Halogen Hybrid Flow Batteries Advances for Stationary Chemical Power Sources Technologies
Abstract
:1. Introduction
2. The Main Energy Storage Technologies Analysis
3. Halogen Hybrid Flow Batteries Perspective Concepts Analysis
3.1. Hybrid Principle Basis
3.2. Halogen Hybrid Flow Batteries
3.3. Halate Hybrid Flow Batteries
- The high reversibility of the electrochemical step (5) of the EC″ mechanism for the bromine/bromide redox couple used in the system, even on carbon electrodes. That is a prerequisite for high current density, sufficient voltage for under load conditions and the specific power of the system;
- Irreversibility of the chemical step (5) of the EC″ mechanism due to sufficiently high solution acidity (acid volume concentration approximately in the molar range);
- The first order of the comproportionation reaction (5) with respect to the anions of bromate and bromide, the second order—with respect to the activity of protons:
- where aH = 10−pH proton activity (in the form of hydroxonium ions), fBrO3 and fBr are activity coefficients of the bromate and the bromide anions, correspondingly.
- The six-electron nature of the electrochemical process (the Br atom changes its oxidation state from +5, which corresponds to BrO3−, to −1, which corresponds to Br−) during the cycle is a prerequisite for ensuring a high specific energy capacity of the system.
3.4. Zinc-Based Halogen Hybrid Flow Batteries
4. Challenges and Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Acronym or Designation | Definition |
| CPS | Chemical power sources |
| DER | Distributed energy resources |
| EMF | Electromotive force |
| EPS | Electrochemical power sources |
| ESS | Energy storage system |
| FC | Fuel cell |
| HRFB | Hybrid redox flow battery |
| MEA | Membrane electrode assembly |
| RDE | Rotating disc electrode |
| RFB | Redox flow battery |
| SB | Solid battery |
| SDG or SDGs | Sustainable development goal(s) |
| SHE | Standard hydrogen electrode |
| VRFB | Vanadium redox flow battery |
| ZIFB | Zinc-iodide flow battery |
| ZFB | Zinc flow battery |
References
- Desa, U.N. Transforming Our World: The 2030 Agenda for Sustainable Development. Available online: https://sdgs.un.org/2030agenda (accessed on 8 September 2022).
- Nikam, V.; Kalkhambkar, V. A Review on Control Strategies for Microgrids with Distributed Energy Resources, Energy Storage Systems, and Electric Vehicles. Int. Trans. Electr. Energy Syst. 2021, 31, 1–26. [Google Scholar] [CrossRef]
- Sánchez-Díez, E.; Ventosa, E.; Guarnieri, M.; Trovò, A.; Flox, C.; Marcilla, R.; Soavi, F.; Mazur, P.; Aranzabe, E.; Ferret, R. Redox Flow Batteries: Status and Perspective towards Sustainable Stationary Energy Storage. J. Power Sources 2021, 481, 228804. [Google Scholar] [CrossRef]
- Zigouras, P. 1000 Mile Battery. Available online: http://www.epc-corporation.com/ (accessed on 8 September 2022).
- Tang, L.; Lu, W.; Zhang, H.; Li, X. Progress and Perspective of the Cathode Materials towards Bromine-Based Flow Batteries. Energy Mater. Adv. 2022, 2022, 9850712. [Google Scholar] [CrossRef]
- Lin, G.; Chong, P.Y.; Yarlagadda, V.; Nguyen, T.V.; Wycisk, R.J.; Pintauro, P.N.; Bates, M.; Mukerjee, S.; Tucker, M.C.; Weber, A.Z. Advanced Hydrogen-Bromine Flow Batteries with Improved Efficiency, Durability and Cost. J. Electrochem. Soc. 2016, 163, A5049–A5056. [Google Scholar] [CrossRef] [Green Version]
- Braff, W.A.; Bazant, M.Z.; Buie, C.R. Membrane-Less Hydrogen Bromine Flow Battery. Nat. Commun. 2013, 4, 2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, K.T.; Tucker, M.C.; Weber, A.Z. A Review of Hydrogen/Halogen Flow Cells. Energy Technol. 2016, 4, 655–678. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.; Savinell, R.F. Flow Batteries. Electrochem. Soc. Interface 2010, 19, 54–56. [Google Scholar] [CrossRef]
- Khor, A.; Leung, P.; Mohamed, M.R.; Flox, C.; Xu, Q.; An, L.; Wills, R.G.A.; Morante, J.R.; Shah, A.A. Review of Zinc-Based Hybrid Flow Batteries: From Fundamentals to Applications. Mater. Today Energy 2018, 8, 80–108. [Google Scholar] [CrossRef]
- Soloveichik, G.L. Flow Batteries: Current Status and Trends. Chem. Rev. 2015, 115, 11533–11558. [Google Scholar] [CrossRef]
- Yang, F.; Mousavie, S.M.A.; Oh, T.K.; Yang, T.; Lu, Y.; Farley, C.; Bodnar, R.J.; Niu, L.; Qiao, R.; Li, Z. Sodium–Sulfur Flow Battery for Low-Cost Electrical Storage. Adv. Energy Mater. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Wang, W.; Luo, Q.; Li, B.; Wei, X.; Li, L.; Yang, Z. Recent Progress in Redox Flow Battery Research and Development. Adv. Funct. Mater. 2013, 23, 970–986. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, P.; Chen, H. Organic Multiple Redox Semi-Solid-Liquid Suspension for Li-Based Hybrid Flow Battery. ChemSusChem 2021, 14, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Tolmachev, Y.V.; Piatkivskyi, A.; Ryzhov, V.V.; Konev, D.V.; Vorotyntsev, M.A. Energy Cycle Based on a High Specific Energy Aqueous Flow Battery and Its Potential Use for Fully Electric Vehicles and for Direct Solar-to-Chemical Energy Conversion. J. Solid State Electrochem. 2015, 19, 2711–2722. [Google Scholar] [CrossRef]
- Ragone, D.V. Review of Battery Systems for Electrically Powered Vehicles; SAE Technical Paper 680453; SAE International: Warrendale, PA, USA, 1968. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, H.; Wu, X.; Wang, L.; Zhang, A.; Xia, T.; Dong, H.; Li, X.; Zhang, L. Progress of Electrochemical Capacitor Electrode Materials: A Review. Int. J. Hydrogen Energy 2009, 438, 213910. [Google Scholar] [CrossRef]
- Guan, C.; Liu, J.; Wang, Y.; Mao, L.; Fan, Z.; Shen, Z.; Zhang, H.; Wang, J. Iron Oxide-Decorated Carbon for Supercapacitor Anodes with Ultrahigh Energy Density and Outstanding Cycling Stability. ACS Nano 2015, 9, 5198–5207. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cong, T.N.; Yang, W.; Tan, C.; Li, Y.; Ding, Y. Progress in Electrical Energy Storage System: A Critical Review. Prog. Nat. Sci. 2009, 19, 291–312. [Google Scholar] [CrossRef]
- Masuda, M.; Shintomi, T. Superconducting Magnetic Energy Storage. Cryogenics 1977, 17, 607–612. [Google Scholar] [CrossRef]
- Gravity Battery Concept. Available online: http://www.gravitybattery.info/ (accessed on 8 September 2022).
- Gravity System Storaging. LLC “Energozapas”. Available online: http://www.energosovet.ru/stat/skolkovo_914.pdf (accessed on 8 September 2022).
- Ibrahim, H.; Ilinca, A.; Perron, J. Energy Storage Systems-Characteristics and Comparisons. Renew. Sustain. Energy Rev. 2008, 12, 1221–1250. [Google Scholar] [CrossRef]
- Alotto, P.; Guarnieri, M.; Moro, F. Redox Flow Batteries for the Storage of Renewable Energy: A Review. Renew. Sustain. Energy Rev. 2014, 29, 325–335. [Google Scholar] [CrossRef]
- Sabihuddin, S.; Kiprakis, A.E.; Mueller, M. A Numerical and Graphical Review of Energy Storage Technologies. Energies 2015, 8, 172–216. [Google Scholar] [CrossRef] [Green Version]
- Hadjipaschalis, I.; Poullikkas, A.; Efthimiou, V. Overview of Current and Future Energy Storage Technologies for Electric Power Applications. Renew. Sustain. Energy Rev. 2009, 13, 1513–1522. [Google Scholar] [CrossRef]
- Ghoniem, A.F. Needs, Resources and Climate Change: Clean and Efficient Conversion Technologies. Prog. Energy Combust. Sci. 2011, 37, 15–51. [Google Scholar] [CrossRef]
- Van den Bossche, P.; Vergels, F.; van Mierlo, J.; Matheys, J.; van Autenboer, W. SUBAT: An Assessment of Sustainable Battery Technology. J. Power Sources 2006, 162, 913–919. [Google Scholar] [CrossRef]
- Lu, Y.C.; Gallant, B.M.; Kwabi, D.G.; Harding, J.R.; Mitchell, R.R.; Whittingham, M.S.; Shao-Horn, Y. Lithium-Oxygen Batteries: Bridging Mechanistic Understanding and Battery Performance. Energy Env. Sci. 2013, 6, 750–768. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the Development of Advanced Li-Ion Batteries: A Review. Energy Env. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Mendez, A.; Leo, T.J.; Herreros, M.A. Current State of Technology of Fuel Cell Power Systems for Autonomous Underwater Vehicles. Energies 2014, 7, 4676–4693. [Google Scholar] [CrossRef] [Green Version]
- Narayan, S.R.; Valdez, T.I. High-Energy Portable Fuel Cell Power Sources. Electrochem. Soc. Interface 2008, 17, 40–45. [Google Scholar] [CrossRef]
- Van Biert, L.; Godjevac, M.; Visser, K.; Aravind, P.V. A Review of Fuel Cell Systems for Maritime Applications. J. Power Sources 2016, 327, 345–364. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.K.; Zhao, T.S.; An, L.; Zhou, X.L.; Wei, L. A Comparative Study of All-Vanadium and Iron-Chromium Redox Flow Batteries for Large-Scale Energy Storage. J. Power Sources 2015, 300, 438–443. [Google Scholar] [CrossRef]
- Scamman, D.P.; Reade, G.W.; Roberts, E.P.L. Numerical Modelling of a Bromide-Polysulphide Redox Flow Battery. Part 2: Evaluation of a Utility-Scale System. J. Power Sources 2009, 189, 1231–1239. [Google Scholar] [CrossRef]
- Scamman, D.P.; Reade, G.W.; Roberts, E.P.L. Numerical Modelling of a Bromide-Polysulphide Redox Flow Battery. Part 1: Modelling Approach and Validation for a Pilot-Scale System. J. Power Sources 2009, 189, 1220–1230. [Google Scholar] [CrossRef]
- Jameson, A.; Gyenge, E. Halogens as Positive Electrode Active Species for Flow Batteries and Regenerative Fuel Cells; Springer: Singapore, 2020; Volume 3, ISBN 0123456789. [Google Scholar]
- Zhao, X.; Liu, H.; Li, A.; Shen, Y.; Qu, J. Bromate Removal by Electrochemical Reduction at Boron-Doped Diamond Electrode. Electrochim. Acta 2012, 62, 181–184. [Google Scholar] [CrossRef]
- Leung, P.; Shah, A.A.; Sanz, L.; Flox, C.; Morante, J.R.; Xu, Q.; Mohamed, M.R.; Ponce de León, C.; Walsh, F.C. Recent Developments in Organic Redox Flow Batteries: A Critical Review. J. Power Sources 2017, 360, 243–283. [Google Scholar] [CrossRef] [Green Version]
- Kougias, I.; Szabó, S. Pumped Hydroelectric Storage Utilization Assessment: Forerunner of Renewable Energy Integration or Trojan Horse? Energy 2017, 140, 318–329. [Google Scholar] [CrossRef]
- Joseph, A.; Chelliah, T.R. A Review of Power Electronic Converters for Variable Speed Pumped Storage Plants: Configurations, Operational Challenges, and Future Scopes. IEEE J. Emerg. Sel. Top Power Electron. 2018, 6, 103–119. [Google Scholar] [CrossRef]
- Taylor, P.; Bolton, R.; Stone, D.; Zhang, X.-P.; Martin, C.; Upham, P. Pathways for Energy Storage in the UK; Technical Report; Centre for Low Carbon Futures: York, UK, 2012; pp. 1–56. [Google Scholar]
- Houssainy, S.; Janbozorgi, M.; Ip, P.; Kavehpour, P. Thermodynamic Analysis of a High Temperature Hybrid Compressed Air Energy Storage (HTH-CAES) System. Renew. Energy 2018, 115, 1043–1054. [Google Scholar] [CrossRef]
- Thomas, J. Heat & Electricity Storage Annual Report 2017 Annual Report 2017; Swiss Competence Center for Energy Research—Heat and Electricity Storage: Zurich, Switzerland, 2018. [Google Scholar]
- Arani, A.A.K.; Karami, H.; Gharehpetian, G.B.; Hejazi, M.S.A. Review of Flywheel Energy Storage Systems Structures and Applications in Power Systems and Microgrids. Renew. Sustain. Energy Rev. 2017, 69, 9–18. [Google Scholar] [CrossRef]
- May, G.J.; Davidson, A.; Monahov, B. Lead Batteries for Utility Energy Storage: A Review. J. Energy Storage 2018, 15, 145–157. [Google Scholar] [CrossRef]
- Moore, T.; Douglas, J. Energy Storage, Big Opportunities on a Smaller Scale. EPRI J. 2006, 16–23. [Google Scholar]
- Yekini Suberu, M.; Wazir Mustafa, M.; Bashir, N. Energy Storage Systems for Renewable Energy Power Sector Integration and Mitigation of Intermittency. Renew. Sustain. Energy Rev. 2014, 35, 499–514. [Google Scholar] [CrossRef]
- Ovshinsky, S.R.; Fetcenko, M.A.; Ross, J. A Nickel Metal Hydride Battery for Electric Vehicles. In Stanford R. Ovshinsky: The Science and Technology of an American Genius; World Scientific: Singapore, 2008; pp. 214–219. [Google Scholar] [CrossRef]
- Gifford, P.; Adams, J.; Corrigan, D.; Venkatesan, S. Development of Advanced Nickel/Metal Hydride Batteries for Electric and Hybrid Vehicles. J. Power Sources 1999, 80, 157–163. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X. Ambient Temperature Sodium-Sulfur Batteries. Small 2015, 11, 2108–2114. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Manthiram, A. Capacity Enhancement and Discharge Mechanisms of Room-Temperature Sodium-Sulfur Batteries. ChemElectroChem 2014, 1, 1275–1280. [Google Scholar] [CrossRef]
- Zheng, S.; Han, P.; Han, Z.; Li, P.; Zhang, H.; Yang, J. Nano-Copper-Assisted Immobilization of Sulfur in High-Surface-Area Mesoporous Carbon Cathodes for Room Temperature Na-S Batteries. Adv. Energy Mater. 2014, 4, 1–7. [Google Scholar] [CrossRef]
- Fan, X.; Yue, J.; Han, F.; Chen, J.; Deng, T.; Zhou, X.; Hou, S.; Wang, C. High-Performance All-Solid-State Na-S Battery Enabled by Casting-Annealing Technology. ACS Nano 2018, 12, 3360–3368. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Manthiram, A. Highly Reversible Room-Temperature Sulfur/Long-Chain Sodium Polysulfide Batteries. J. Phys. Chem. Lett. 2014, 5, 1943–1947. [Google Scholar] [CrossRef] [PubMed]
- Delmas, C. Sodium and Sodium-Ion Batteries: 50 Years of Research. Adv. Energy Mater. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Sudworth, J.L. Zebra Batteries. J. Power Sources 1994, 51, 105–114. [Google Scholar] [CrossRef]
- Dustmann, C.H. Advances in ZEBRA Batteries. J. Power Sources 2004, 127, 85–92. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-Ion Battery Materials: Present and Future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Coplin, J.; Yang, A.; Poppe, A.R.; Burtscher, M. Increasing Telemetry Throughput Using Customized and Adaptive Data Compression. In Proceedings of the AIAA Space and Astronautics Forum and Exposition, SPACE 2016, Las Vegas, NV, USA, 12–15 September 2016. [Google Scholar] [CrossRef] [Green Version]
- Blomgren, G.E. The Development and Future of Lithium Ion Batteries. J. Electrochem. Soc. 2017, 164, A5019–A5025. [Google Scholar] [CrossRef] [Green Version]
- Borup, R.; Meyers, J.; Pivovar, B.; Kim, Y.S.; Mukundan, R.; Garland, N.; Myers, D.; Wilson, M.; Garzon, F.; Wood, D.; et al. Scientific Aspects of Polymer Electrolyte Fuel Cell Durability and Degradation. Chem. Rev. 2007, 107, 3904–3951. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yuan, X.Z.; Martin, J.J.; Wang, H.; Zhang, J.; Shen, J.; Wu, S.; Merida, W. A Review of PEM Fuel Cell Durability: Degradation Mechanisms and Mitigation Strategies. J. Power Sources 2008, 184, 104–119. [Google Scholar] [CrossRef]
- Cheng, X.; Shi, Z.; Glass, N.; Zhang, L.; Zhang, J.; Song, D.; Liu, Z.S.; Wang, H.; Shen, J. A Review of PEM Hydrogen Fuel Cell Contamination: Impacts, Mechanisms, and Mitigation. J. Power Sources 2007, 165, 739–756. [Google Scholar] [CrossRef]
- Freni, S.; Cavallaro, S.; Aquino, M.; Ravida, D.; Giordano, N. Lifetime-Limiting Factors for a Molten Carbonate Fuel Cell. Int. J. Hydrogen Energy 1994, 19, 337–341. [Google Scholar] [CrossRef]
- Mehrpooya, M.; Sayyad, S.; Zonouz, M.J. Energy, Exergy and Sensitivity Analyses of a Hybrid Combined Cooling, Heating and Power (CCHP) Plant with Molten Carbonate Fuel Cell (MCFC) and Stirling Engine. J. Clean Prod. 2017, 148, 283–294. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, H.; Ni, M.; Chen, J. Performance Evaluation and Parametric Optimum Design of a Syngas Molten Carbonate Fuel Cell and Gas Turbine Hybrid System. Renew. Energy 2015, 80, 407–414. [Google Scholar] [CrossRef]
- Sorensen, B.; Spazzafumo, G. Hydrogen and Fuel Cells. Emerging Technologies and Applications; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9781119130536. [Google Scholar]
- Dicks, A.L.; Rand, D.A.J. Fuel Cell Systems Explained; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; ISBN 9781118706978. [Google Scholar]
- Blackburn, B. Affordable, High Performance, Intermediate Temperature Solid Oxide Fuel Cells. Ph.D. Dissertation, Redox Power Systems LLC, Beltsville, MD, USA, 2015. [Google Scholar]
- McPhail, S.; Leto, L.; Boigues-Muñoz, C. The Yellow Pages of SOFC Technology—International Status of SOFC Deployment 2012–2013; ENEA: Roma, Italy, 2013; ISBN 978-88-8286-290-9. [Google Scholar]
- High-Temperature Fuel Cell Systems. Bosch Company Announcement. Available online: https://www.bosch.com/research/know-how/success-stories/high-temperature-fuel-cell-systems/ (accessed on 8 September 2022).
- Enervault. Final Technical Report Flow Battery Solution for Smart Grid Applications; Enervault: Sunnyvale, CA, USA, 2015. [Google Scholar]
- DOE Global Energy Storage Database. Available online: https://sandia.gov/ess-ssl/gesdb/public/projects.html (accessed on 8 September 2022).
- Weber, A.Z.; Mench, M.M.; Meyers, J.P.; Ross, P.N.; Gostick, J.T.; Liu, Q. Redox Flow Batteries: A Review. J Appl. Electrochem. 2011, 41, 1137–1164. [Google Scholar] [CrossRef]
- Manohar, A.K.; Kim, K.M.; Plichta, E.; Hendrickson, M.; Rawlings, S.; Narayanan, S.R. A High Efficiency Iron-Chloride Redox Flow Battery for Large-Scale Energy Storage. J. Electrochem. Soc. 2016, 163, A5118–A5125. [Google Scholar] [CrossRef]
- Hruska, L.W.; Savinell, R.F. Investigation of Factors Affecting Performance of the Iron-Redox Battery. J. Electrochem. Soc. 1981, 128, 18–25. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Kazacos, G.; Poon, G.; Verseema, H. Application of Graphene and Graphene-Based Materials in Clean Energy-Related Devices Minghui. Recent. Adv. UNSW Vanadium-Based Redox Flow Batter. 2010, 34, 182–189. [Google Scholar] [CrossRef]
- Zhang, H. Polysulfide-Bromine Flow Batteries (PBBs) for Medium- and Large-Scale Energy Storage; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; ISBN 9781782420224. [Google Scholar]
- Leung, P.; Li, X.; Ponce De León, C.; Berlouis, L.; Low, C.T.J.; Walsh, F.C. Progress in Redox Flow Batteries, Remaining Challenges and Their Applications in Energy Storage. RSC Adv. 2012, 2, 10125–10156. [Google Scholar] [CrossRef]
- Lotspeich, C. A Comparative Assessment of Flow Battery Technologies. In Proceedings of the 2002 EESAT Conference, San Francisco, CA, USA, 11–14 November 2002; pp. 15–17. [Google Scholar]
- Manufacturing Cost Analysis of 100 and 250 KW Fuel Cell Systems for Primary Power and Combined Heat and Power Applications; United States Department of Energy: Washington, DC, USA, 2016.
- Bradbury, K. Energy Storage Technology Review; Duke University: Durham, NC, USA, 2010; pp. 1–34. [Google Scholar]
- Hart, D.M.; Bonvillian, W.B.; Austin, N. Energy Storage for the Grid: Policy Options for Sustaining Innovation. MIT Energy Initiat. Work. Pap. 2018, 1, 33. [Google Scholar]
- Lazard. Lazard’s Levelized Cost of Storage Analysis—Version 7.0; Lazard: New York, NY, USA, 2022. [Google Scholar]
- Weber, A.Z.; Perry, M.L.; Zawodzinski, T.A.; Stetson, N.; Johnson, M.; Gyuk, I. Flow Cells for Energy Storage Workshop Summary Report; US Department of Energy: Washington, DC, USA, 2012; p. 39. [Google Scholar]
- Ralon, P.; Taylor, M.; Ilas, A.; Diaz-Bone, H.; Kairies, K. Electricity Storage and Renewables: Costs and Markets to 2030; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2017; ISBN 978-92-9260-038-9. [Google Scholar]
- Howard, W.F.; Spotnitz, R.M. Theoretical Evaluation of High-Energy Lithium Metal Phosphate Cathode Materials in Li-Ion Batteries. J Power Sources 2007, 165, 887–891. [Google Scholar] [CrossRef]
- Zang, X.; Shen, C.; Kao, E.; Warren, R.; Zhang, R.; Teh, K.S.; Zhong, J.; Wei, M.; Li, B.; Chu, Y.; et al. Titanium Disulfide Coated Carbon Nanotube Hybrid Electrodes Enable High Energy Density Symmetric Pseudocapacitors. Adv. Mater. 2018, 30, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Xu, Z.; Li, Z.; Cui, K.; Ding, J.; Kohandehghan, A.; Tan, X.; Zahiri, B.; Olsen, B.C.; Holt, C.M.B.; et al. Hybrid Device Employing Three-Dimensional Arrays of MnO in Carbon Nanosheets Bridges Battery-Supercapacitor Divide. Nano Lett. 2014, 14, 1987–1994. [Google Scholar] [CrossRef]
- Choudhary, N.; Li, C.; Chung, H.S.; Moore, J.; Thomas, J.; Jung, Y. High-Performance One-Body Core/Shell Nanowire Supercapacitor Enabled by Conformal Growth of Capacitive 2D WS2 Layers. ACS Nano 2016, 10, 10726–10735. [Google Scholar] [CrossRef]
- Izadi-Najafabadi, A.; Yamada, T.; Futaba, D.N.; Yudasaka, M.; Takagi, H.; Hatori, H. High-Power Supercapacitor Electrodes Nanotube Composite. ACS Nano 2011, 5, 811–819. [Google Scholar] [CrossRef]
- Sun, S.; Zhai, T.; Liang, C.; Savilov, S.V.; Xia, H. Boosted Crystalline/Amorphous Fe2O3-δ Core/Shell Heterostructure for Flexible Solid-State Pseudocapacitors in Large Scale. Nano Energy 2018, 45, 390–397. [Google Scholar] [CrossRef]
- Ahmadi, S.; Bathaee, S.M.T.; Hosseinpour, A.H. Improving Fuel Economy and Performance of a Fuel-Cell Hybrid Electric Vehicle (Fuel-Cell, Battery, and Ultra-Capacitor) Using Optimized Energy Management Strategy. Energy Convers. Manag. 2018, 160, 74–84. [Google Scholar] [CrossRef]
- Pan, H.; Han, K.S.; Engelhard, M.H.; Cao, R.; Chen, J.; Zhang, J.G.; Mueller, K.T.; Shao, Y.; Liu, J. Addressing Passivation in Lithium–Sulfur Battery Under Lean Electrolyte Condition. Adv. Funct. Mater. 2018, 28, 1–7. [Google Scholar] [CrossRef]
- Evers, S.; NaZar, L. New Approaches for High Energy Density Lithium -Sulfur Battery Cathodes. Acc. Chem. Res. 2012, 46, 1135–1143. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, W.; Fan, H.; Cheng, F.; Su, D.; Wang, G. Promoting Lithium Polysulfide/Sulfide Redox Kinetics by the Catalyzing of Zinc Sulfide for High Performance Lithium-Sulfur Battery. Nano Energy 2018, 51, 73–82. [Google Scholar] [CrossRef]
- Zhang, S.S. Liquid Electrolyte Lithium/Sulfur Battery: Fundamental Chemistry, Problems, and Solutions. J. Power Sources 2013, 231, 153–162. [Google Scholar] [CrossRef]
- Aurbach, D. Introduction to the Focus Issue on Lithium-Sulfur Batteries: Materials, Mechanisms, Modeling, and Applications. J. Electrochem. Soc. 2018, 165, Y1. [Google Scholar] [CrossRef]
- Du, H.; Li, S.; Qu, H.; Lu, B.; Wang, X.; Chai, J.; Zhang, H.; Ma, J.; Zhang, Z.; Cui, G. Stable Cycling of Lithium-Sulfur Battery Enabled by a Reliable Gel Polymer Electrolyte Rich in Ester Groups. J. Memb. Sci. 2018, 550, 399–406. [Google Scholar] [CrossRef]
- Carbone, L.; Coneglian, T.; Gobet, M.; Munoz, S.; Devany, M.; Greenbaum, S.; Hassoun, J. A Simple Approach for Making a Viable, Safe, and High-Performances Lithium-Sulfur Battery. J. Power Sources 2018, 377, 26–35. [Google Scholar] [CrossRef]
- Liu, Q.; Chang, Z.; Li, Z.; Zhang, X. Flexible Metal—Air Batteries: Progress, Challenges, and Perspectives. Small Methods 2017, 1700231, 1–16. [Google Scholar] [CrossRef]
- Pan, J.; Li, H.; Sun, H.; Zhang, Y.; Wang, L.; Liao, M.; Sun, X. A Lithium—Air Battery Stably Working at High Temperature with High Rate Performance. Small 2017, 1703454, 1–6. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, J.; Peng, Z. Achilles’ Heel of Li-Air Batteries: Li2CO3. Angew. Chem.-Int. Ed. 2017, 57, 3874–3886. [Google Scholar] [CrossRef]
- Guo, Z.; Li, C.; Liu, J.; Wang, Y.; Xia, Y. A Long-Life Lithium—Air Battery in Ambient Air with a Polymer Electrolyte Containing a Redox Mediator. Angew. Chem. 2017, 129, 7613–7617. [Google Scholar] [CrossRef]
- Wu, F.; Yu, Y. Toward True Lithium-Air Batteries. Joule 2018, 2, 815–817. [Google Scholar] [CrossRef] [Green Version]
- Aurbach, D.; Mccloskey, B.D.; Nazar, L.F.; Bruce, P.G. Underpinning Lithium—Air Batteries. Nat. Publ. Group 2016, 1, 1–11. [Google Scholar] [CrossRef]
- Asadi, M.; Sayahpour, B.; Abbasi, P.; Ngo, A.T.; Karis, K.; Jokisaari, J.R.; Liu, C.; Narayanan, B.; Gerard, M.; Yasaei, P.; et al. A Lithium-Oxygen Battery with a Long Cycle Life in an Air-like Atmosphere. Nature 2018, 555, 502–506. [Google Scholar] [CrossRef]
- Xu, S.; Yao, Y.; Guo, Y.; Zeng, X.; Lacey, S.D.; Song, H.; Chen, C.; Li, Y.; Dai, J.; Wang, Y.; et al. Textile Inspired Lithium–Oxygen Battery Cathode with Decoupled Oxygen and Electrolyte Pathways. Adv. Mater. 2017, 30, 1704907. [Google Scholar] [CrossRef]
- Torres, A.E.; Balbuena, P.B. Exploring the LiOH Formation Reaction Mechanism in Lithium-Air Batteries. Chem. Mater. 2018, 30, 708–717. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, M.; Zhang, L.; Dai, L.; Xia, Z. Design Principles for Heteroatom-Doped Carbon Nanomaterials as Highly Efficient Catalysts for Fuel Cells and Metal-Air Batteries. Adv. Mater. 2015, 27, 6834–6840. [Google Scholar] [CrossRef]
- Cui, Z.; Fu, G.; Li, Y.; Goodenough, J.B. Ni3FeN-Supported Fe3Pt Intermetallic Nanoalloy as a High-Performance Bifunctional Catalyst for Metal–Air Batteries. Angew. Chem. 2017, 129, 10033–10037. [Google Scholar] [CrossRef]
- Hardwick, L.J.; León, C.P. de Rechargeable Multi-Valent Metal-Air Batteries. Johns. Matthey Technol. Rev. 2018, 62, 134–149. [Google Scholar] [CrossRef]
- Liu, G.; Kim, J.Y.; Wang, M.; Woo, J.Y.; Wang, L.; Zou, D.; Lee, J.K. Soft, Highly Elastic, and Discharge-Current-Controllable Eutectic Gallium–Indium Liquid Metal–Air Battery Operated at Room Temperature. Adv. Energy Mater. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Wu, X.; Meng, G.; Liu, W.; Li, T.; Yang, Q.; Sun, X.; Liu, J. Metal-Organic Framework-Derived, Zn-Doped Porous Carbon Polyhedra with Enhanced Activity as Bifunctional Catalysts for Rechargeable Zinc-Air Batteries. Nano Res 2018, 11, 163–173. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Song, L.; Wang, D.; Zhang, Z. Three-Dimensional Graphene Network Supported Ultrathin CeO2 Nanoflakes for Oxygen Reduction Reaction and Rechargeable Metal-Air Batteries. Electrochim. Acta 2018, 263, 561–569. [Google Scholar] [CrossRef]
- Wang, Y.J.; Fang, B.; Zhang, D.; Li, A.; Wilkinson, D.P.; Ignaszak, A.; Zhang, L.; Zhang, J. A Review of Carbon-Composited Materials as Air-Electrode Bifunctional Electrocatalysts for Metal–Air Batteries; Springer: Singapore, 2018; Volume 1, ISBN 0123456789. [Google Scholar]
- Lee, D.U.; Park, M.G.; Cano, Z.P.; Ahn, W.; Chen, Z. Hierarchical Core–Shell Nickel Cobaltite Chestnut-like Structures as Bifunctional Electrocatalyst for Rechargeable Metal–Air Batteries. ChemSusChem 2018, 11, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; He, G.; He, Y.; Zhang, J.; Zheng, X.; Li, L.; Zhong, C.; Hu, W.; Deng, Y.; Ma, T.Y. Engineering Catalytic Active Sites on Cobalt Oxide Surface for Enhanced Oxygen Electrocatalysis. Adv. Energy Mater. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Wang, Z.L.; Xu, D.; Xu, J.J.; Zhang, X.B. Oxygen Electrocatalysts in Metal-Air Batteries: From Aqueous to Nonaqueous Electrolytes. Chem. Soc. Rev. 2014, 43, 7746–7786. [Google Scholar] [CrossRef]
- Wu, Z.P.; Caracciolo, D.T.; Maswadeh, Y.; Wen, J.; Kong, Z.; Shan, S.; Vargas, J.A.; Yan, S.; Hopkins, E.; Park, K.; et al. Alloying–Realloying Enabled High Durability for Pt–Pd-3d-Transition Metal Nanoparticle Fuel Cell Catalysts. Nat. Commun. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Chong, L.; Wen, J.; Kubal, J.; Sen, F.G.; Zou, J.; Greeley, J.; Chan, M.; Barkholtz, H.; Ding, W.; Liu, D.J. Ultralow-Loading Platinum-Cobalt Fuel Cell Catalysts Derived from Imidazolate Frameworks. Science 2018, 362, 1276–1281. [Google Scholar] [CrossRef]
- Ren, X.; Lv, Q.; Liu, L.; Liu, B.; Wang, Y.; Liu, A.; Wu, G. Current Progress of Pt and Pt-Based Electrocatalysts Used for Fuel Cells. Sustain. Energy Fuels 2019, 4, 15–30. [Google Scholar] [CrossRef]
- Dowd, R.P.; Zeets, M.; Van Nguyen, T. Effect of Br2 Complexation on a Hydrogen-Bromine Flow Battery Performance. ECS Meet. Abstr. 2015, 47, 1886. [Google Scholar] [CrossRef]
- Cho, K.T.; Albertus, P.; Battaglia, V.; Kojic, A.; Srinivasan, V.; Weber, A.Z. Optimization and Analysis of High-Power Hydrogen/Bromine-Flow Batteries for Grid-Scale Energy Storage. Energy Technol. 2013, 1, 596–608. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, C.; Li, X. Bromine–Graphite Intercalation Enabled Two-Electron Transfer for a Bromine-Based Flow Battery. Trans. Tianjin Univ. 2022, 28, 186–192. [Google Scholar] [CrossRef]
- Livshits, V.; Ulus, A.; Peled, E. High-Power H2/Br2 Fuel Cell. Electrochem. Commun. 2006, 8, 1358–1362. [Google Scholar] [CrossRef]
- Cho, K.T.; Ridgway, P.; Weber, A.Z.; Haussener, S.; Battaglia, V.; Srinivasan, V. High Performance Hydrogen/Bromine Redox Flow Battery for Grid-Scale Energy Storage. J. Electrochem. Soc. 2012, 159, A1806–A1815. [Google Scholar] [CrossRef]
- Kim, J.; Park, H. Recent Advances in Porous Electrodes for Vanadium Redox Flow Batteries in Grid-Scale Energy Storage Systems: A Mass Transfer Perspective. J. Power Sources 2022, 545, 231904. [Google Scholar] [CrossRef]
- Parasuraman, A.; Lim, T.M.; Menictas, C.; Skyllas-Kazacos, M. Review of Material Research and Development for Vanadium Redox Flow Battery Applications. Electrochim. Acta 2013, 101, 27–40. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, W.; Li, X. Progress and Perspectives of Flow Battery Technologies. Electrochem. Energy Rev. 2019, 2, 492–506. [Google Scholar] [CrossRef]
- Kear, G.; Shah, A.A.; Walsh, F.C. Development of the All-vanadium Redox Flow Battery for Energy Storage: A Review of Technological, Financial and Policy Aspects. Int. J. Energy Res. 2012, 36, 1105–1120. [Google Scholar] [CrossRef] [Green Version]
- Wlodarczyk, J.K.; Küttinger, M.; Friedrich, A.K.; Schumacher, J.O. Exploring the Thermodynamics of the Bromine Electrode in Concentrated Solutions for Improved Parametrisation of Hydrogen–Bromine Flow Battery Models. J. Power Sources 2021, 508, 230202. [Google Scholar] [CrossRef]
- Ronen, R.; Atlas, I.; Suss, M.E. Theory of Flow Batteries with Fast Homogeneous Chemical Reactions. J. Electrochem. Soc. 2018, 165, A3820–A3827. [Google Scholar] [CrossRef]
- Ronen, R.; Gloukhovski, R.; Suss, M.E. Single-Flow Multiphase Flow Batteries: Experiments. J. Power Sources 2022, 540, 231567. [Google Scholar] [CrossRef]
- Hou, S.; Chen, L.; Fan, X.; Fan, X.; Ji, X.; Wang, B.; Cui, C.; Chen, J.; Yang, C.; Wang, W.; et al. High-Energy and Low-Cost Membrane-Free Chlorine Flow Battery. Nat. Commun. 2022, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Küttinger, M.; Loichet Torres, P.A.; Meyer, E.; Fischer, P. Properties of Bromine Fused Salts Based on Quaternary Ammonium Molecules and Their Relevance for Use in a Hydrogen Bromine Redox Flow Battery. Chem. Eur. J. 2022, 28, e202103491. [Google Scholar] [CrossRef] [PubMed]
- Küttinger, M.; Riasse, R.; Wlodarczyk, J.; Fischer, P.; Tübke, J. Improvement of Safe Bromine Electrolytes and Their Cell Performance in H2/Br2 Flow Batteries Caused by Tuning the Bromine Complexation Equilibrium. J. Power Sources 2022, 520, 230804. [Google Scholar] [CrossRef]
- Wu, W.; Xu, S.; Lin, Z.; Lin, L.; He, R.; Sun, X. A Polybromide Confiner with Selective Bromide Conduction for High Performance Aqueous Zinc-Bromine Batteries. Energy Storage Mater. 2022, 49, 11–18. [Google Scholar] [CrossRef]
- Cantu, B.B.M.; Fischer, P.; Zitoun, D.; Tübke, J.; Pinkwart, K. In Situ Measurement of Localized Current Distribution in H2-br2 Redox Flow Batteries. Energies 2021, 14, 4945. [Google Scholar] [CrossRef]
- Tolmachev, Y.V. Hydrogen-Halogen Electrochemical Cells: A Review of Applications and Technologies. Russ. J. Electrochem. 2014, 50, 301–316. [Google Scholar] [CrossRef]
- Naresh, R.P.; Surendran, A.; Ragupathy, P.; Dixon, D. Enhanced Electrochemical Performance of Zinc/Bromine Redox Flow Battery with Carbon-Nanostructured Felt Generated by Cobalt Ions. J. Energy Storage 2022, 52, 104913. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Modestov, A.D.; Konev, D.V.; Tripachev, O.V.; Antipov, A.E.; Tolmachev, Y.V.; Vorotyntsev, M.A. A Hydrogen–Bromate Flow Battery for Air-Deficient Environments. Energy Technol. 2018, 6, 242–245. [Google Scholar] [CrossRef]
- Petrov, M.M.; Modestov, A.D.; Konev, D.V.; Antipov, A.E.; Loktionov, P.A.; Pichugov, R.D.; Kartashova, N.V.; Glazkov, A.T.; Abunaeva, L.Z.; Andreev, V.N.; et al. Redox Flow Batteries: Role in Modern Electric Power Industry and Comparative Characteristics of the Main Types. Russ. Chem. Rev. 2021, 90, 677–702. [Google Scholar] [CrossRef]
- Vorotyntsev, M.A.; Konev, D.V.; Tolmachev, Y.V. Electroreduction of Halogen Oxoanions via Autocatalytic Redox Mediation by Halide Anions: Novel EC" Mechanism. Theory for Stationary 1D Regime. Electrochim. Acta 2015, 173, 779–795. [Google Scholar] [CrossRef]
- Vorotyntsev, M.A.; Antipov, A.E.; Tolmachev, Y.V. One-Dimensional Model of Steady-State Discharge Process in Hydrogen-Bromate Flow Battery. Electrochim. Acta 2016, 222, 1555–1561. [Google Scholar] [CrossRef]
- Vorotyntsev, M.A.; Antipov, A.E. Bromate Electroreduction from Acidic Solution at Spherical Microelectrode under Steady-State Conditions: Theory for the Redox-Mediator Autocatalytic (EC”) Mechanism. Electrochim. Acta 2017, 258, 544–553. [Google Scholar] [CrossRef]
- Modestov, A.D.; Konev, D.V.; Antipov, A.E.; Petrov, M.M.; Pichugov, R.D.; Vorotyntsev, M.A. Bromate Electroreduction from Sulfuric Acid Solution at Rotating Disk Electrode: Experimental Study. Electrochim. Acta 2018, 259, 346–360. [Google Scholar] [CrossRef]
- Cho, K.T.; Razaulla, T. Redox-Mediated Bromate Based Electrochemical Energy System. J. Electrochem. Soc. 2019, 166, A286–A296. [Google Scholar] [CrossRef]
- Chinannai, M.F.; Ju, H. Analysis of Performance Improvement of Hydrogen/Bromine Flow Batteries by Using Bromate Electrolyte. Int. J. Hydrogen Energy 2021, 46, 13760–13774. [Google Scholar] [CrossRef]
- Modestov, A.D.; Konev, D.V.; Antipov, A.E.; Vorotyntsev, M.A. Hydrogen-Bromate Flow Battery: Can One Reach Both High Bromate Utilization and Specific Power? J. Solid State Electrochem. 2019, 23, 3075–3088. [Google Scholar] [CrossRef]
- Pichugov, R.; Konev, D.; Speshilov, I.; Abunaeva, L.; Petrov, M.; Vorotyntsev, M.A. Analysis of the Composition of Bromide Anion Oxidation Products in Aqueous Solutions with Different PH via Rotating Ring-Disk Electrode Method. Membranes 2022, 12, 820. [Google Scholar] [CrossRef]
- Modestov, A.; Kartashova, N.; Pichugov, R.; Petrov, M.; Antipov, A.; Abunaeva, L. Bromine Crossover in Operando Analysis of Proton Exchange Membranes in Hydrogen−Bromate Flow Batteries. Membranes 2022, 12, 815. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Cao, L.; Kazacos, M.; Kausar, N.; Mousa, A. Vanadium electrolyte studies for the vanadium redox battery—A review. ChemSusChem 2011, 9, 1521–1543. [Google Scholar] [CrossRef]
- Service, R.F. Tanks for the Batteries. Science 2014, 344, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Kordesch, K.; Daniel-Ivad, J. Rechargeable Zinc/Alkaline/ Manganese Dioxide Batteries. In Handbook of Batteries; Linden, D., Reddy, T., Eds.; McGraw. Hill: New York, NY, USA, 2002; ISBN 0-07-135978-8. [Google Scholar]
- McComsey, D.W. Zinc-Carbon Batteries (Leclanche’ and Zinc Chloride Cell Systems). In Handbook of Batteries; Linden, D., Reddy, T.B., Eds.; McGraw. Hill: New York, NY, USA, 2002; pp. 184–228. ISBN 0-07-135978-8. [Google Scholar]
- London Metal Exchange. Available online: https://www.lme.com/Metals/Non-ferrous/Zinc#tabIndex=0 (accessed on 6 September 2022).
- Fleischmann, M.; Hill, I.R.; Sundholm, G. A Raman Spectroscopic Study of Thiourea Adsorbed on Silver and Copper Electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1983, 157, 359–368. [Google Scholar] [CrossRef]
- Yuan, Z.; Yin, Y.; Xie, C.; Zhang, H.; Yao, Y.; Li, X. Advanced Materials for Zinc-Based Flow Battery: Development and Challenge. Adv. Mater. 2019, 31, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Jonshagen, B. Zincbromine Battery for Energy Storage. J. Power Sources 1991, 35, 405–410. [Google Scholar] [CrossRef]
- Wu, M.; Zhao, T.; Zhang, R.; Jiang, H.; Wei, L. A Zinc–Bromine Flow Battery with Improved Design of Cell Structure and Electrodes. Energy Technol. 2018, 6, 333–339. [Google Scholar] [CrossRef]
- Jiang, T.; Lin, H.; Sun, Q.; Zhao, G.; Shi, J. Recent Progress of Electrode Materials for Zinc Bromide Flow Battery. Int. J. Electrochem. Sci. 2018, 13, 5603–5611. [Google Scholar] [CrossRef]
- Kim, R.; Kim, H.G.; Doo, G.; Choi, C.; Kim, S.; Lee, J.H.; Heo, J.; Jung, H.Y.; Kim, H.T. Ultrathin Nafion-Filled Porous Membrane for Zinc/Bromine Redox Flow Batteries. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.C.; Zhao, T.S.; Jiang, H.R.; Zeng, Y.K.; Ren, Y.X. High-Performance Zinc Bromine Flow Battery via Improved Design of Electrolyte and Electrode. J. Power Sources 2017, 355, 62–68. [Google Scholar] [CrossRef]
- Biswas, S.; Senju, A.; Mohr, R.; Hodson, T.; Karthikeyan, N.; Knehr, K.W.; Hsieh, A.G.; Yang, X.; Koel, B.E.; Steingart, D.A. Minimal Architecture Zinc-Bromine Battery for Low Cost Electrochemical Energy Storage. Energy Env. Sci 2017, 10, 114–120. [Google Scholar] [CrossRef]
- Wu, M.C.; Zhao, T.S.; Wei, L.; Jiang, H.R.; Zhang, R.H. Improved Electrolyte for Zinc-Bromine Flow Batteries. J. Power Sources 2018, 384, 232–239. [Google Scholar] [CrossRef]
- Jiang, H.R.; Wu, M.C.; Ren, Y.X.; Shyy, W.; Zhao, T.S. Towards a Uniform Distribution of Zinc in the Negative Electrode for Zinc Bromine Flow Batteries. Appl. Energy 2018, 213, 366–374. [Google Scholar] [CrossRef]
- Lin, H.; Jiang, T.; Sun, Q.; Zhao, G.; SHI, J. The Research Progress of Zinc Bromine Flow Battery. J. New Mater. Electrochem. Syst. 2018, 21, 063–070. [Google Scholar] [CrossRef]
- Turney, D.E.; Gallaway, J.W.; Yadav, G.G.; Ramirez, R.; Nyce, M.; Banerjee, S.; Chen-Wiegart, Y.C.K.; Wang, J.; D’Ambrose, M.J.; Kolhekar, S.; et al. Rechargeable Zinc Alkaline Anodes for Long-Cycle Energy Storage. Chem. Mater. 2017, 29, 4819–4832. [Google Scholar] [CrossRef]
- Lai, Q.; Zhang, H.; Li, X.; Zhang, L.; Cheng, Y. A Novel Single Flow Zinc-Bromine Battery with Improved Energy Density. J. Power Sources 2013, 235, 1–4. [Google Scholar] [CrossRef]
- Rajarathnam, G.P.; Vassallo, A.M. The Zinc/Bromine Flow Battery Practical Solutions Challenges and Materials for Technology Advancement; Springer: Singapore, 2016; ISBN 978-981-287-646-1. [Google Scholar]
- Jorné, J.; Kim, J.T.; Kralik, D. The Zinc-Chlorine Battery: Half-Cell Overpotential Measurements. J. Appl. Electrochem. 1979, 9, 573–579. [Google Scholar] [CrossRef]
- Leung, P.K.; Ponce De León, C.; Walsh, F.C. An Undivided Zinc-Cerium Redox Flow Battery Operating at Room Temperature (295 K). Electrochem. Commun. 2011, 13, 770–773. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, S.; Zhou, J. Progress and Prospect of the Zinc–Iodine Battery. Curr. Opin. Electrochem. 2021, 30, 100761. [Google Scholar] [CrossRef]
- Lin, D.; Li, Y. Recent Advances of Aqueous Rechargeable Zinc-Iodine Batteries: Challenges, Solutions, and Prospects. Adv. Mater. 2022, 34, 1–28. [Google Scholar] [CrossRef]
- Pan, H.; Li, B.; Mei, D.; Nie, Z.; Shao, Y.; Li, G.; Li, X.S.; Han, K.S.; Mueller, K.T.; Sprenkle, V.; et al. Controlling Solid-Liquid Conversion Reactions for a Highly Reversible Aqueous Zinc-Iodine Battery. ACS Energy Lett. 2017, 2, 2674–2680. [Google Scholar] [CrossRef]
- Xie, C.; Zhang, H.; Xu, W.; Wang, W.; Li, X. A Long Cycle Life, Self-Healing Zinc-Iodine Flow Battery with High Power Density. Angew. Chem. 2018, 130, 11341–11346. [Google Scholar] [CrossRef]
- Li, B.; Nie, Z.; Vijayakumar, M.; Li, G.; Liu, J.; Sprenkle, V.; Wang, W. Ambipolar Zinc-Polyiodide Electrolyte for a High-Energy Density Aqueous Redox Flow Battery. Nat. Commun. 2015, 6, 63031–603038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, G.M.; Li, Z.; Cong, G.; Zhou, Y.; Lu, Y.C. Unlocking the Capacity of Iodide for High-Energy-Density Zinc/Polyiodide and Lithium/Polyiodide Redox Flow Batteries. Energy Env. Sci. 2017, 10, 735–741. [Google Scholar] [CrossRef]
- Eyal, E.; Treinin, A. A Spectrophotometric Study of the System I2 + Br-. J. Am. Chem. Soc. 1964, 86, 4287–4290. [Google Scholar] [CrossRef]
- Xie, C.; Liu, Y.; Lu, W.; Zhang, H.; Li, X. Highly Stable Zinc-Iodine Single Flow Batteries with Super High Energy Density for Stationary Energy Storage. Energy Env. Sci. 2019, 12, 1834–1839. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, G.; Xu, P.; Ghorbani Kashkooli, A.; Mousavi, M.; Yu, A.; Chen, Z. An All-Aqueous Redox Flow Battery with Unprecedented Energy Density. Energy Env. Sci. 2018, 11, 2010–2015. [Google Scholar] [CrossRef]
- Xu, Z.; Fan, Q.; Li, Y.; Wang, J.; Lund, P.D. Review of Zinc Dendrite Formation in Zinc Bromine Redox Flow Battery. Renew. Sustain. Energy Rev. 2020, 127, 109838. [Google Scholar] [CrossRef]
- Yang, M.; Xu, Z.; Xiang, W.; Xu, H.; Ding, M.; Li, L.; Tang, A.; Gao, R.; Zhou, G.; Jia, C. High Performance and Long Cycle Life Neutral Zinc-Iron Flow Batteries Enabled by Zinc-Bromide Complexation. Energy Storage Mater. 2022, 44, 433–440. [Google Scholar] [CrossRef]
- Arenas, L.F.; Loh, A.; Trudgeon, D.P.; Li, X.; Ponce de León, C.; Walsh, F.C. The Characteristics and Performance of Hybrid Redox Flow Batteries with Zinc Negative Electrodes for Energy Storage. Renew. Sustain. Energy Rev. 2018, 90, 992–1016. [Google Scholar] [CrossRef]
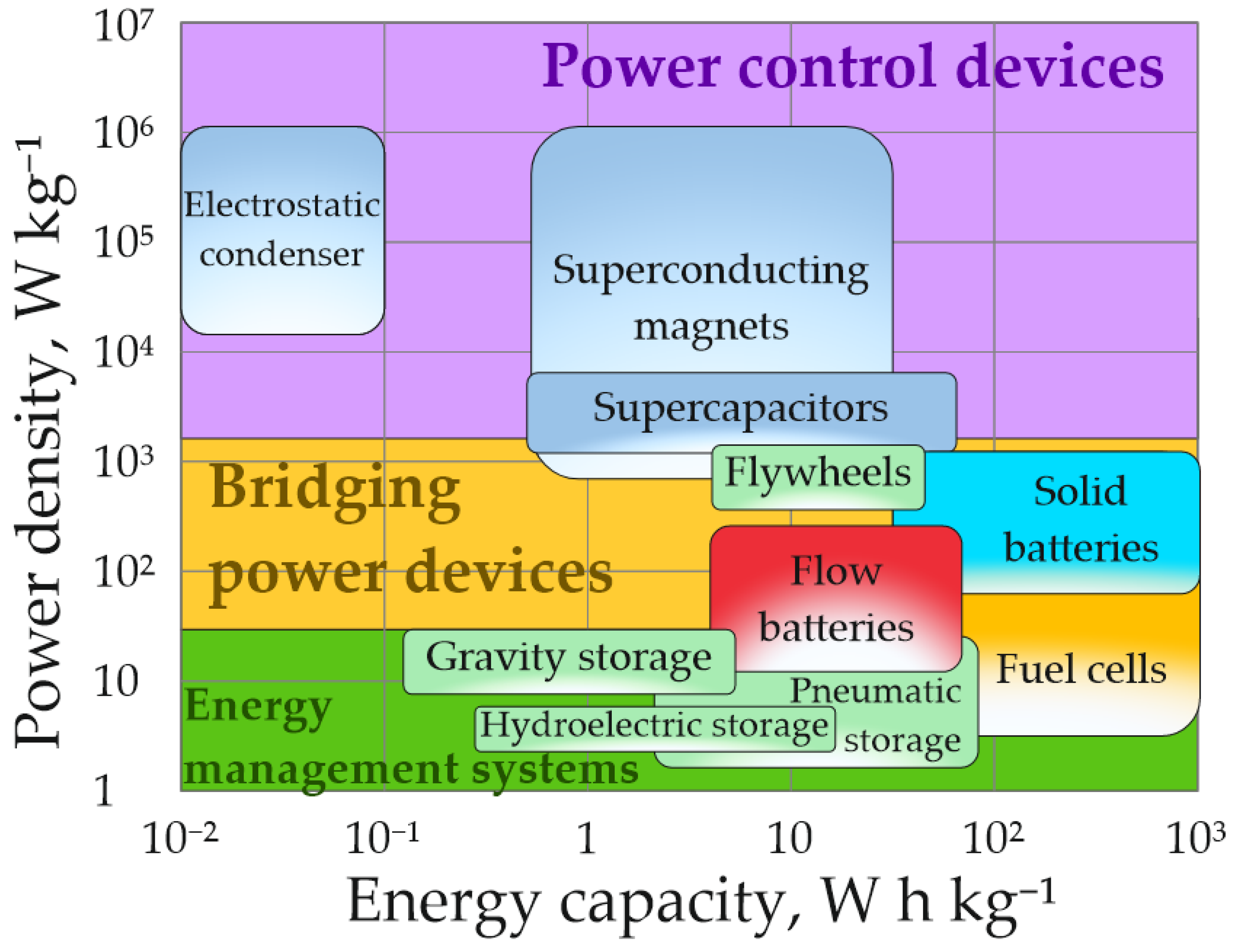
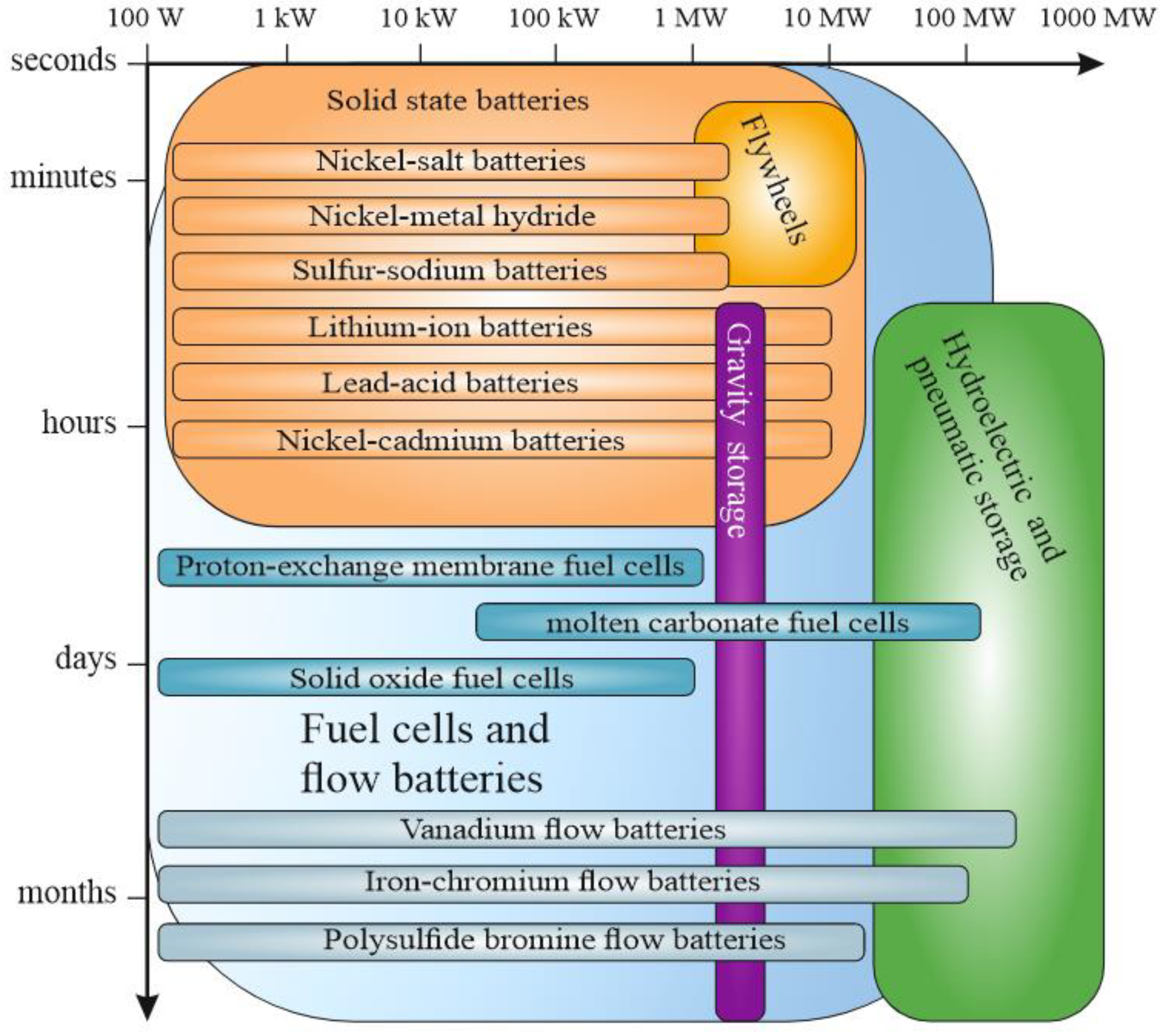
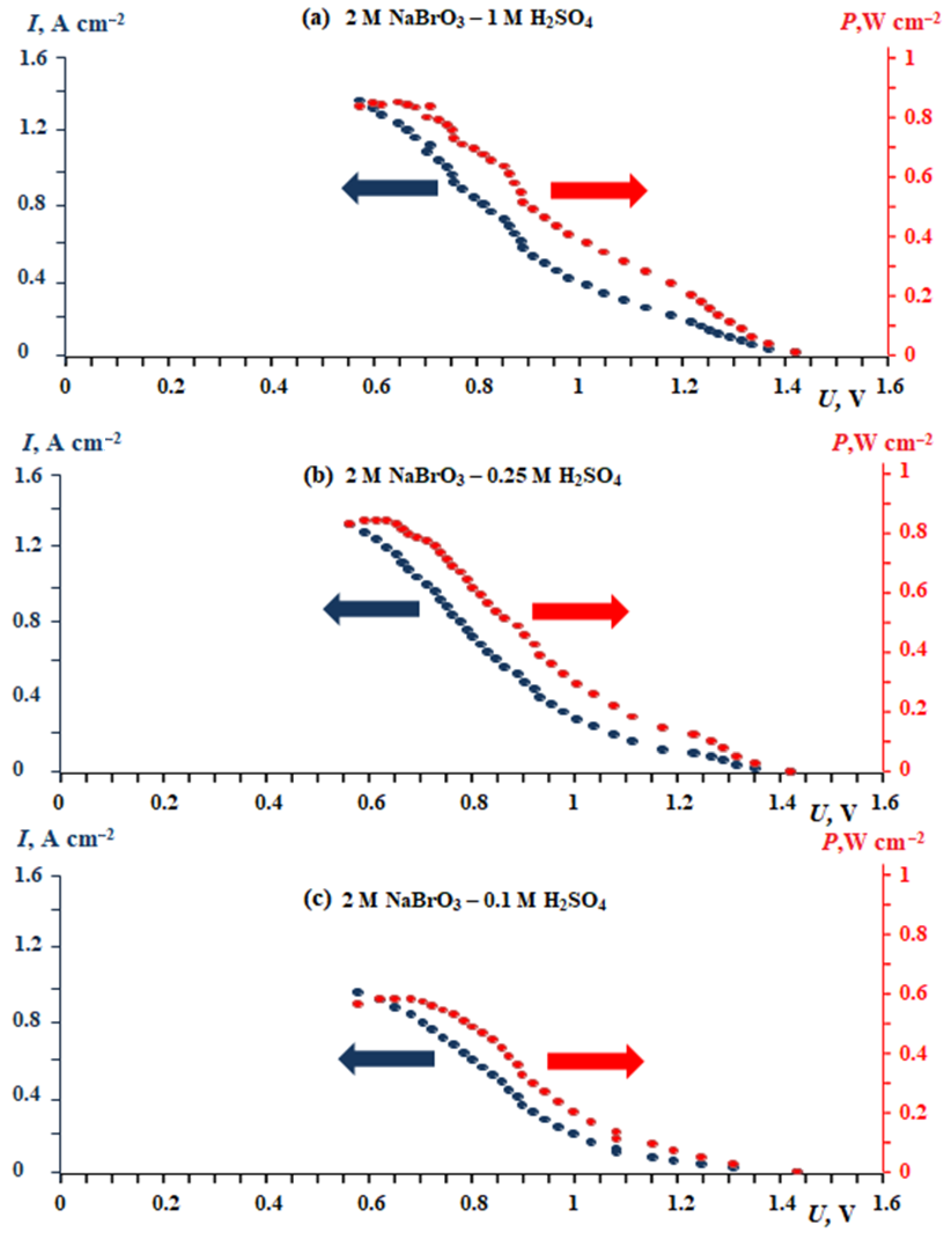

| Power Source Operating Principle | Gravimetric Energy Density, W h kg−1 | Specific Power Density, W kg−1 | Source |
|---|---|---|---|
| Electrostatic capacitor | 0.01–0.1 | 1·104–1·106 | [17] |
| Supercapacitor | 1–75 | 1·103–1.4·103 | [17,18] |
| Superconducting magnet | 0.5–30 | 5·102–1·106 | [19,20] |
| Gravity storage | 0.1–0.5 | 0.5–1.5 | [21,22] |
| Hydroelectric storage | 0.2–0.5 | 0.5–5 | [19,23] |
| Pneumatic accumulator | 3–60 | 2–24 | [24,25] |
| Flywheel | 10–30 | 400–1500 | [19,26,27] |
| Lead acid battery | 33–42 | 80–300 | [19,25,28] |
| Nickel-cadmium battery | 50–75 | 150–500 | [19,24,25,28] |
| Nickel-metal hydride battery | 60–70 | 200–1500 | [24,25,28] |
| Sodium sulfur battery | 150–240 | 150–250 | [19,24,25] |
| Nickel salt battery | 100–125 | 150–200 | [19,24,25,28] |
| Li-ion battery | 75–265 | 80–250 | [19,25,28,29,30] |
| Fuel cell with proton exchange membrane | 300–1000 | 4–675 | [19,24,25,31] |
| Methanol fuel cell | 140–960 | 2–20 | [25,32] |
| Fuel cell based on molten carbonate | 100–610 | 8–36 | [25,33] |
| Solid oxide fuel cell | 410–1500 | 10–80 | [25,33] |
| Vanadium flow battery | 10–50 | 30–170 | [19,25,34] |
| Iron-chromium flow battery | 5–25 | 22–116 | [34] |
| Polysulfide-bromine flow battery | 10–45 | 5–28 | [8,35,36,37] |
| Iron-titanium redox flow battery | 10–50 | 14–74 | [38] |
| Organic flow redox battery | 10–40 | 1–100 | [39] |
| Power Source Operating Principle | Average System Efficiency, % | Total Energy Capacity 1, MW h | Characteristic Energy Storage Times, Sec–Months | Average Service Life, Years (Number of Cycles) | Source |
|---|---|---|---|---|---|
| Gravity storage | 70–80 | 0.1–3 | 1–24 h and more | 50 | [22] |
| Hydroelectric storage | 70–87 | 100–3000 | 1–24 h and more | 30–60 | [40,41,42] |
| Pneumatic storage | 70–89 | 50–300 | 1–24 h and more | 20–40 | [19,42,43,44] |
| Flywheel | 70–90 | 1–20 | Less than 15 min | 15–20 | [19,42,45] |
| Lead-acid battery | 80–90 | 1·10−5–10 | sec–days | 5–10 (200–1000) | [42,46,47] |
| Nickel-cadmium battery | 60–80 | 1·10−5–30 | sec–hours | 10–15 (500–2500) | [42,48] |
| Nickel-metal hydride battery | 65–90 | 1·10−5–5 | sec–hours | 10–15 (1000–1800) | [49,50] |
| Sodium-sulfur battery | 75–90 | 1·10−5–5 | sec–up to 10 h | 5–15 (2500–5000) | [51,52,53,54,55] |
| Nickel-salt battery | 75–90 | 1·10−5–5 | sec–hours | 5–15 (2500–4500) | [56,57,58] |
| Lithium-ion battery | 75–92 | 1·10−5–10 | sec–days | 5–15 (2000–5000) | [19,59,60,61] |
| Fuel cell with proton exchange membrane | 20–85 | 1·10−5–2 | sec–months | 1–3 | [62,63,64] |
| Molten carbonate fuel cell | 70–80 | 1–100 | min–months | 5–10 | [65,66,67,68] |
| Solid oxide fuel cell | 60–85 | 1·10−5–1 | min–days | 2–5 | [68,69,70,71,72] |
| Vanadium flow battery | 65–85 | 1·10−5–200 | sec–months | 10–20 (10,000) | [73,74] |
| Iron-chromium flow battery | 70–80 | 1·10−5–100 | sec–months | 10–20 | [34,75,76,77] |
| Polysulfide-bromine flow battery | 70–80 | 1·10−5–12 | sec–months | 5–15 | [8,78,79,80,81] |
| Type | Power Source Operating Principle | Main Features | Cost of Power, $ kW−1 | Cost of Energy, $ kW−1 h−1 | Source |
|---|---|---|---|---|---|
| Solid batteries | Lead acid | Low cost | 3.5–12 | 75–150 | [83,85] |
| Nickel-cadmium | High pulse power performance | 3–30 | 100–350 | [82,85,87] | |
| Li-ion | Fast and safe charging unprecedented manufacturability | 3.5–80 | 180–950 | [84,85] | |
| Fuel cells | With proton exchange membrane | fast startup, flexibility in input fuel, compact | 34–62 | 600–1500 | [82,85,87] |
| Melt-carbonate | high fuel utilization and power generation efficiency | 40–60 | 500–650 | [85,86,87] | |
| Solid oxide | combined heat and power efficiency, long-term stability | 25–34 | 650–850 | [82,85,86] | |
| Redox flow batteries | Vanadium | no limit on energy capacity, no penalty for mixing electrolytes | 3.5–30 | 75–100 | [82,84,85] |
| Iron-chromium | abundance of Fe and Cr resources, low energy storage cost | 3.1–28 | 50–100 | [82,84,85] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antipov, A.; Pichugov, R.; Abunaeva, L.; Tong, S.; Petrov, M.; Pustovalova, A.; Speshilov, I.; Kartashova, N.; Loktionov, P.; Modestov, A.; et al. Halogen Hybrid Flow Batteries Advances for Stationary Chemical Power Sources Technologies. Energies 2022, 15, 7397. https://doi.org/10.3390/en15197397
Antipov A, Pichugov R, Abunaeva L, Tong S, Petrov M, Pustovalova A, Speshilov I, Kartashova N, Loktionov P, Modestov A, et al. Halogen Hybrid Flow Batteries Advances for Stationary Chemical Power Sources Technologies. Energies. 2022; 15(19):7397. https://doi.org/10.3390/en15197397
Chicago/Turabian StyleAntipov, Anatoly, Roman Pichugov, Lilia Abunaeva, Shengfu Tong, Mikhail Petrov, Alla Pustovalova, Ivan Speshilov, Natalia Kartashova, Pavel Loktionov, Alexander Modestov, and et al. 2022. "Halogen Hybrid Flow Batteries Advances for Stationary Chemical Power Sources Technologies" Energies 15, no. 19: 7397. https://doi.org/10.3390/en15197397





