Fabrication and Characterization of a Flexible Ag/AgCl-Zn Battery for Biomedical Applications
Abstract
:1. Introduction
2. Methodology and Materials
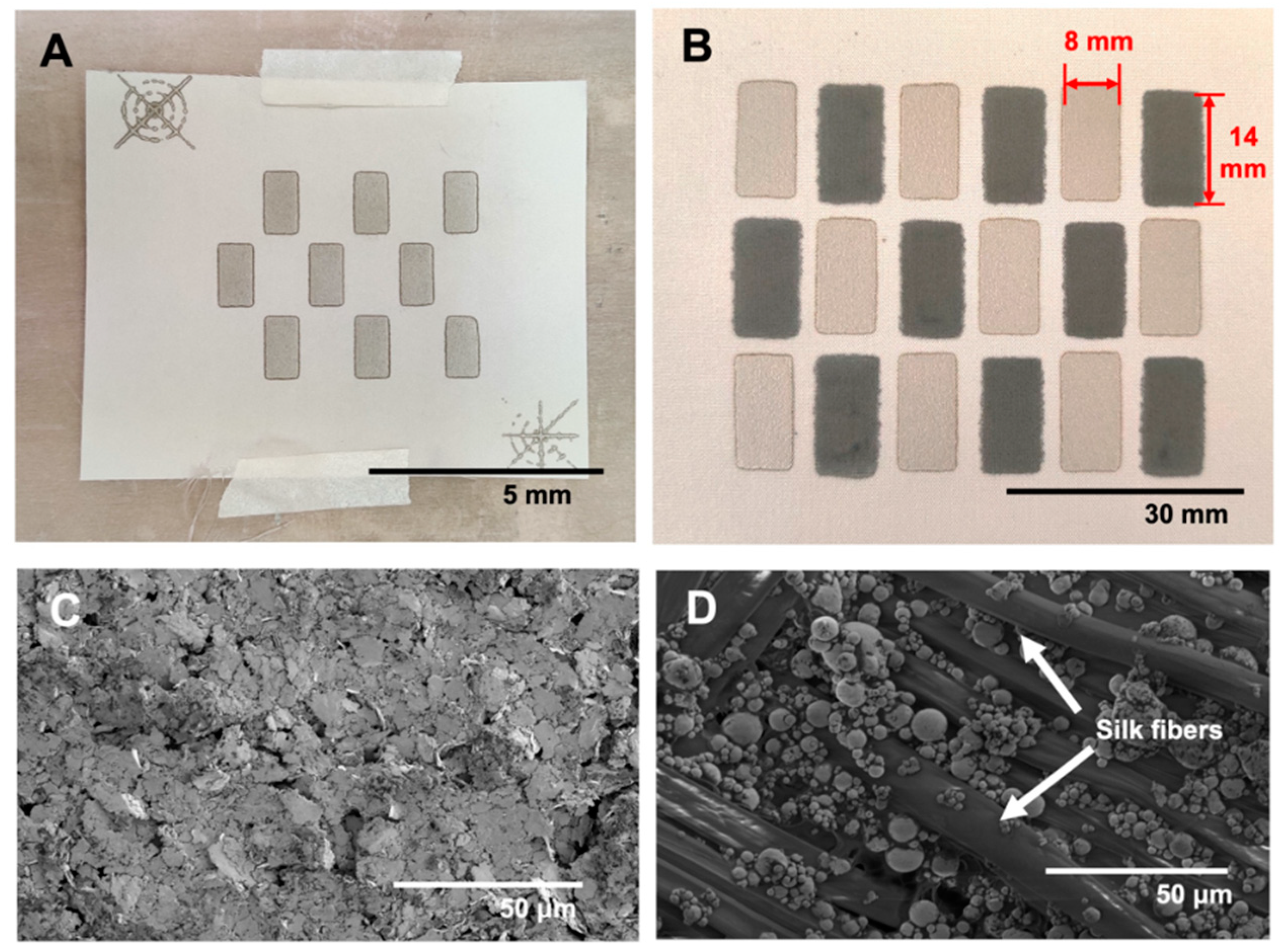
2.1. Single Cell Battery Fabrication
2.2. Nine Cell Battery Fabrication
2.3. Cyclic Voltammetry Characterization
2.4. Electrochemical Impedance Spectroscopy Characterization
2.5. Single Cell Discharge and Nine-Cell Battery Operation
2.6. Sample Application on Soft Tissue Mimic
3. Results and Discussion
3.1. Cyclic Voltammetry Characterization
3.2. Electrochemical Impedance Spectroscopy Characterization
3.3. Cell Discharge and Battery Flexibility Evaluation
3.4. Sample Application on Soft Tissue Mimic
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heald, R.; Bennett, M.; Subramaniam, V.V.; Dusane, D.; Lochab, V.; Sundaram, P.M.; Salyer, S.; West, J.; Stoodley, P.; Prakash, S. Printed Electroceutical Dressings for the Inhibition of Biofilms and Treatment of Chronic Wounds. J. Microelectromech. Syst. 2020, 29, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Leal, C.; Lopes, P.A.; Serra, A.; Coelho, J.F.; de Almeida, A.T.; Tavakoli, M. Untethered disposable health monitoring electronic patches with an integrated Ag2O–Zn battery, a AgInGa current collector, and hydrogel electrodes. ACS Appl. Mater. Interfaces 2019, 12, 3407–3414. [Google Scholar] [CrossRef]
- Best, J. Wearable technology: COVID-19 and the rise of remote clinical monitoring. BMJ 2021, 372, n413. [Google Scholar] [CrossRef] [PubMed]
- Nag, A.; Mukhopadhyay, S.C.; Kosel, J. Wearable flexible sensors: A review. IEEE Sens. J. 2017, 17, 3949–3960. [Google Scholar] [CrossRef]
- Gao, J.; Shang, K.; Ding, Y.; Wen, Z. Material and configuration design strategies towards flexible and wearable power supply devices: A review. J. Mater. Chem. A 2021, 9, 8950–8965. [Google Scholar] [CrossRef]
- Howe, D.; Dunning, J.; Zorman, C.; Garverick, S.; Bogie, K. Development of an integrated surface stimulation device for systematic evaluation of wound electrotherapy. Ann. Biomed. Eng. 2015, 43, 306–313. [Google Scholar] [CrossRef]
- Barki, K.G.; Das, A.; Dixith, S.; Ghatak, P.D.; Mathew-Steiner, S.; Schwab, E.; Khanna, S.; Wozniak, D.J.; Roy, S.; Sen, C.K. Electric field based dressing disrupts mixed-species bacterial biofilm infection and restores functional wound healing. Ann. Surg. 2019, 269, 756. [Google Scholar] [CrossRef]
- Kambouris, M.E.; Markogiannakis, A.; Arabatzis, M.; Manoussopoulos, Y.; Kantzanou, M.; Velegraki, A. Wireless electrostimulation: A new approach in combating infection? Future Microbiol. 2017, 12, 255–265. [Google Scholar] [CrossRef]
- Banerjee, J.; Ghatak, P.D.; Roy, S.; Khanna, S.; Hemann, C.; Deng, B.; Das, A.; Zweier, J.L.; Wozniak, D.; Sen, C.K. Silver-zinc redox-coupled electroceutical wound dressing disrupts bacterial biofilm. PLoS ONE 2015, 10, e0119531. [Google Scholar] [CrossRef]
- Lochab, V.; Jones, T.H.; Dusane, D.H.; Peters, C.W.; Stoodley, P.; Wozniak, D.J.; Subramaniam, V.V.; Prakash, S. Ultrastructure imaging of Pseudomonas aeruginosa lawn biofilms and eradication of the tobramycin-resistant variants under in vitro electroceutical treatment. Sci. Rep. 2020, 10, 9879. [Google Scholar] [CrossRef]
- Dowlatababdi, F.H.; Amiri, G.; Mohammadi Sichani, M. Investigation of the antimicrobial effect of silver doped Zinc Oxide nanoparticles. Nanomed. J. 2017, 4, 50–54. [Google Scholar]
- Xiao, X.; Liu, E.; Shao, J.; Ge, S. Advances on biodegradable zinc-silver-based alloys for biomedical applications. J. Appl. Biomater. Funct. Mater. 2021, 19, 22808000211062407. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.S.; Park, S.K.; Roh, K.C.; Park, H.S. Electrode materials for biomedical patchable and implantable energy storage devices. Energy Storage Mater. 2020, 24, 113–128. [Google Scholar] [CrossRef]
- Garcia, D.M.E.; Pereira, A.S.T.M.; Almeida, A.C.; Roma, U.S.; Soler, A.B.A.; Lacharmoise, P.D.; Ferreira, I.M.d.M.; Simão, C.C.D. Large-Area Paper Batteries with Ag and Zn/Ag Screen-Printed Electrodes. ACS Omega 2019, 4, 16781–16788. [Google Scholar] [CrossRef]
- Braam, K.T.; Volkman, S.K.; Subramanian, V. Characterization and optimization of a printed, primary silver–zinc battery. J. Power Sources 2012, 199, 367–372. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem compound summary for CID 14797, potassium hydroxide. In PubChem; National Center for Biotechnology Information: Bethesda, MD, USA, 2022. [Google Scholar]
- Karpinski, A.; Makovetski, B.; Russell, S.; Serenyi, J.; Williams, D. Silver–zinc: Status of technology and applications. J. Power Sources 1999, 80, 53–60. [Google Scholar] [CrossRef]
- Mauger, A.; Julien, C. Critical review on lithium-ion batteries: Are they safe? Sustainable? Ionics 2017, 23, 1933–1947. [Google Scholar] [CrossRef]
- Sepúlveda, A.; Speulmanns, J.; Vereecken, P.M. Bending impact on the performance of a flexible Li4Ti5O12-based all-solid-state thin-film battery. Sci. Technol. Adv. Mater. 2018, 19, 454–464. [Google Scholar] [CrossRef]
- Hu, L.; Wu, H.; La Mantia, F.; Yang, Y.; Cui, Y. Thin, flexible secondary Li-ion paper batteries. ACS Nano 2010, 4, 5843–5848. [Google Scholar] [CrossRef]
- Kang, K.-Y.; Lee, Y.-G.; Shin, D.O.; Kim, J.-C.; Kim, K.M. Performance improvements of pouch-type flexible thin-film lithium-ion batteries by modifying sequential screen-printing process. Electrochim. Acta 2014, 138, 294–301. [Google Scholar] [CrossRef]
- Fang, Z.; Wang, J.; Wu, H.; Li, Q.; Fan, S.; Wang, J. Progress and challenges of flexible lithium ion batteries. J. Power Sources 2020, 454, 227932. [Google Scholar] [CrossRef]
- Gellner, S.; Schwarz-Pfeiffer, A.; Nannen, E. Textile-Based Battery Using a Biodegradable Gel-Electrolyte. Proceedings 2021, 68, 17. [Google Scholar]
- Cheng, Q.; Song, Z.; Ma, T.; Smith, B.B.; Tang, R.; Yu, H.; Jiang, H.; Chan, C.K. Folding paper-based lithium-ion batteries for higher areal energy densities. Nano Lett. 2013, 13, 4969–4974. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, Y.; Grundish, N.S.; Goodenough, J.B.; Chen, Y.; Guo, L.; Peng, Z.; Qi, X.; Yang, F.; Qie, L. The 2021 battery technology roadmap. J. Phys. D Appl. Phys. 2021, 54, 183001. [Google Scholar] [CrossRef]
- Li, Y.; Yong, S.; Hillier, N.; Arumugam, S.; Beeby, S. Screen printed flexible water activated battery on woven cotton textile as a power supply for e-textile applications. IEEE Access 2020, 8, 206958–206965. [Google Scholar] [CrossRef]
- Joung, Y.-H. Development of implantable medical devices: From an engineering perspective. Int. Neurourol. J. 2013, 17, 98. [Google Scholar] [CrossRef]
- Park, H.-K.; Kong, B.-S.; Oh, E.-S. Effect of high adhesive polyvinyl alcohol binder on the anodes of lithium ion batteries. Electrochem. Commun. 2011, 13, 1051–1053. [Google Scholar] [CrossRef]
- Chaouat, M.; Le Visage, C.; Baille, W.E.; Escoubet, B.; Chaubet, F.; Mateescu, M.A.; Letourneur, D. A novel cross-linked poly (vinyl alcohol)(PVA) for vascular grafts. Adv. Funct. Mater. 2008, 18, 2855–2861. [Google Scholar] [CrossRef]
- Abdal-Hay, A.; Hussein, K.H.; Casettari, L.; Khalil, K.A.; Hamdy, A.S. Fabrication of novel high performance ductile poly (lactic acid) nanofiber scaffold coated with poly (vinyl alcohol) for tissue engineering applications. Mater. Sci. Eng. C 2016, 60, 143–150. [Google Scholar] [CrossRef]
- Vilkhu, R.; Thio, W.J.-C.; Ghatak, P.D.; Sen, C.K.; Co, A.C.; Kiourti, A. Power generation for wearable electronics: Designing electrochemical storage on fabrics. IEEE Access 2018, 6, 28945–28950. [Google Scholar] [CrossRef]
- Fan, L.; Guo, Z.; Zhang, Y.; Wu, X.; Zhao, C.; Sun, X.; Yang, G.; Feng, Y.; Zhang, N. Stable artificial solid electrolyte interphase films for lithium metal anode via metal–organic frameworks cemented by polyvinyl alcohol. J. Mater. Chem. A 2020, 8, 251–258. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, D.; Gao, Y.; Chen, T.; Huang, Q.; Wang, D. Stable Li metal anode by a polyvinyl alcohol protection layer via modifying solid-electrolyte interphase layer. Nano Energy 2019, 64, 103893. [Google Scholar] [CrossRef]
- Heald, R. Development and Evaluation of a Smart Platform for Engineered Electroceutical Dressings (SPEEDs). Master’s Thesis, The Ohio State University, Columbus, OH, USA, 2021. [Google Scholar]
- Porter, F.C. Zinc Handbook: Properties, Processing, and Use in Design; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Dusane, D.H.; Lochab, V.; Jones, T.; Peters, C.W.; Sindeldecker, D.; Das, A.; Roy, S.; Sen, C.K.; Subramaniam, V.V.; Wozniak, D.J. Electroceutical treatment of Pseudomonas aeruginosa biofilms. Sci. Rep. 2019, 9, 2008. [Google Scholar] [CrossRef] [PubMed]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A practical beginner’s guide to cyclic voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- McBreen, J.; Gannon, E. The electrochemistry of metal oxide additives in pasted zinc electrodes. Electrochim. Acta 1981, 26, 1439–1446. [Google Scholar]
- Lǎzǎrescu, V.; Radovici, O.; Vass, M. Voltammetric studies on anodic oxidation of silver. Electrochim. Acta 1985, 30, 1407–1408. [Google Scholar] [CrossRef]
- Ozgit, D.; Hiralal, P.; Amaratunga, G.A. Improving performance and cyclability of zinc–silver oxide batteries by using graphene as a two dimensional conductive additive. ACS Appl. Mater. Interfaces 2014, 6, 20752–20757. [Google Scholar] [PubMed]
- Lochab, V.; Jones, T.H.; Alkandry, E.; West, J.D.; Abdel-Rahman, M.H.; Subramaniam, V.V.; Prakash, S. Evaluation of electrical properties of ex vivo human hepatic tissue with metastatic colorectal cancer. Physiol. Meas. 2020, 41, 085005. [Google Scholar] [CrossRef]
- Vitarelli Jr, M.J.; Prakash, S.; Talaga, D.S. Determining nanocapillary geometry from electrochemical impedance spectroscopy using a variable topology network circuit model. Anal. Chem. 2011, 83, 533–541. [Google Scholar] [CrossRef]
- Kota, A.; Gogia, A.; Neidhard-Doll, A.T.; Chodavarapu, V.P. Printed Textile-Based Ag2O–Zn Battery for Body Conformal Wearable Sensors. Sensors 2021, 21, 2178. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical methods fundamentals and applications. Surf. Technol. 1983, 20, 91–92. [Google Scholar]
- Peng, J.; Deng, Y.; Wang, D.; Jin, X.; Chen, G.Z. Cyclic voltammetry of electroactive and insulative compounds in solid state: A revisit of AgCl in aqueous solutions assisted by metallic cavity electrode and chemically modified electrode. J. Electroanal. Chem. 2009, 627, 28–40. [Google Scholar] [CrossRef]
- Heald, R.; Salyer, S.; Ham, K.; Wilgus, T.A.; Subramaniam, V.V.; Prakash, S. Electroceutical treatment of infected chronic wounds in a dog and a cat. Vet. Surg. 2022, 51, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Gamry Instruments, I. Application Note Rev. 1.0. Available online: https://www.gamry.com/application-notes/EIS/category/Electrochemical+Impedance+Spectroscopy (accessed on 25 September 2022).
- Veal, B.W.; Baldo, P.M.; Paulikas, A.P.; Eastman, J.A. Understanding Artifacts in Impedance Spectroscopy. J. Electrochem. Soc. 2014, 162, H47–H57. [Google Scholar] [CrossRef]
- Middlemiss, L.A.; Rennie, A.J.R.; Sayers, R.; West, A.R. Characterisation of batteries by electrochemical impedance spectroscopy. Energy Rep. 2020, 6, 232–241. [Google Scholar] [CrossRef]
- Zamarayeva, A.M.; Gaikwad, A.M.; Deckman, I.; Wang, M.; Khau, B.; Steingart, D.A.; Arias, A.C. Fabrication of a High-Performance Flexible Silver–Zinc Wire Battery. Adv. Electron. Mater. 2016, 2, 1500296. [Google Scholar] [CrossRef]
- Chang, B.-Y.; Park, S.-M. Integrated Description of Electrode/Electrolyte Interfaces Based on Equivalent Circuits and Its Verification Using Impedance Measurements. Anal. Chem. 2006, 78, 1052–1060. [Google Scholar] [CrossRef]
- Franks, W.; Schenker, I.; Schmutz, P.; Hierlemann, A. Impedance Characterization and Modeling of Electrodes for Biomedical Applications. IEEE Trans. Bio-Med. Eng. 2005, 52, 1295–1302. [Google Scholar] [CrossRef]
- Kumar, R.; Shin, J.; Yin, L.; You, J.-M.; Meng, Y.; Wang, J. All-Printed, Stretchable Zn-Ag2O Rechargeable Battery via, Hyperelastic Binder for Self-Powering Wearable Electronics. Adv. Energy Mater. 2016, 7, 1602096. [Google Scholar] [CrossRef]
- Berchmans, S.; Bandodkar, A.J.; Jia, W.; Ramírez, J.; Meng, Y.S.; Wang, J. An epidermal alkaline rechargeable Ag–Zn printable tattoo battery for wearable electronics. J. Mater. Chem. A 2014, 2, 15788–15795. [Google Scholar] [CrossRef]
- Stauss, S.; Honma, I. Biocompatible batteries—Materials and chemistry, fabrication, applications, and future prospects. Bull. Chem. Soc. Jpn. 2018, 91, 492–505. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, X.; Wang, Y.; Wu, L.; Hu, Y. Experimental and Modeling of Conductivity for Electrolyte Solution Systems. ACS Omega 2020, 5, 22465–22474. [Google Scholar] [CrossRef] [PubMed]


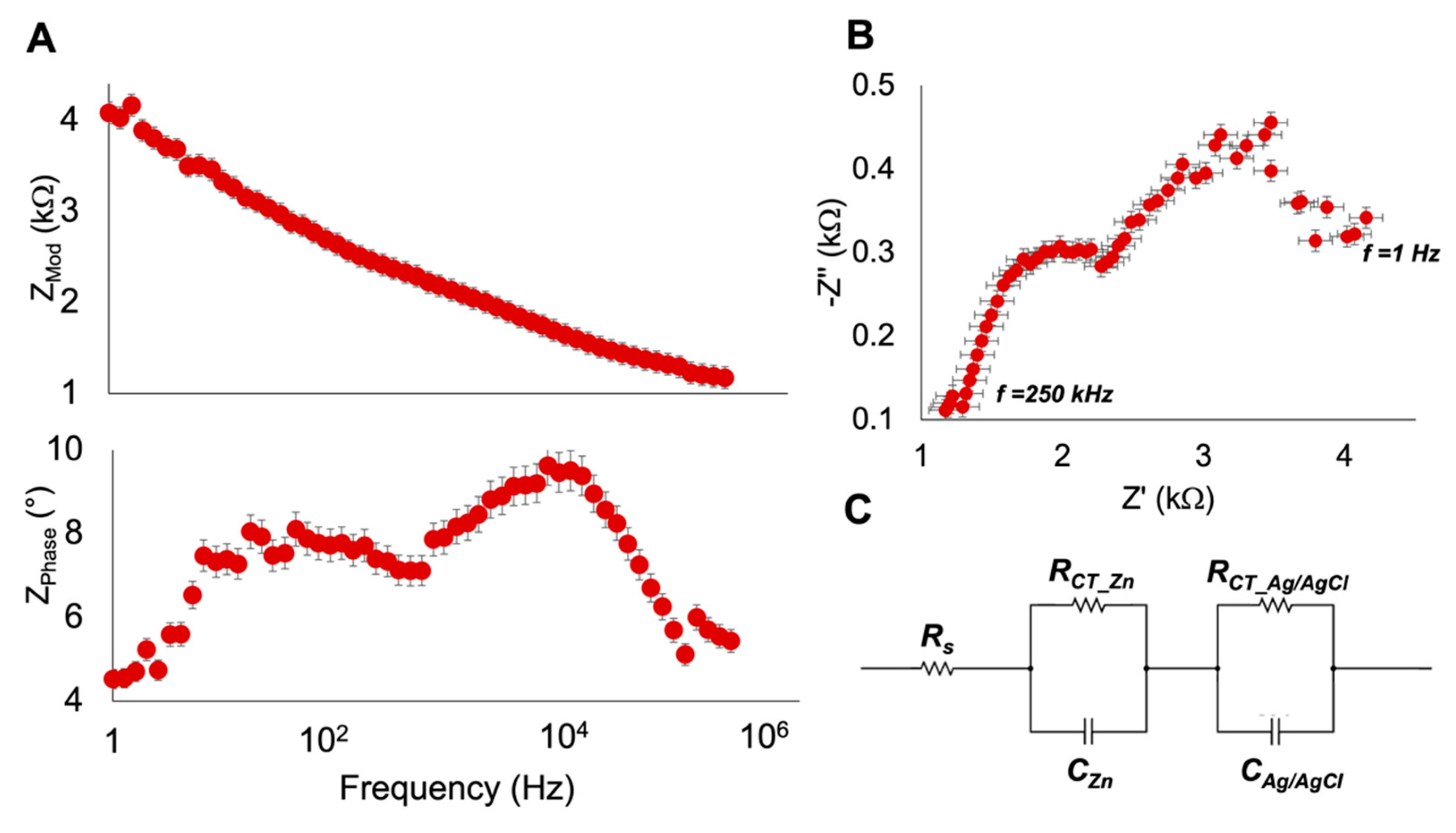

| Component | Value |
|---|---|
| RS | 1.29 × 103 Ω ± 10.9 Ω |
| CZn | 7.18 × 10−8 F ± 5.23 × 10−9 F |
| RCT_Zn | 8.95 × 102 Ω ± 19.8 Ω |
| CAg/AgCl | 3.78 × 10−6 F ± 2.44 × 10−7 F |
| RCT_Ag/AgCl | 1.37 × 103 Ω ± 31.9 Ω |
| Component | Flat | Quadricep | Gluteus Maximus |
|---|---|---|---|
| Discharge Time | 62 min | 48 min | 32 min |
| RS | 4.30 × 103 Ω ± 5.97 × 102 Ω | 8.50 × 103 Ω ± 80.0 Ω | 7.20 × 103 Ω ± 94.5 Ω |
| CZn | 2.71 × 10−8 F ± 7.63 × 10−9 F | 9.09 × 10−9 F ± 9.12 × 10−10 F | 5.06 × 10−9 F ± 2.70 × 10−10 F |
| RCT_Zn | 1.39 × 103 Ω ± 4.18 × 102 Ω | 2.45 × 103 Ω ± 1.13 × 102 Ω | 5.15 × 103 Ω ± 1.30 × 102 Ω |
| CAg/AgCl | 1.98 × 10−6 F ± 3.35 × 10−7 F | 6.44 × 10−7 F ± 5.24 × 10−8 F | 3.59 × 10−7 F ± 2.30 × 10−8 F |
| RCT_Ag/AgCl | 3.09 × 103 Ω ± 88.8 Ω | 3.65 × 103 Ω ± 1.37 × 102 Ω | 5.69 × 103 Ω ± 1.56 × 102 Ω |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bentley, D.; Heald, R.; Daniel, J.F.; Prakash, S. Fabrication and Characterization of a Flexible Ag/AgCl-Zn Battery for Biomedical Applications. Energies 2022, 15, 7167. https://doi.org/10.3390/en15197167
Bentley D, Heald R, Daniel JF, Prakash S. Fabrication and Characterization of a Flexible Ag/AgCl-Zn Battery for Biomedical Applications. Energies. 2022; 15(19):7167. https://doi.org/10.3390/en15197167
Chicago/Turabian StyleBentley, Daria, Rachel Heald, Joseph Fraser Daniel, and Shaurya Prakash. 2022. "Fabrication and Characterization of a Flexible Ag/AgCl-Zn Battery for Biomedical Applications" Energies 15, no. 19: 7167. https://doi.org/10.3390/en15197167






