1. Introduction
In the Tatarstan Republic (Russia), most of the oilfields are at the last stage of development, and about 40% of hydrocarbons remain in carbonate reservoirs [
1,
2]. Most of these carbonate reservoirs are located in the southeast of the Volga region and are confined to the Carboniferous and Devonian systems [
3,
4]. These reservoirs are characterized by high heterogeneity, natural fracture, low hydrophilicity of the rock, and, as a result, the premature breakthrough of water and high water cut. Development of these oilfields is also complicated by the ultra-high salinity of formation water (>200 g/L), high viscosity of oil, and low oilfield temperature [
5,
6]. All these conditions negatively affect the efficiency of the development of these reservoirs.
Improving the profitability of such reservoirs is possible through the use of chemical enhanced oil recovery (EOR) methods. The leading technology of chemical EOR is aqueous solutions of surfactants’ injection or surfactant flooding. Surfactant flooding is a tertiary development method that can help to produce up to 50% residual oil [
7,
8]. Injection of surfactants mobilizes trapped or residual oil stacked in the mature or oil-wet reservoirs by decreasing the interfacial tension (IFT) value and changing rock wettability to hydrophilic [
9,
10,
11]. The addition of surfactants decreases the capillary pressure of water with decreasing IFT according to the Young–Laplace equation. However, there are other mechanisms of oil displacement during spontaneous imbibition, which improves by low IFT values. Adsorption of surfactants in the oil/water interface decreases interfacial tension (N/m), or energy, required to increase the oil/water interfacial surface (J/m
2). This decreasing allows oil drops to stretch or reshape, which mobilizes trapped oil. Moreover, in the case of ultralow IFT, solubilization of oil can occur through the creation of microemulsions [
12]. Another mechanism of surfactant flooding is wettability changing through surfactant adsorption on the rock surface. Carbonate rocks are characterized by hydrophobicity and a high contact angle at the water-rock interface. In this case, the vector of capillary pressure is directed from oil to injected water. Surfactant injection makes it possible to increase hydrophilicity by the adsorption of surfactants on the surface of the rock. Consequently, capillary pressure promotes the penetration of water into the pores, that is, increasing the injectivity of the rock. Thus, changing the direction of capillary pressure mobilizes oil in the pores, activating spontaneous imbibition [
13]. To estimate roughly necessary parameters of surfactants and predict their efficiency, the concept of “capillary number” is used [
14,
15].
Surfactants are amphiphilic molecules, which are conventionally divided into lipophilic (organic tale) and hydrophilic (surfactants head) parts. Depending on the charge of the head, the surfactant can be classified as nonionic (absence of charge) or ionic, such as cationic (positive charge), anionic (negative charge), and amphoteric (zwitterionic) [
16]. The absence of charge reduces the influence of cations and anions of inorganic salts on the nonionic surfactants, which makes it possible to use them in formations with high mineralization [
17]. However, ionic surfactants usually have a higher surface activity and the ability to reduce interfacial tension to ultralow values [
18]. Several works reported about the synergy effect and high efficiency of nonionic-ionic blends [
19,
20], which are preferable for surfactant flooding in reservoirs with high water mineralization, where ionic surfactants effectively decrease IFT, change wettability, and, at the same time, nonionic surfactants can increase the salt tolerance of surfactants’ blend.
High mineralization of formation water limits the use of chemical reagents [
21]; in this regard, the problem of the selection of surfactants arises [
22,
23,
24]. In high salinity carbonate reservoirs, many problems arise, including surfactant sedimentation [
25], high surfactant adsorption [
26], and, in view of this, the selection of an effective surfactant is complicated not only by salinity, reservoir type, temperature, but also by the type and properties of the oil [
27]. A large number of studies such as the “bulk test” (solubility, thermal stability, IFT measures, wettability alteration, and static adsorption tests) and core flooding experiments (spontaneous imbibition, dynamic adsorption, and oil displacement experiments) of surfactants were carried out in conditions of different salinity and temperatures [
13,
28,
29]. However, more often, to reduce the time and costs of research, the screening of surfactants is carried out according to the averaged parameters of the oilfield, not considering production zones and their parameters separately.
Thus, the success of surfactant flooding depends on the individualized selection of surfactants not only for the conditions of the individual oilfield but also separately for each production zone. Despite small differences in the parameters of production zones, upon detailed study, there is a strong influence of various parameters on the effectiveness of the selected surfactants. This work is devoted to the selection of surfactants and the assessment of the effectiveness of surfactant flooding at two production zones (Bashkirian and Vereian) of the Ivinskoe carbonate oilfield complicated by the high salinity of formation water. X-ray scanning of core samples was used to reveal structural differences between both production zones. Two different oil displacement mechanisms of ionic surfactants and ionic-nonionic surfactant blends were evaluated for both production zones.
2. Materials and Methods
2.1. Fluids and Reservoir Conditions
A lot of oilfields in Tatarstan (Russia) are characterized by a low temperature and high mineralization reservoir conditions. Surfactant screening was carried out for Ivinskoe oilfield characterized by high water mineralization and low-temperature carbonate reservoirs. Two reservoirs of the Ivinskoe oilfield were considered—Bashkirian stage (B) and Vereian horizon (V). Both of them are characterized by low temperatures, 21 °C and 22 °C (in the tests we used 22 °C), and ultra-high mineralization of water, 268 g/L, with a high divalent cations (Ca
2+ and Mg
2+) content (
Table 1). The formation water type is chloride-calcium-sodium.
Crude oil from both production zones is characterized as heavy and high-viscosity (
Table 2). Nevertheless, there is a difference in these parameters for the presented production zones which can affect the interaction between surfactant and oil.
2.2. Core Study
Absolute permeability and porosity of cores were measured by porosimeter and permeameter (
Table A1). Average permeability did not differ a lot and was 800 mD of Vereian and 700 mD for Bashkirian cores. The D2 Phaser diffractometer (Brucker, Germany) was used for determining mineral compounds. The quantity of mineral compound was determined by the interpretation of the intensity of the diffraction signal which recalculates to the volume percent concentration. Results showed that there is no significant difference in rock samples’ mineral composition, and both rock samples are 99% calcite (
Figure 1).
To determine the characteristics of the pore space of standard core samples from the presented production zones, a micro and nanofocus research X-ray system for computed tomography General Electric v|tome| XS 240 was used. The survey of standard core samples for this study was carried out using a microfocus tube at a voltage of 140 kV, a current of 130 mA, and a survey resolution of 57 μm.
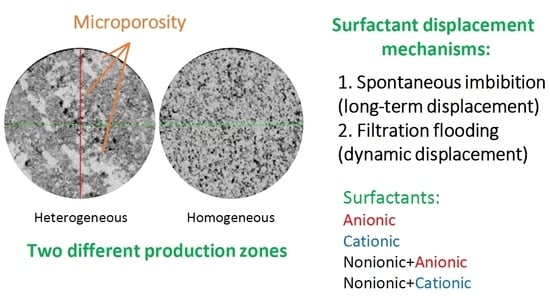
Despite the same mineral composition, the configuration of the pore space in the presented horizons is different. According to the results of computed tomography, the void-pore space of the samples of the (V) is more homogeneous, in contrast to the (B), where there are areas with microporosity (
Figure 2a,b).
2.3. Surfactants
The list of the tested surfactants’ samples includes commercial surfactants that were already successfully applied in various oilfields of the Russian Federation, as well as surfactant compositions evaluated in Kazan Federal University (
Table 3).
The main parameters that affect the choice and efficiency of the surfactant used to improve waterflooding efficiency [
15]:
reservoir type (sandstone, carbonate, etc.);
temperature;
pH, salinity, and composition of formation water;
chemical composition (average length of the alkyl tail, acid number, physicochemical properties of oil, content of paraffin and resins).
According to these parameters, two compositions and two individual surfactants were selected for the screening (
Table 3).
2.4. Solubility and Thermal Stability
To assess solubility and long-term stability, 100 mL of 0.3 wt% surfactant solutions by active content were prepared. Such a surfactant concentration is sufficient to achieve maximum efficiency, since an increase in concentration does not affect a decrease in IFT and is economically unprofitable. The visual method was used to assess the homogeneity of the solution at a reservoir temperature for 14 days.
2.5. IFT Measurements
The investigation of interfacial tension (IFT) in the surfactant solution/crude oil (two types for (B) and (V)) system was carried out by the spinning drop method on the Kruss SDT tensiometer at 22 °C and a surfactant concentration of 0.3 wt%. The spinning drop method makes it possible to determine ultralow values of IFT. This is an important condition in the analysis of the effectiveness of surfactant solutions, which significantly reduce the values of IFT relative to the initial level. Initial IFT between brine and crude oil was measured at the formation water-oil interface, which was considered a reference sample. The measurement of interfacial tension at the surfactant solution-oil continued until stabilization.
2.6. Wettability Measurements
Wettability is another important factor that strongly influences oil displacement efficiency in surfactant flooding, especially for oil-wet carbonate formations. Wettability was measured by the contact angle method (KRUSS DSA 100) at surfactants’ concentration of 0.3 wt% by the active substance.
To achieve the highest quality of the studied image, the investigated water droplet is shone from one side, and the contour of the droplet is observed on the other side. Thus, in the course of a laboratory experiment, a scaled image of a drop with a graphically applied wetting edge angle is observed on the monitor screen. On the wetted surface, the aqueous phase spreads, while the contact angle tends to zero. If the surface is not wetted, a spherical drop forms, and the contact angle approaches 180°. Therefore, the most effective wetting agents provide the smallest contact angle.
All cores were aged in oil. For each core, three contact angle measurements were performed. The first measurement of wettability was water drop and surfactant solution drop on the aged core. Then, cores were placed in surfactant solution for 1, 4, and 24 h, and the contact angle of water was measured again.
2.7. Imbibition in Amott Cells
Generally, surfactants increase oil displacement during spontaneous imbibition by wettability alteration to water-wet (increasing hydrophilicity) and increasing water capillary pressure. The process of capillary displacement of oil with a surfactant solution is enhanced by the effect of oil solubilization in a surfactant solution with the formation of a microemulsion [
12]. This process is accompanied by phase inversion at the moment when the interfacial tension is extremely low. This effect contributes to the transfer of residual oil into a mobile state, which increases oil recovery. The formation of microemulsions in the cells confirms this phenomenon and determines the best properties of certain surfactant models.
The oil displacement coefficient during spontaneous imbibition (
KIORF) was determined as the ratio of the sum of the total volume of displaced oil (
VIo) and volume of oil contained in the microemulsion (
VIme) to the
OOIPI—original oil in place (1):
2.8. Filtration Experiments
The multicomponent composition of the rock, which is represented by core samples, as well as complex reservoir conditions, can have a great impact on the effectiveness of aqueous surfactant solutions when used in a real field. Therefore, to compare the best surfactant samples, a series of filtration studies was carried out for the experimental modeling of the surfactant flooding technology.
Filtration experiments were carried out on a typical filtration setup (
Figure 3).
Dried core samples, cleaned of hydrocarbons and inorganic impurities, were saturated with formation water under vacuum for 24 h. Water saturated cores were placed in the core holder, and reservoir conditions were created. After that, water was injected through the cores with volumetric rates 5, 10, and 20 mL/min to determine phase permeability by water (
kw) (2). After that, the cores were saturated with kerosene and aged for 2 h. Kerosene is a more mobile liquid than oil, displaces water from the smallest pores, and also restores the wettability of the extracted rock (according to Russian standards). Thus, at the next stage, the oil not only displaces kerosene but also replaces it in the smallest pores as a result of diffusion. After oil saturation, the cores were aged in reservoir conditions for 16 h for the final restoration of wettability.
where
q—volumetric flow,
μw—viscosity of water,
L—length of core,
F—cross-section area of core, ∆
P—pressure drop.
The first stage of the filtration experiment was the oil displacement stage by 0.2 mL/min water injection. Water injection continued until 100% water cut at the outlet, but not less than 5 pore volumes (PV). Based on the results of this stage, the Oil recovery factor (
ORF) (3) and injectivity parameter of the core samples (
I1) (4) were calculated.
where
Vd—volumes of oil displaced by water,
OOIP—original oil in place.
The second stage involved injection of 3 PV surfactant solution at 0.2 mL/min; the third—water injection at the same rate until full water cut at the outlet, but not less than 5 pore volumes (PV). At this stage, the additional oil recovery factor (
AORF) and injectivity parameter (
I2) were calculated by the Formula (5):
where
Vro—the volume of displaced oil after surfactant solution injection.
The efficiency of surfactants was estimated by the coefficient (
KORF) which was defined as the ratio of the total volume of displaced oil at both stages (before and after surfactant slug injection) to the volume of displaced oil after surfactant injection (6):
The effect of the surfactant on the change in the rock wettability was also assessed by the relative parameter, which considered the injectivity before and after the surfactant injection—the coefficient of injectivity change (
KI) (7):
where
I1 is injectivity of the sample during water flooding;
I2 is injectivity during subsequent water flooding after the surfactant slug injection.