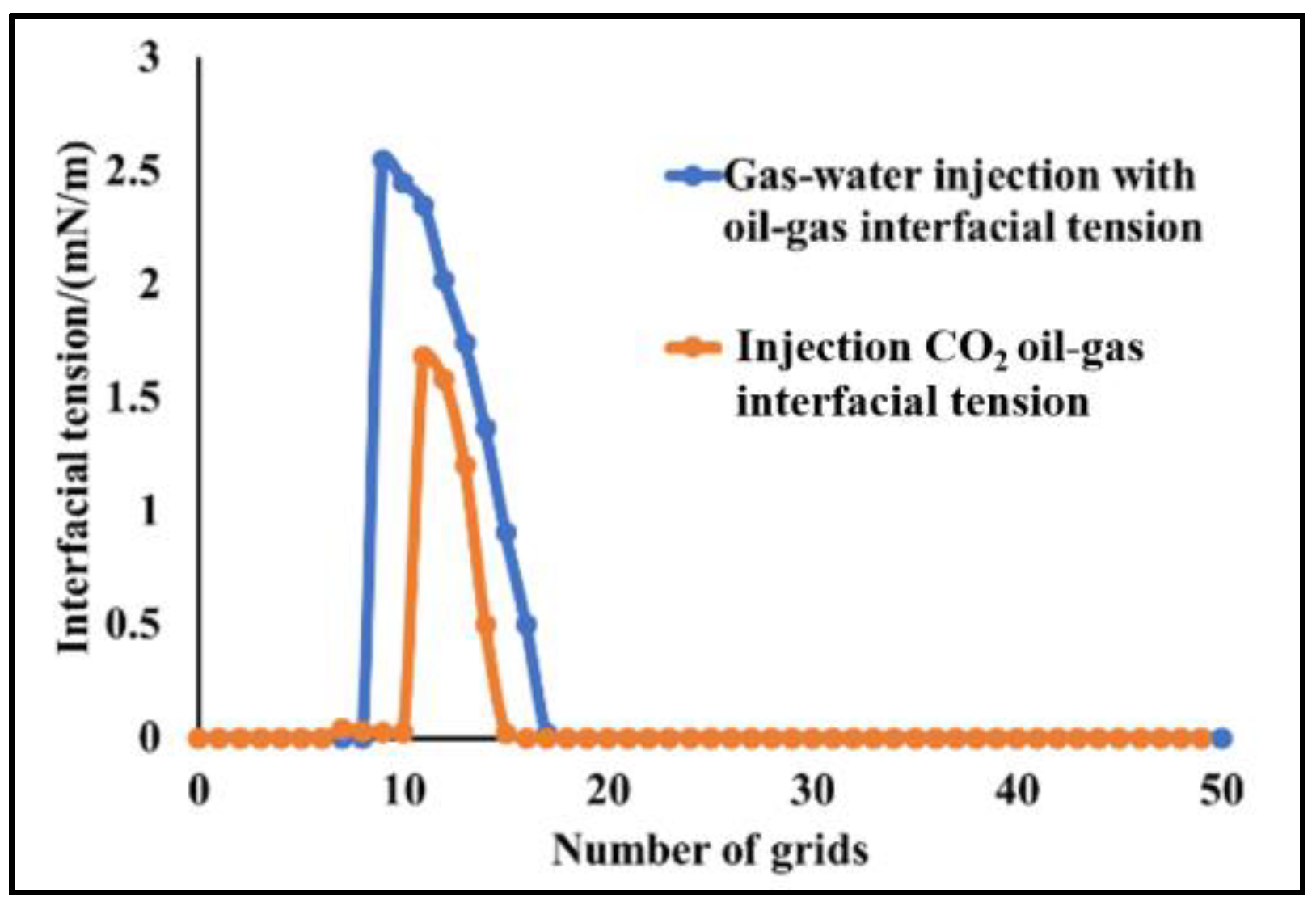
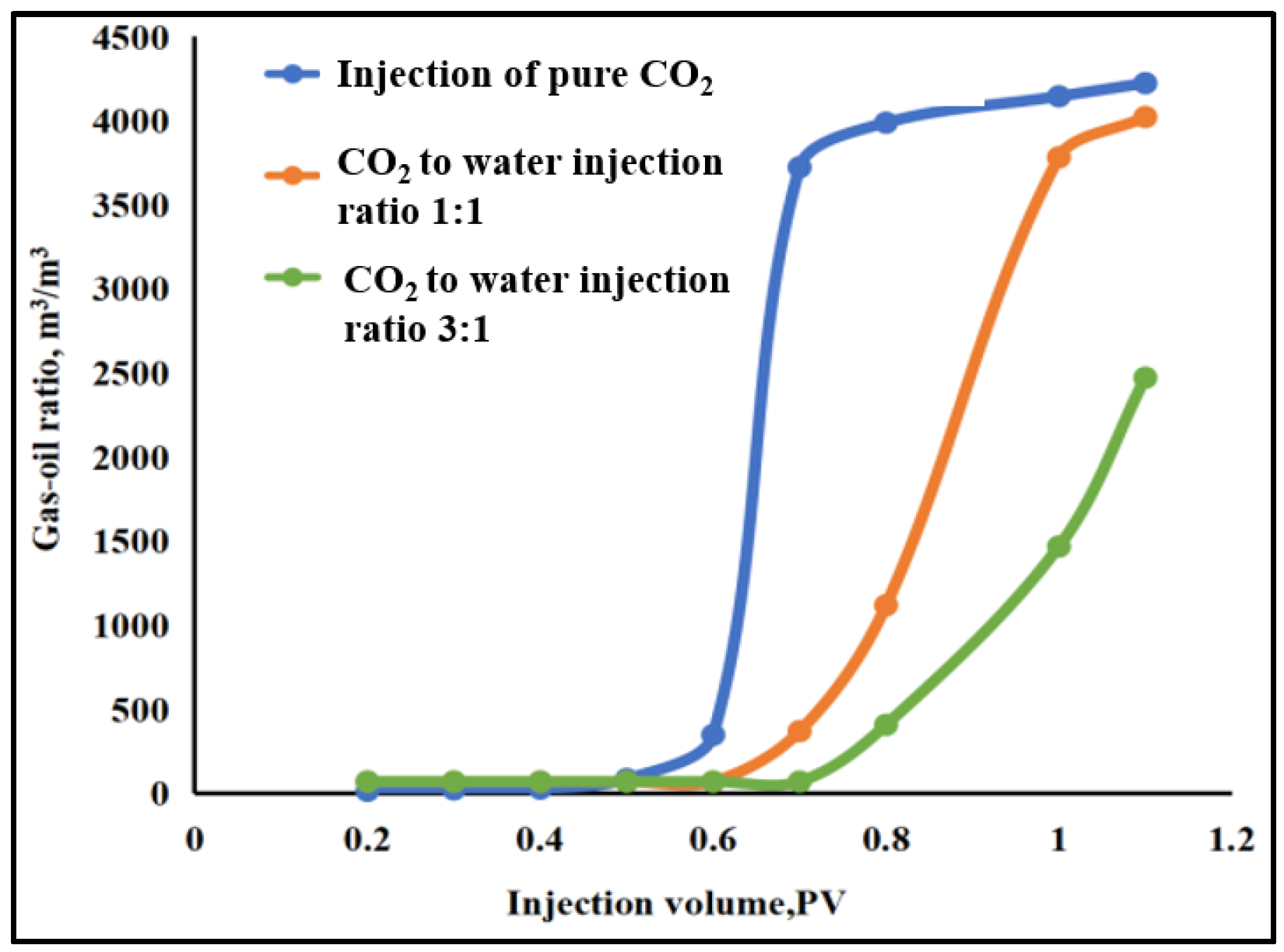
4.2.2. Slim-Tube Simulation Result Analysis with Pure CO2 Injection and CO2–Water Co-Injection
4.2.3. Study on the Miscible Characteristics of Pure CO2 Injection and CO2–Water Co-Injection
5. Conclusions
- (1)
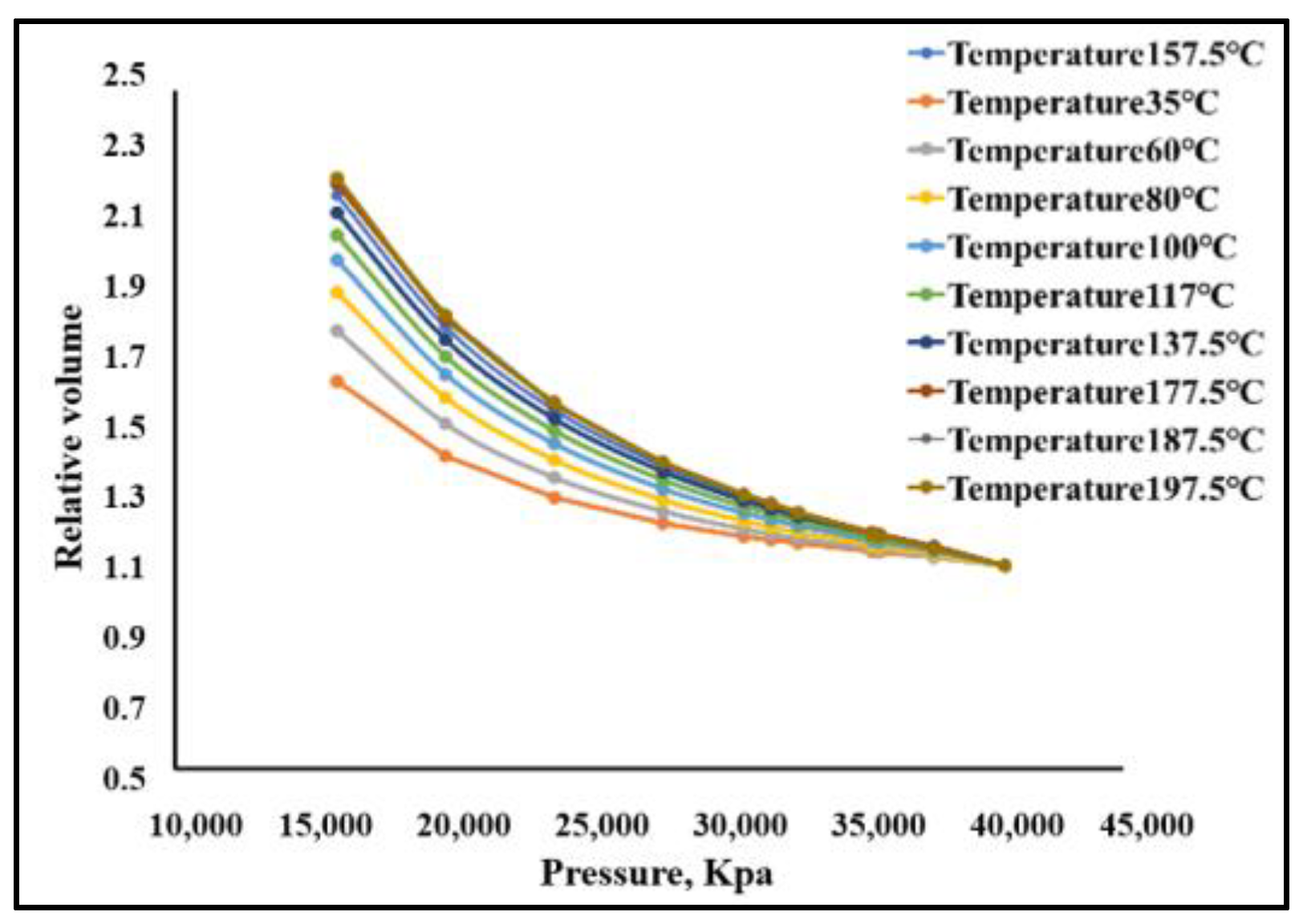
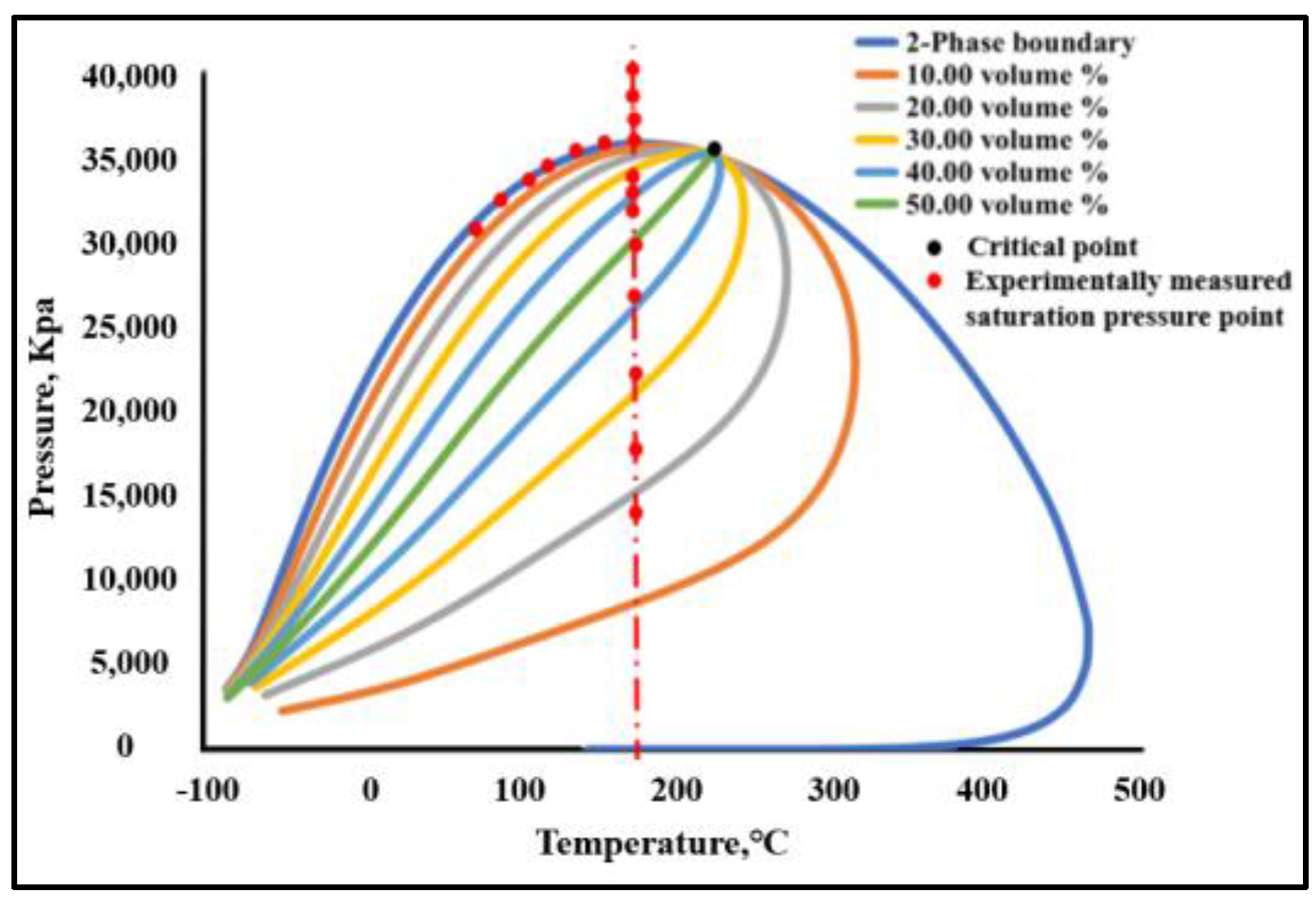
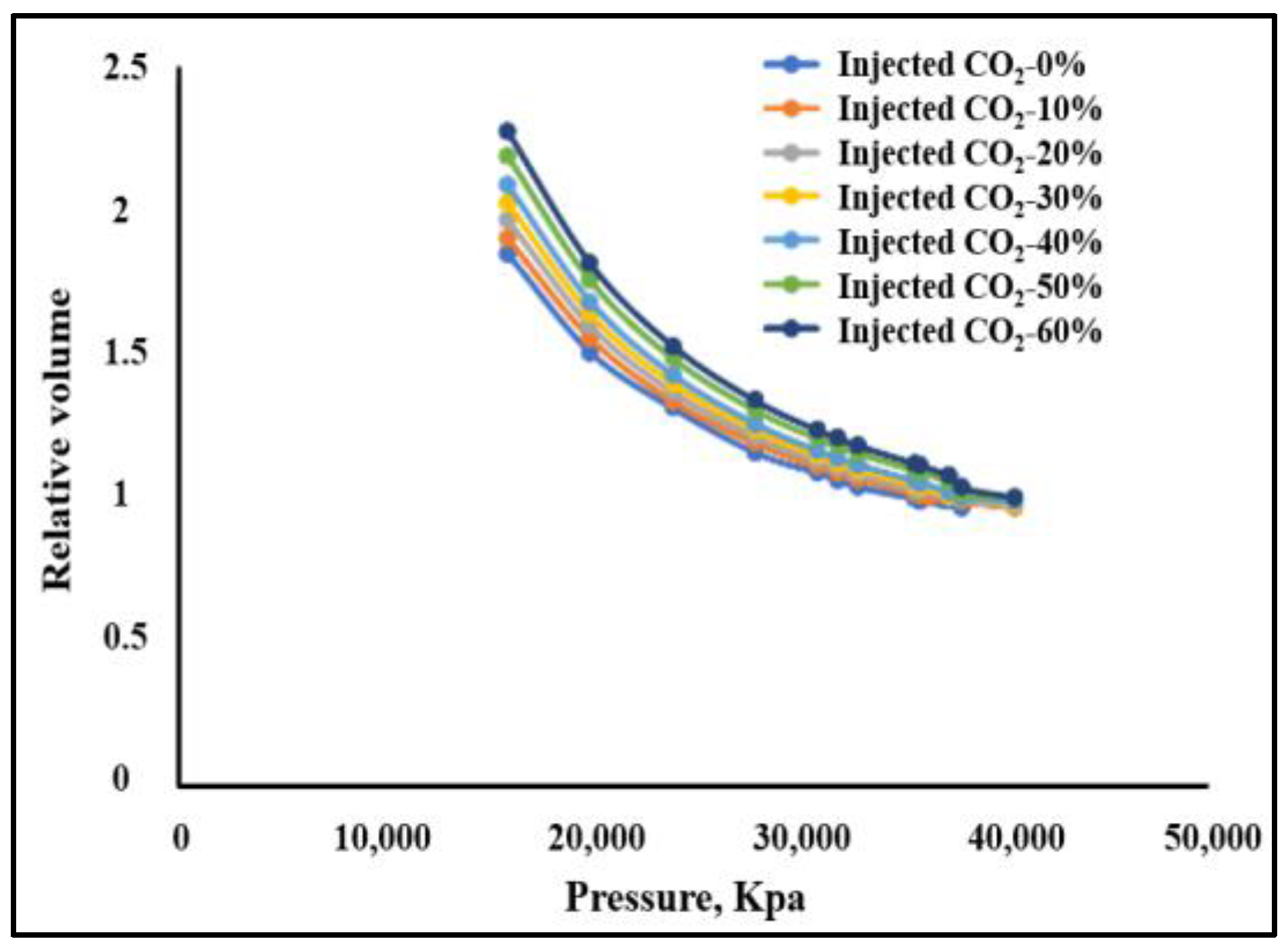
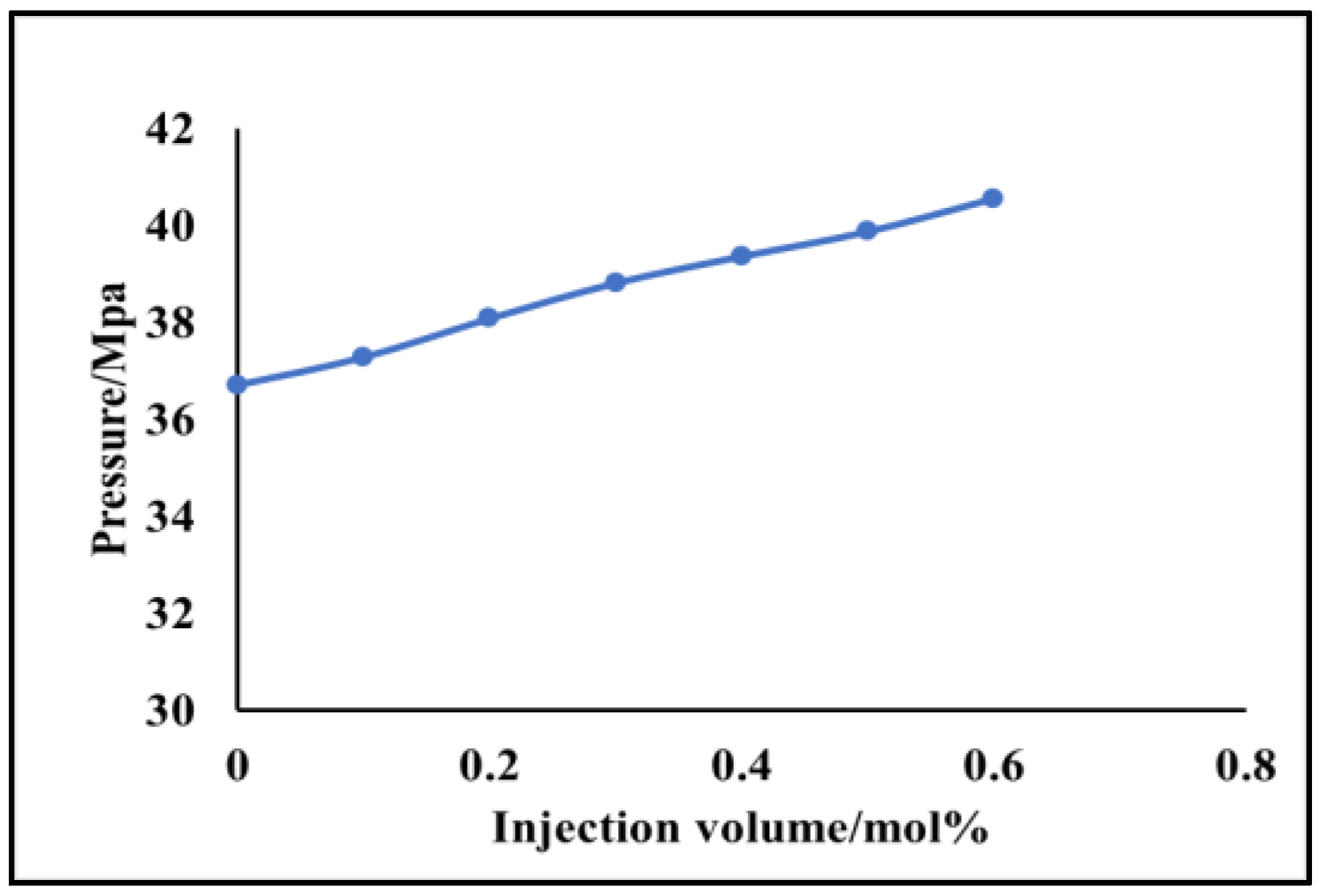
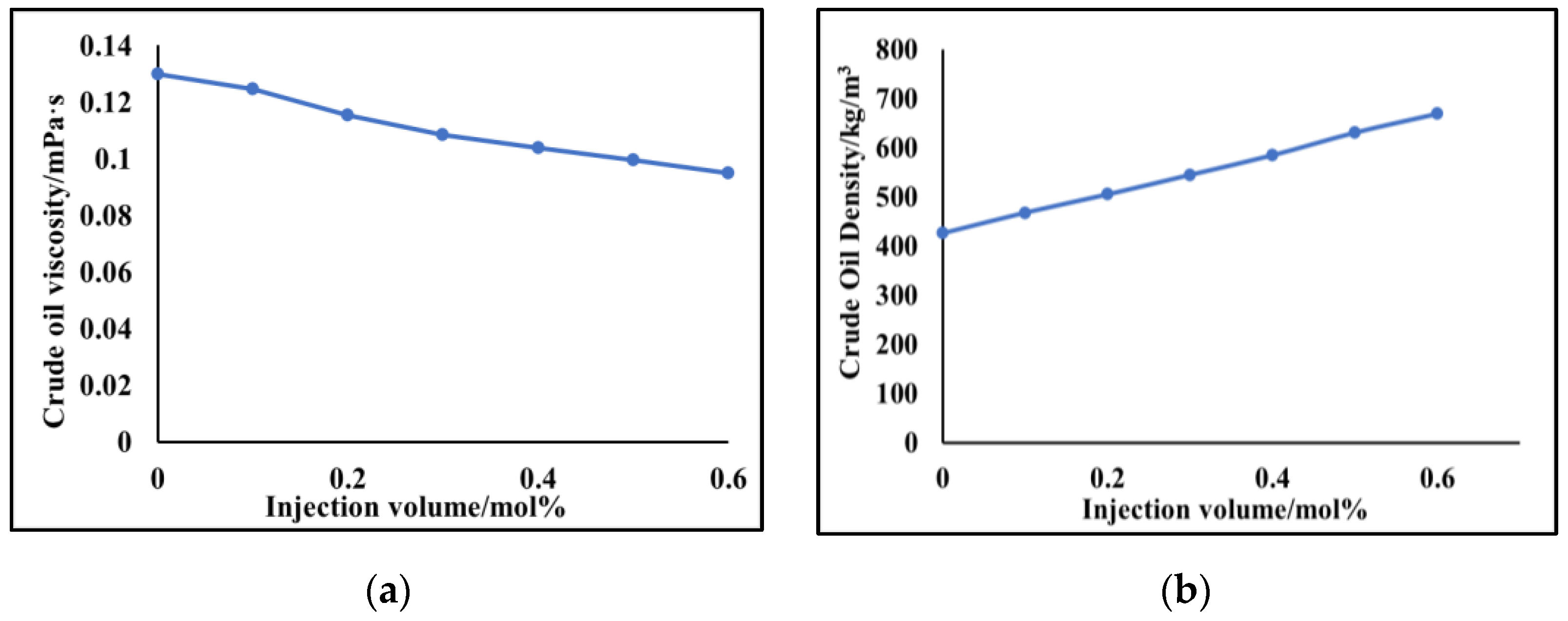
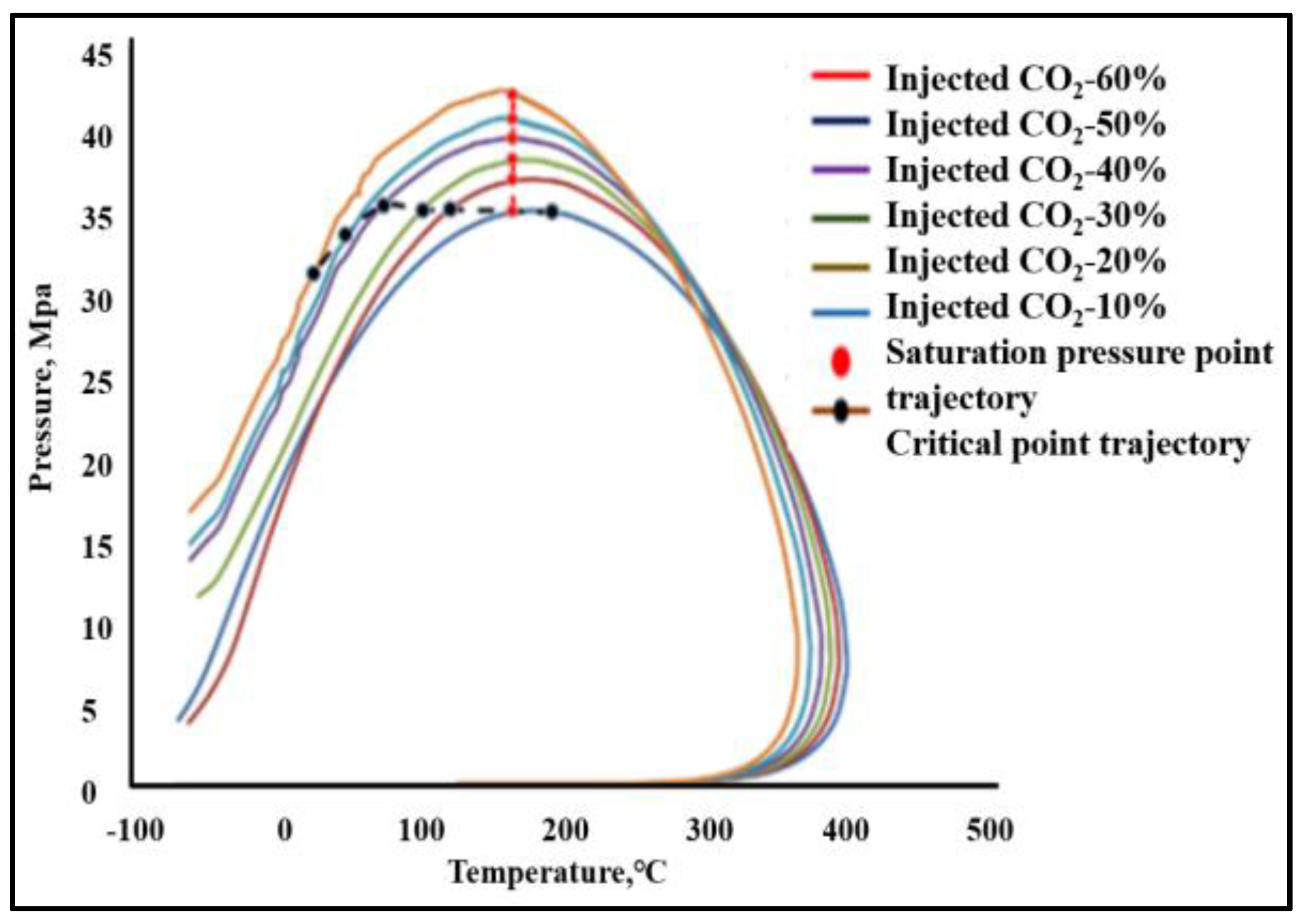
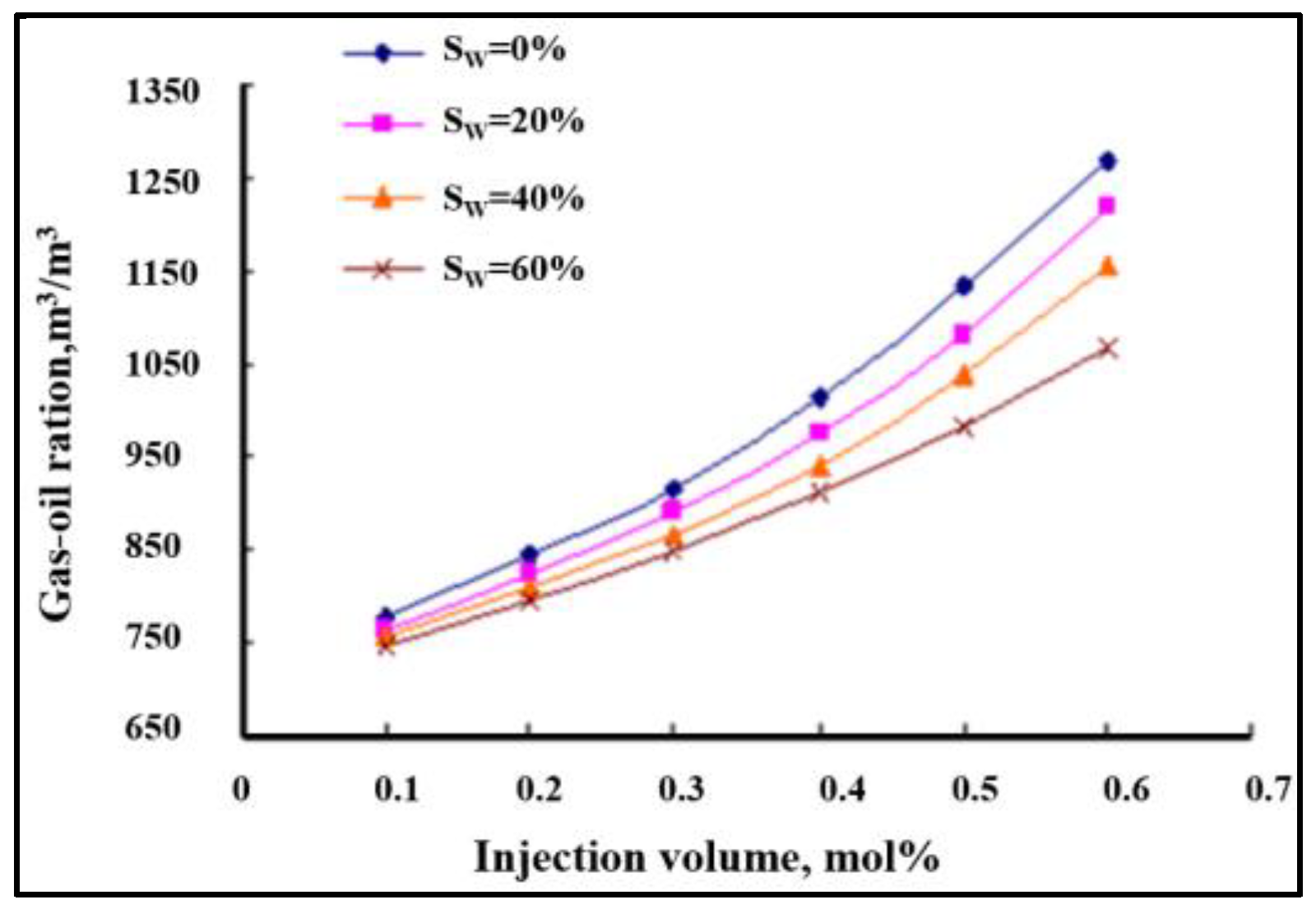
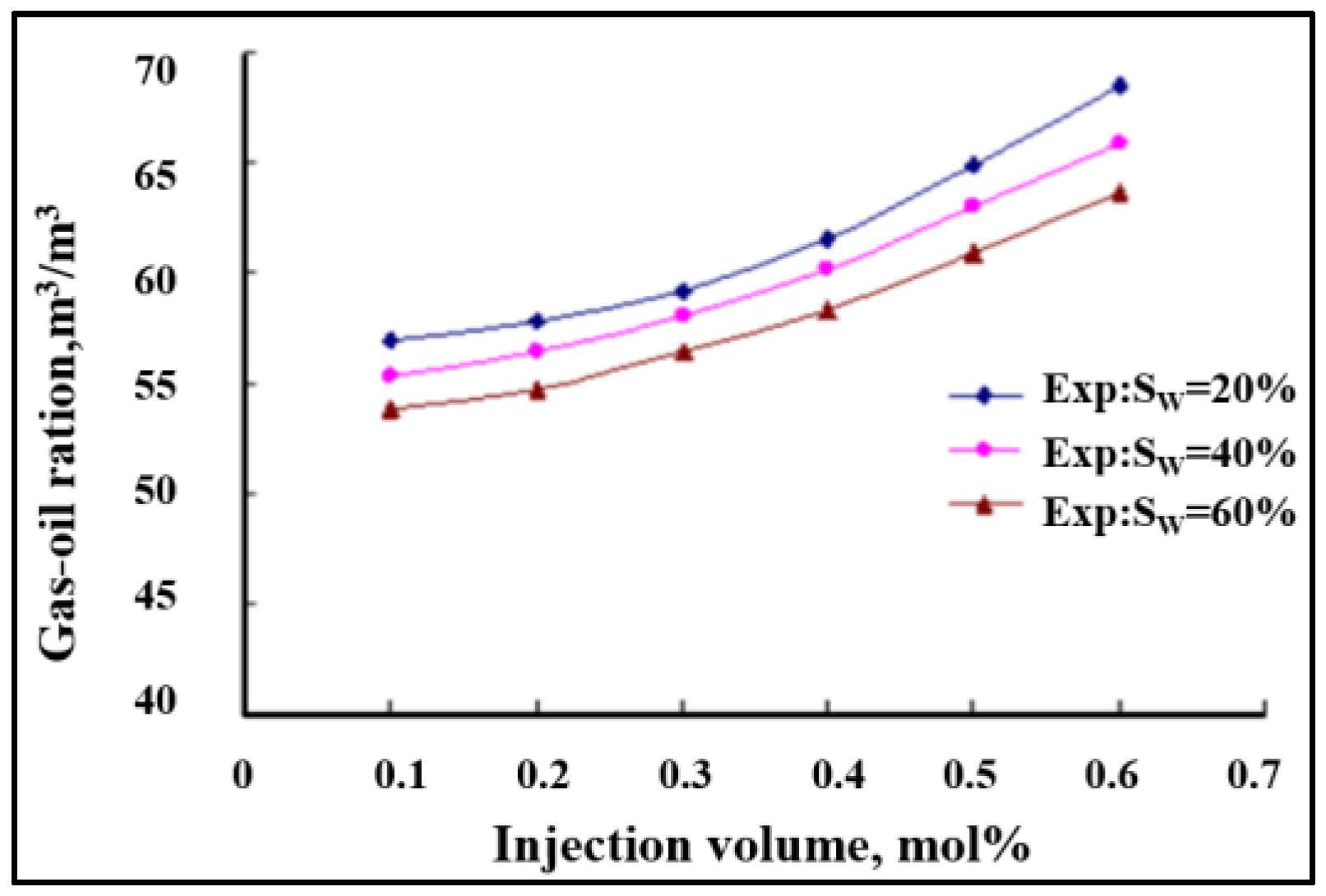
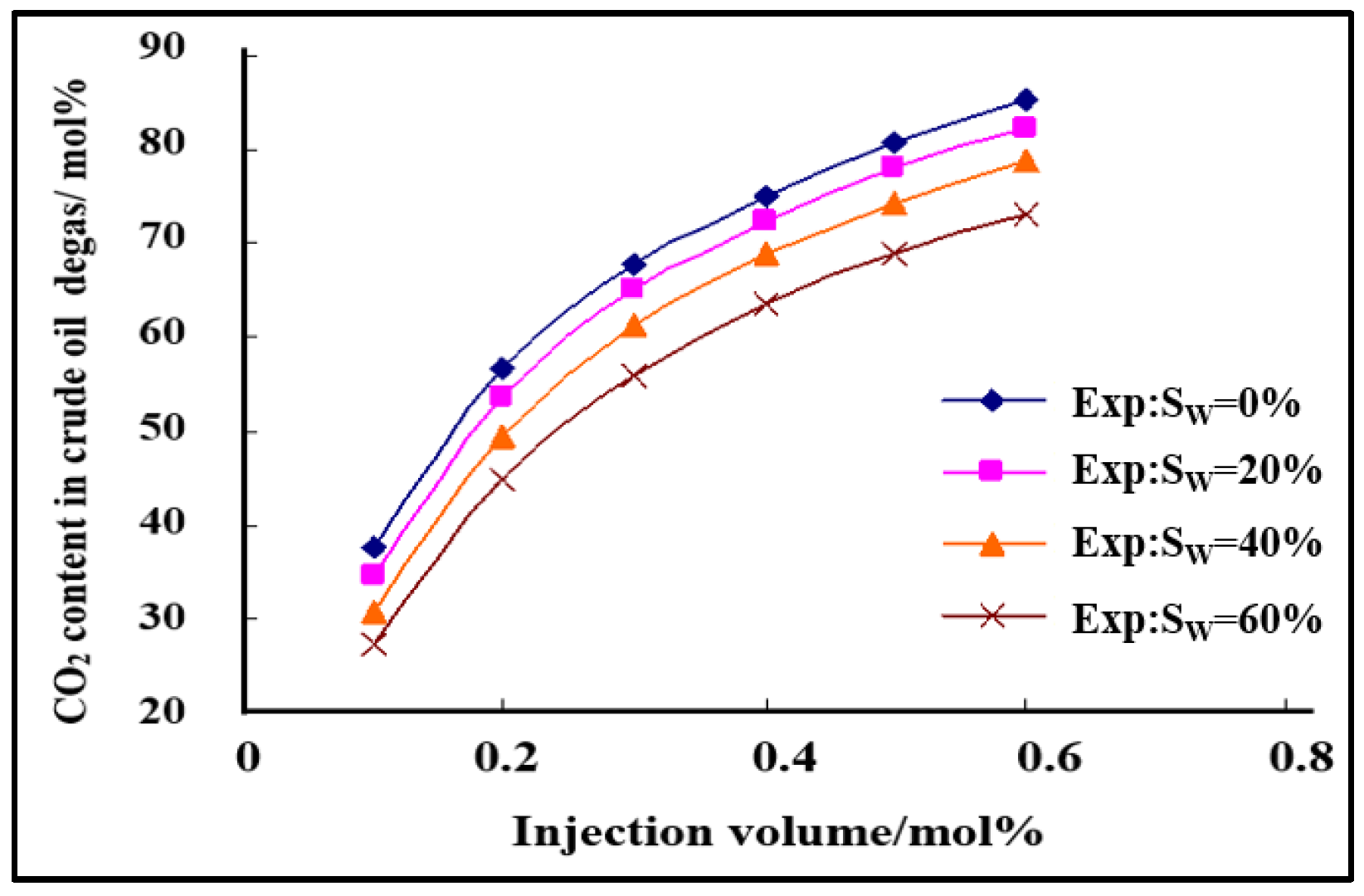
- The phase equilibrium experimental and simulation results for CO2-near-critical volatile oil and CO2-near-critical volatile oil-formation water show that with the continuous injection of CO2, the saturation pressure of the system gradually increases, the density increases and the viscosity decreases; when the injected CO2 amount reaches 10 mol%~20 mol%, the phase inversion occurs and the near-critical volatile oil system is transformed into a condensate gas system; as the water saturation increases, the gas–oil ratio, the gas–water ratio and the CO2 content in the degassing decrease.
- (2)
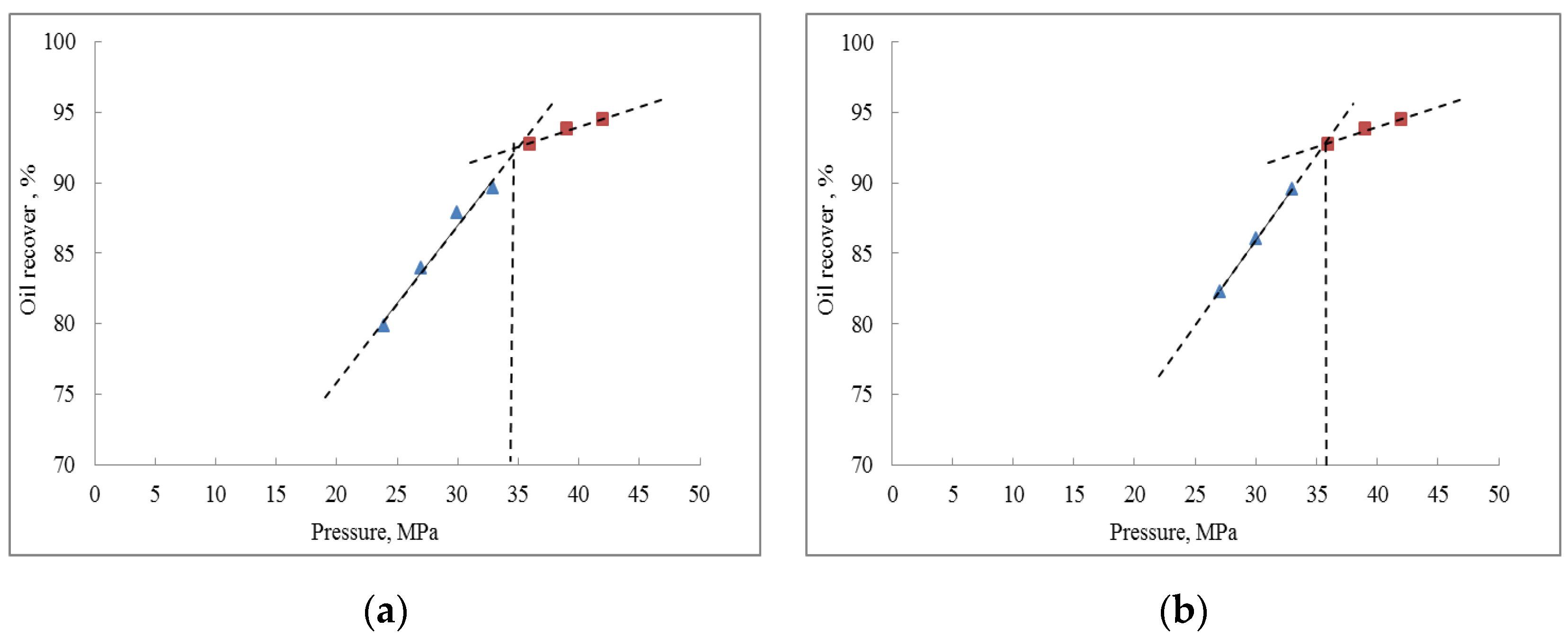
- The results of the slim-tube experiment show that when the injection pressure is greater than 36 MPa, both pure CO2 injection and CO2–water co-injection reach a miscible-phase state; the recovery rate of crude oil increases with the increase in injection pressure, and after reaching a certain pressure point, the increase of in recovery rate will slow down; the minimum miscible pressure under CO2–water co-injection is higher than that under pure CO2 injection. This is due to the fact that when CO2–water is co-injected, part of the CO2 is dissolved in water, resulting in less CO2 in contact with the crude oil and a higher miscible pressure.
- (3)
- The results of slim-tube simulation show that the degree of crude oil recovery under CO2–water co-injection is higher than that of pure CO2 injection, and the transition period of CO2 dissolution in oil and gas is shorter and the gas breakthrough time is later under CO2–water co-injection, which effectively increases the sweep volume and improves the degree of crude oil recovery. When CO2–water is injected together, the higher the proportion of water, the later the gas–oil ratio rises, the later the CO2 breaks through, and the higher the degree of crude oil recovery. It shows that the gas–water co-injection increases the sweep area and effectively inhibits the occurrence of gas scramble, thus improving the degree of crude oil recovery.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| 𝑉 | molar volume (dm3 mol−1) |
| 𝑏 | covolume parameter (dm3 mol−1) |
| 𝑎 | attraction parameter (kPa dm3 mol−2) |
| 𝑅 | universal gas constant (kPa dm3 mol−1 K−1) |
| 𝑃 | pressure (MPa) |
| 𝑇 | absolute temperature (K) |
| 𝑇𝑐𝑖 | critical temperature of component 𝑖 (K) |
| 𝑇𝑟𝑖 | reduced temperature of component 𝑖 |
| 𝜔𝑖 | acentric factor of component 𝑖 |
| 𝑃𝑐𝑖 | critical pressure of component 𝑖 (MPa) |
| 𝑥𝑖 | mole fraction of component 𝑖 |
| 𝑥𝑗 | mole fraction of component 𝑗 |
| 𝑘𝑖𝑗 | binary interaction parameter for 𝑖-𝑗 contacts |
References
- Lv, C.; Lun, Z.; Wang, J.; Zhao, S.; Wang, X.; Xu, X. Dynamic miscible characteristics of CO2 drive in volatile reservoirs. Oil Gas Geol. Recovery 2020, 27, 92. [Google Scholar]
- Lu, K.; Su, C.; Cheng, C. An improved method for predicting crude oil recovery from volatile reservoirs. Pet. Explor. Dev. 2021, 48, 95–1003. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, L.; Ouyang, C.; Wang, Z.; Zhu, H. Optimization of alternating gas-water drive parameters in low-permeability reservoirs. Contemp. Chem. Ind. 2018, 47, 1380–1386. [Google Scholar]
- Li, L. Dynamic production performance in a natural depletion and evaluation on remaining production potential of Hua 3 near-critical volatile reservoir. Petrochem. Ind. Appl. 2015, 34, 4–10. [Google Scholar]
- Chen, M.; Ren, L.; Li, M.; Yue, Y. Study on seepage law of Chang 7 ultra-low permeability reservoir in Ordos Basin. Fault-Block Oil Gas Field 2013, 20, 191–195. [Google Scholar]
- Reza, B.; Mohsen, M. A novel correlation for modeling interfacial tension in binary mixtures of CH4, CO2 and N2+normal alkanes systems: Application to gas injection EOR process. Fuel 2022, 325, 124622. [Google Scholar]
- Li, H.; Li, X.; Zhao, L.; Li, J. Characterization of dew point pressure change during gas injection in condensate reservoirs. J. Shanxi Univ. Sci. Technol. Nat. Sci. Ed. 2009, 27, 12–16. [Google Scholar]
- Zhou, X.; Wang, Y.; Zhang, L.; Zhang, K.; Jiang, Q.; Pu, H.; Wang, L.; Yuane, Q. Evaluation of enhanced oil recovery potential using gas/water flooding in a tight oil reservoir. Fuel 2020, 272, 117706. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, F.; Hou, J.; Duan, X.; Li, S. Physical simulation of enhancing oil recovery by simultaneous water and gas injection flooding. Pet. Geol. Recovery Effic. 2015, 22, 89–98. [Google Scholar]
- Qu, X.; Lei, Q.; Gao, W.; Zhang, L.; He, Y.; Wang, B. Experimental study on imbibition of Chang7 tight oil cores in Erdos Basin. J. China Univ. Pet. 2018, 42, 102–109. [Google Scholar]
- Wang, J.; Yang, S.; Cao, Y.; Wang, M.; Xu, Y. Influencing Factors of Water Injection Huff and Puff of Tight Oil and Establishment of Mathematical Model. J. Petrochem. Univ. 2020, 33, 26–31. [Google Scholar]
- Fan, J.; Wang, C.; Qu, X.; Cheng, L.; Xue, T. Development and practice of water flooding huff-puff in tight oil horizontal well, Ordos Basin: A case study of Yanchang Formation Chang 7 oil layer. Acta Pet. Sin. 2019, 40, 706–715. [Google Scholar]
- Ghaderi, S.M.; Clarkson, C.R.; Ghanizadeh, A.; Barry, K.; Fiorentino, R. Improved oil recovery in tight oil formations: Result of water injection operations and gas injection sensitivities in the Bakken formation of southeast Saskatchewan. In Proceedings of the SPE Unconventional Resources Conference, Calgary, AB, Canada, 15–16 February 2017. SPE185030-MS. [Google Scholar]
- Chen, M.; Dai, J.; Liu, X.; Qin, M.; Pei, Y.; Wang, Z. Differences in the Fluid Characteristics between Spontaneous Imbibition and Drainage in Tight Sandstone Cores from Nuclear Magnetic Resonance. Energy Fuels 2018, 32, 10333–10343. [Google Scholar] [CrossRef]
- Wang, X.; Peng, X.; Zhang, S.; Du, Z.; Zeng, F. Characteristics of oil distributions in forced and spontaneous imbibition of tight oil reservoir. Fuel 2018, 224, 280–288. [Google Scholar] [CrossRef]
- Cheng, Z.; Ning, Z.; Yu, X.; Wang, Q.; Zhang, W. New insights into spontaneous imbibition in tight oil sandstones with NMR. J. Pet. Sci. Eng. 2019, 179, 455–464. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Qian, G.; Peng, Y.; Gao, Y. Investigations on spontaneous imbibition and the influencing factors in tight oil reservoirs. Fuel 2019, 236, 755–768. [Google Scholar]
- Yang, Z.; Liu, X.; Li, H.; Lei, Q.; Luo, Y.; Wang, X. Analysis on the influencing factors of imbibition and the effect evaluation of imbibition in tight reservoirs. Pet. Explor. Dev. 2019, 46, 779–785. [Google Scholar] [CrossRef]
- Zou, J.; Liao, X.; Chen, Z.; Zhao, X.; Mu, L.; Chu, H.; Dong, P.; Guan, C. Integrated PVT and Coreflooding Studies of Carbonated Water Injection in Tight Oil Reservoirs: A Case Study. Energy Fuels 2019, 33, 8852–8863. [Google Scholar] [CrossRef]
- Lijun, L.Y.L.; Leung, J.Y.; Wu, K.; Moridis, G. Coupled flow/geomechanics modeling of interfracture water injection to enhance oil recovery in tight reservoirs. SPE J. 2021, 26, 1–21. [Google Scholar]
- Liang, Y.; Lai, F.; Dai, Y.; Shi, H.; Shi, G. An experimental study of imbibition process and fluid distribution in tight oil reservoir under different pressures and temperatures. Capillarity 2021, 4, 66–75. [Google Scholar] [CrossRef]
- Chen, T.; Yang, Z.; Wang, X.; Xiao, P.; Wang, X. Study on micro displacement mechanism with gas flooding in tight oil reservoir. China Sci. 2017, 12, 1038–1043. [Google Scholar]
- Dai, Y.; Guo, H.; Li, H.; Yang, Z.; Meng, L.; Zhang, Y.; Zeng, Q.; Meng, H. NMR experiment study of gas flooding of tight oil reservoir. China Sci. 2020, 15, 105–111. [Google Scholar]
- Wei, B.; Shang, J.; Pu, W.; Valeriy, K.; Zhao, J. Predicting the diffusive front of supercritical CO2 in tight oil reservoirs. J. Southwest Pet. Univ. Sci. Technol. Ed. 2020, 42, 94–102. [Google Scholar]
- Ren, B.; Zhang, L.; Huang, H.; Ren, S.; Chen, G.; Zhang, H. Performance evaluation and mechanisms study of near-miscible CO2 flooding in a tight oil reservoir of Jilin Oilfield China. J. Nat. Gas Sci. Eng. 2015, 27, 1796–1805. [Google Scholar] [CrossRef]
- Yang, D.; Song, C.; Zhang, J.; Zhang, G.; Ji, Y.; Gao, J. Performance evaluation of injectivity for water-alternating-CO2 processes in tight oil formations. Fuel 2015, 139, 292–300. [Google Scholar] [CrossRef]
- Yu, W.; Lashgari, H.R.; Wua, K.; Sepehrnoori, K. CO2 injection for enhanced oil recovery in Bakken tight oil reservoirs. Fuel 2015, 159, 354–363. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, W.; Li, Z.; Sepehrnoori, K. Simulation study of factors affecting CO2 Huff-n-Puff process in tight oil reservoirs. J. Pet. Sci. Eng. 2018, 163, 264–269. [Google Scholar] [CrossRef]
- Song, Z.; Song, Y.; Li, Y.; Baic, B.; Song, K.; Hou, J. A critical review of CO2 enhanced oil recovery in tight oil reservoirs of North America and China. Fuel 2020, 276, 1–12. [Google Scholar] [CrossRef]
- Yu, H.; Fu, W.; Zhang, Y.; Lu, X.; Cheng, S.; Xie, Q.; Qu, X.; Yang, W.; Lu, J. Experimental study on EOR performance of CO2-based flooding methods on tight oil. Fuel 2021, 290, 119988. [Google Scholar] [CrossRef]
- Quale, E.A.; Lv, W.; Han, R. Optimization of process of CO2-water co-injection. Foreign Oilfield Eng. 2001, 8, 13–14. [Google Scholar]
- Pisharody, N.; Hussain, F. Techno-Economic Analysis of Co-Injection of CO2 and Water into Formations with Open Boundary. In Proceedings of the 14th Greenhouse Gas Control Technologies Conference (GHGT-14), Melbourne, VIC, Australia, 21–26 October 2018. [Google Scholar]
- Ajoma, E.; Kamali, F.; Ge, J.; Hussain, F. Effect of CO2-Water Injection Ratio on the Co-Optimization of Oil Recovery and CO2 Storage in a Swag Displacement Process. In Proceedings of the 14th Greenhouse Gas Control Technologies Conference (GHGT-14), Melbourne, VIC, Australia, 21–26 October 2018. [Google Scholar]
- GB/T 26981-2020; Method for Fluid Physical Properties Analysis of Oil and Gas Reservoirs. Standardization Administration of China: Beijing, China, 2020.
- SY/T 6573 2016; Minimum Miscible Pressure Experiment Determination Method-Slim Tube Method. China Petroleum Industry Standard; Petroleum Industry Press: Beijing, China, 2016.
- Peng, D.-Y.; Robinson, D.B. A new two-constant equation of state. Ind. Eng. Chem. Fundam. 1976, 15, 59–64. [Google Scholar] [CrossRef]






| Composition | Molar Percentage Content, mol% |
|---|---|
| CO2 | 4.69 |
| N2 | 0.74 |
| C1 | 63.61 |
| C2 | 11.01 |
| C3 | 4.98 |
| iC4 | 0.76 |
| nC4 | 1.52 |
| iC 5 | 0.45 |
| nC5 | 0.43 |
| C6 | 0.46 |
| C7 | 0.45 |
| C8 | 1.01 |
| C9 | 0.99 |
| C10 | 1.02 |
| C11+ | 7.88 |
| Length, m | Diameter, m | Porosity, % | Pore Volume, cm3 | Permeability, mD |
|---|---|---|---|---|
| 20 | 0.0044 | 9.43 | 28.663 | 9.7 |
| Pseudo-Component | Molar Percentage Content, mol% |
|---|---|
| CO2 | 4.69 |
| N2 | 0.74 |
| C1 | 63.607 |
| C2~C6 | 19.612 |
| C7~C10 | 3.47 |
| C11~C15 | 3.938 |
| C16~C27 | 3.194 |
| C28+ | 0.748 |
| Pseudo-Component | CO2 | N2 | C1 | C2~C6 | C7~C10 | C11~C15 | C16~C24 | C28+ |
|---|---|---|---|---|---|---|---|---|
| CO2 | 0 | 0.0200 | 0.1030 | 0.1315 | 0.1500 | 0.1500 | 0.1500 | 0.1500 |
| N2 | 0.0200 | 0 | 0.0310 | 0.0648 | 0.1200 | 0.1200 | 0.1200 | 0.1200 |
| C1 | 0.1030 | 0.0310 | 0 | 0.0027 | 0.0253 | 0.0314 | 0.0426 | 0.0561 |
| C2~C6 | 0.1315 | 0.0648 | 0.0027 | 0 | 0.0117 | 0.0160 | 0.0245 | 0.0352 |
| C7~C10 | 0.1500 | 0.1200 | 0.0253 | 0.0117 | 0 | 0.0075 | 0.0136 | 0.0219 |
| C11~C15 | 0.1500 | 0.1200 | 0.0314 | 0.0160 | 0.0075 | 0 | 0.0069 | 0.0133 |
| C16~C27 | 0.1500 | 0.1200 | 0.0426 | 0.0245 | 0.0136 | 0.0069 | 0 | 0.001 |
| C28+ | 0.1500 | 0.1200 | 0.0561 | 0.0352 | 0.0219 | 0.0133 | 0.001 | 0 |
| Composition | Critical Temperature Tc, °C | Critical Pressure Pc, MPa | |
|---|---|---|---|
| C11~C15 | 416.72 | 2.13 | 0.487 |
| C16~C27 | 527.64 | 1.58 | 0.717 |
| C28+ | 684.51 | 1.08 | 1.086 |
| Physical Property Parameter | Experimental Value | Simulated Value | Relative Error, % |
|---|---|---|---|
| Gas–oil ratio, m3/m3 | 712 | 713 | 0.14 |
| Density of formation crude oil, g/cm3 | 0.439 | 0.44 | 0.23 |
| Degassed crude oil density (20 °C, 0.1 MPa), g/cm3 | 0.8006 | 0.8022 | 0.20 |
| Molecular weight of degassed crude oil, g/mol | 207.18 | 207.8 | 0.30 |
| Bubble point pressure, MPa | 35.72 | 35.68 | 0.11 |
| Temperature, °C | Measured Value, MPa | Calculated Value, MPa | Relative Error, % |
|---|---|---|---|
| 197.50 d | 35.50 | 35.42 | 0.213 |
| 187.50 d | 35.85 | 35.61 | 0.673 |
| 177.50 b | 35.62 | 35.72 | 0.280 |
| 157.50 b | 35.72 | 35.68 | 0.112 |
| 137.50 b | 35.09 | 35.13 | 0.113 |
| 117.50 b | 33.99 | 34.25 | 0.785 |
| 100.00 b | 33.55 | 33.15 | 1.213 |
| 80.00 b | 31.85 | 31.48 | 1.174 |
| 60.00 b | 28.90 | 29.36 | 1.596 |
| 35.00 b | 26.88 | 26.08 | 2.955 |
| Pressure, MPa | 24 | 27 | 30 | 33 | 36 | 39 | 42 |
| Recovery of pure CO2 injection, % | 79.85 | 83.92 | 87.88 | 89.62 | 92.05 | 93.45 | 94.24 |
| Recovery of CO2–water co-injection, % | 82.31 | 86.01 | 89.55 | 92.76 | 93.85 | 94.46 |
| Pressure, MPa | 24 | 27 | 30 | 33 | 36 | 39 | 42 |
| Recovery of CO2 injection, % | 79.58 | 83.43 | 87.55 | 89.55 | 92.55 | 93.87 | 94.75 |
| Recovery of CO2–water co-injection, % (CO2 to water injection ratio of 3:1) | 83.54 | 87.63 | 89.8 | 92.74 | 93.92 | 94.68 | |
| Recovery of CO2–water co-injection, % (CO2 to water injection ratio of 1:1) | 83.79 | 87.98 | 89.95 | 93.05 | 94.08 | 94.85 |
| Minimum Miscible Pressure | Simulated Value, MPa | Experimental Value, MPa | Relative Error, % |
|---|---|---|---|
| CO2 injection | 34.8 | 34.0 | 0.88 |
| CO2–water co-injection | 35.4 | 35.6 | 0.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, D.; Li, J.; Tang, H.; Guo, J.; Xiang, X. Study on CO2–Water Co-Injection Miscible Characteristics in Low-Permeability Near-Critical Volatile Oil Reservoir. Energies 2022, 15, 7131. https://doi.org/10.3390/en15197131
Hou D, Li J, Tang H, Guo J, Xiang X. Study on CO2–Water Co-Injection Miscible Characteristics in Low-Permeability Near-Critical Volatile Oil Reservoir. Energies. 2022; 15(19):7131. https://doi.org/10.3390/en15197131
Chicago/Turabian StyleHou, Dali, Jinghui Li, Hongming Tang, Jianchun Guo, and Xueni Xiang. 2022. "Study on CO2–Water Co-Injection Miscible Characteristics in Low-Permeability Near-Critical Volatile Oil Reservoir" Energies 15, no. 19: 7131. https://doi.org/10.3390/en15197131
APA StyleHou, D., Li, J., Tang, H., Guo, J., & Xiang, X. (2022). Study on CO2–Water Co-Injection Miscible Characteristics in Low-Permeability Near-Critical Volatile Oil Reservoir. Energies, 15(19), 7131. https://doi.org/10.3390/en15197131







