Numerical Study on the Homogeneous Reactions of Mercury in a 600 MW Coal-Fired Utility Boiler
Abstract
:1. Introduction
2. Utility Boiler and Input Parameters
2.1. Boiler Description and Mesh Generation
2.2. Coal Parameters and Boundary Conditions
3. Computational Modeling
3.1. Coal Combustion and NOx Formation
3.2. Homogeneous Oxidation of Hg0
4. Results and Discussion
4.1. Model Validation
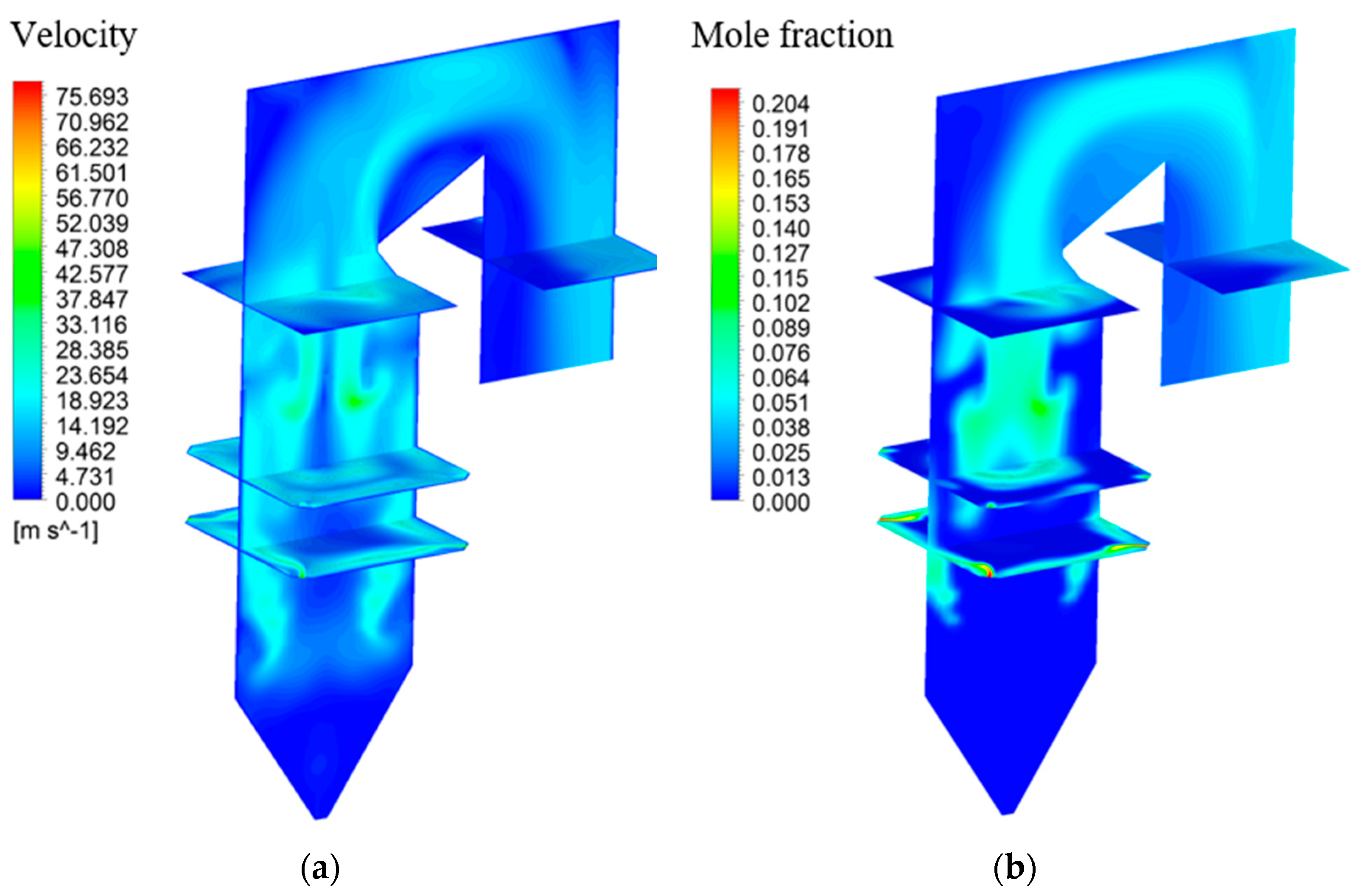
4.2. Simulation Results before Considering Hg Reactions
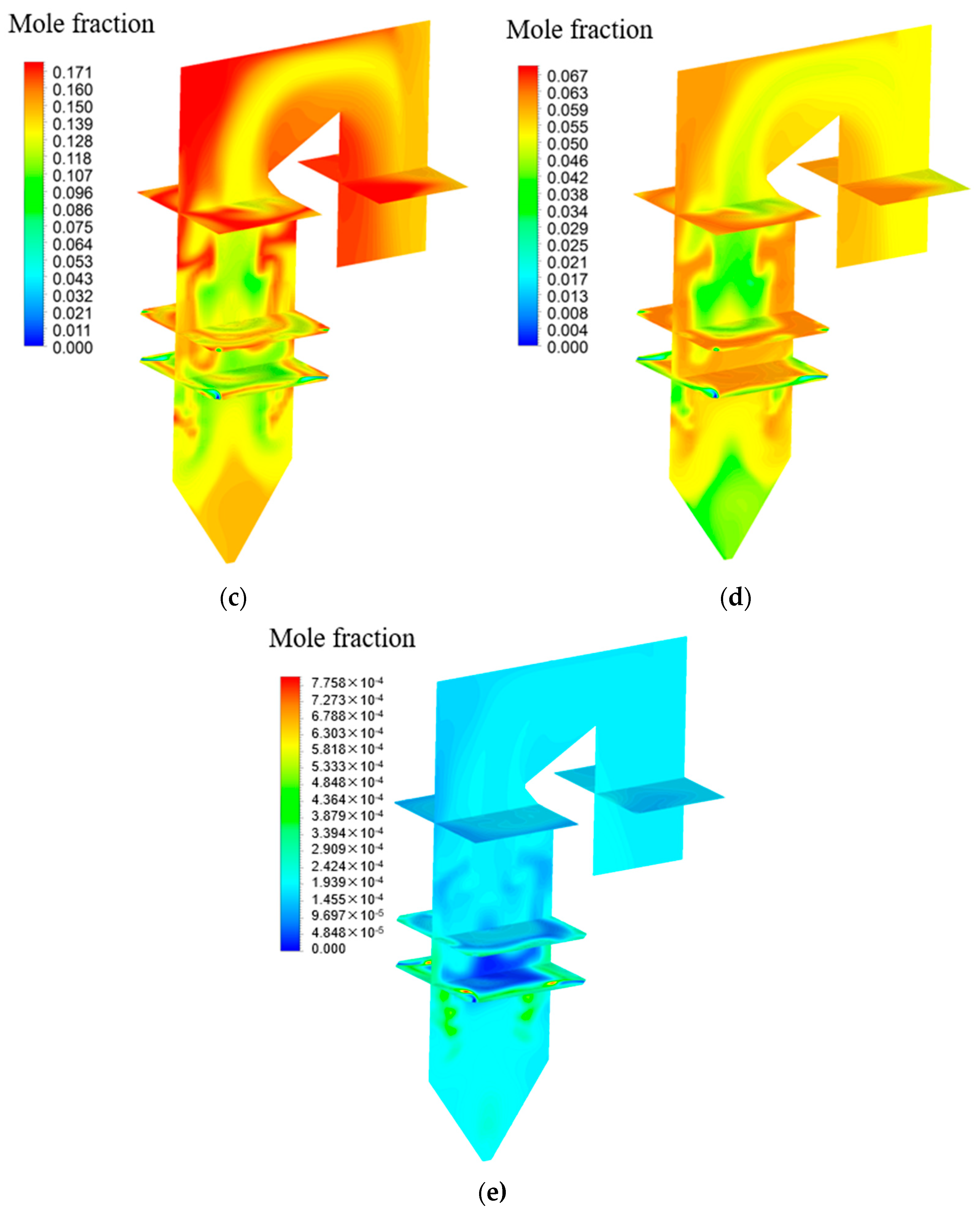
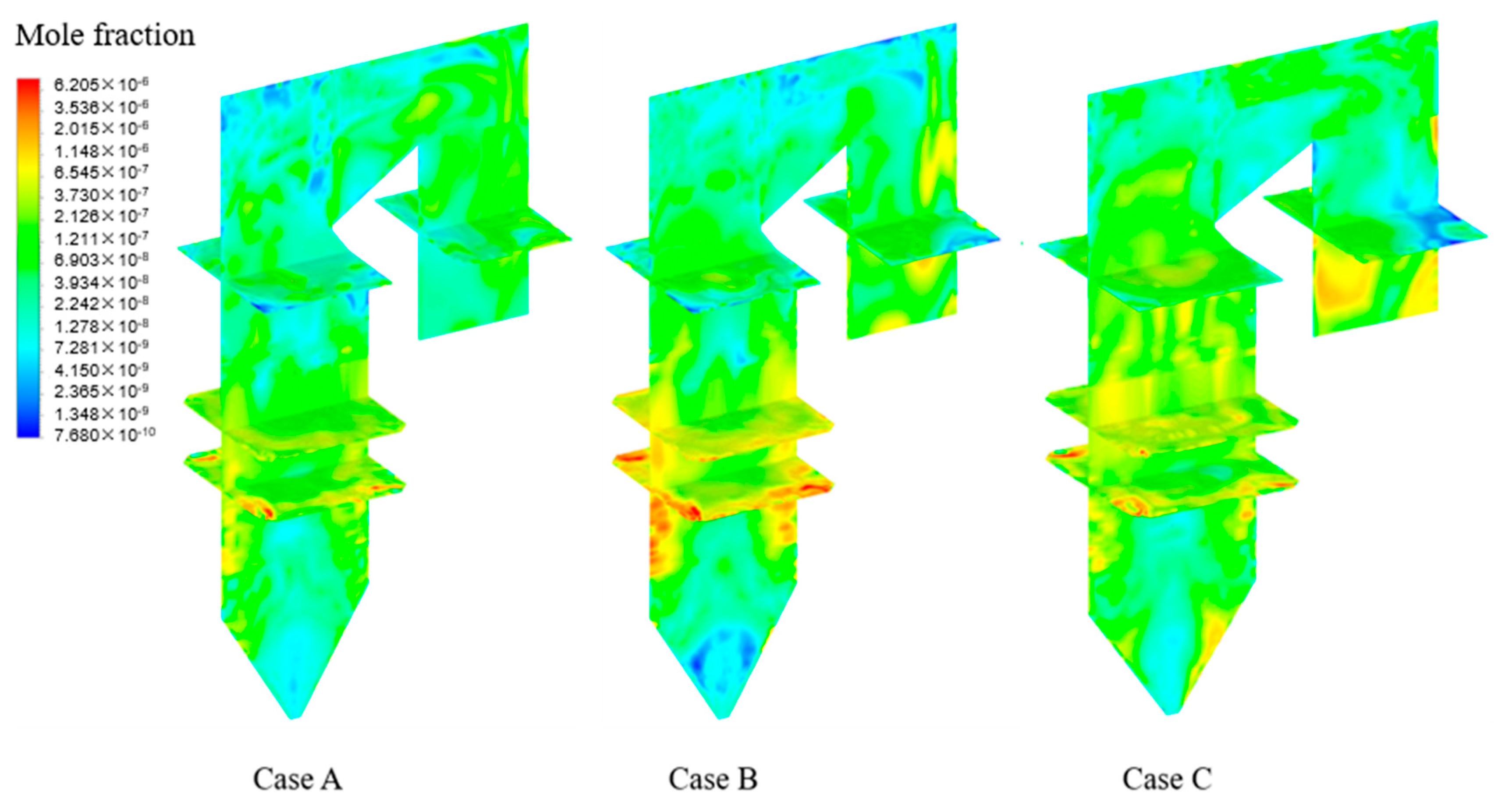
4.3. Effects of Chlorine Content in Coal on Homogeneous Reactions of Hg
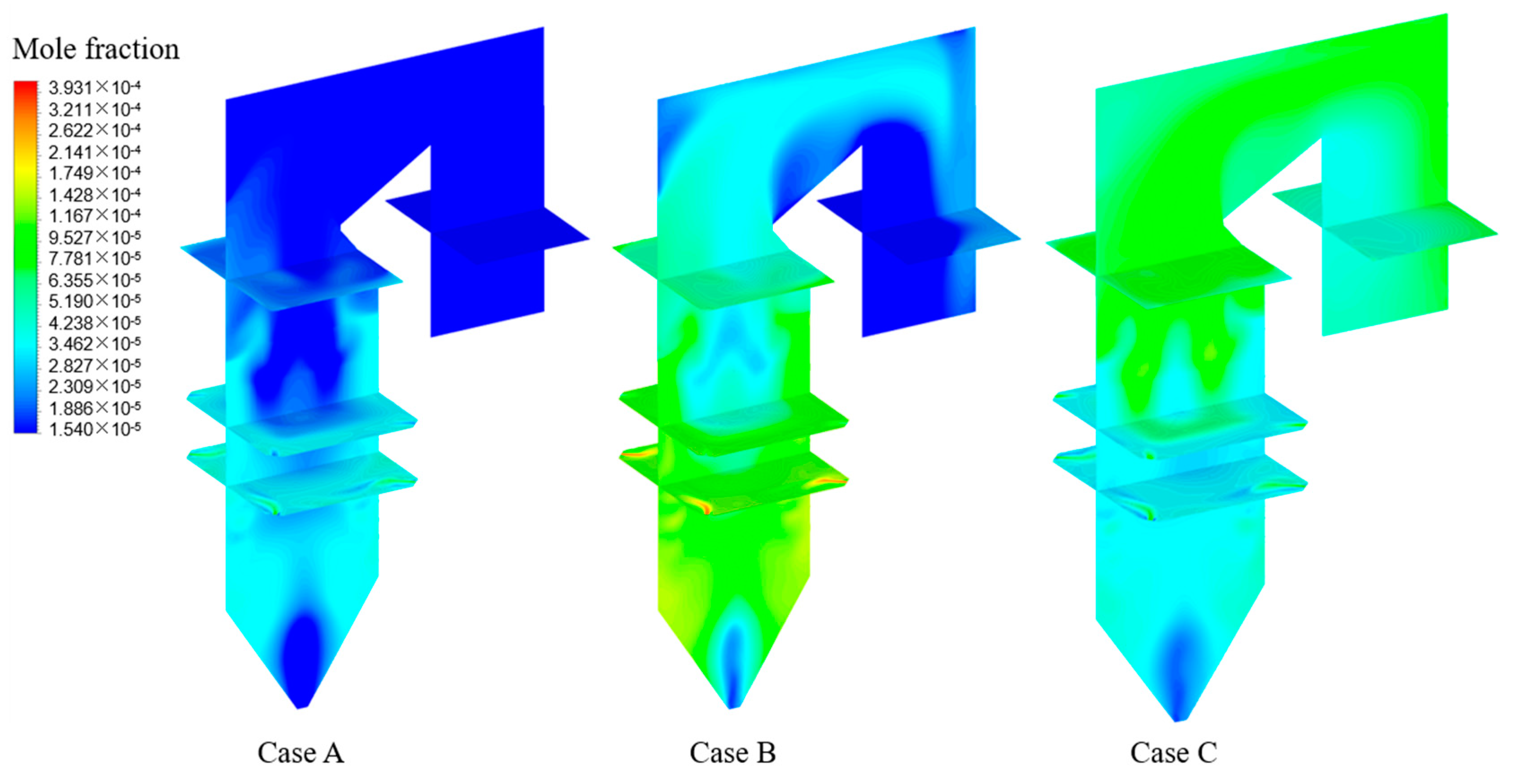
4.4. Effects of Adding HCl on Homogeneous Reactions of Hg
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fernández-Miranda, N.; Rodríguez, E.; Lopez-Anton, M.A.; García, R.; Martínez-Tarazona, M.R. A new approach for retaining mercury in energy generation processes: Regenerable carbonaceous sorbents. Energies 2017, 10, 1311. [Google Scholar] [CrossRef] [Green Version]
- Lv, Q.; Cai, M.; Wang, C.A.; He, Y.; Che, D.F. Investigation on elemental mercury removal by cerium modified semi-coke. J. Energy Inst. 2020, 93, 666–678. [Google Scholar] [CrossRef]
- Li, X.; Teng, Y.; Zhang, K.; Peng, H.; Cheng, F.; Yoshikawa, K. Mercury migration behavior from flue gas to fly ashes in a commercial coal-fired CFB power plant. Energies 2020, 13, 1040. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Liu, J.; Wang, Z. Reaction mechanisms and chemical kinetics of mercury transformation during coal combustion. Prog. Energ. Combust. 2020, 79, 100844. [Google Scholar] [CrossRef]
- Contreras, M.L.; Ganesh, N.; Rodilla, I.; Bahillo, A. Assess of biomass co-firing under oxy-fuel conditions on Hg speciation and ash deposit formation. Fuel 2018, 215, 395–405. [Google Scholar] [CrossRef]
- Galbreath, K.C.; Zygarlicke, C.J. Mercury transformations in coal combustion flue gas. Fuel Process. Technol. 2000, 65, 289–310. [Google Scholar] [CrossRef]
- Senior, C.L.; Sarofim, A.F.; Zeng, T.F.; Helble, J.J.; Mamani-Paco, R. Gas-phase transformations of mercury in coal-fired power plants. Fuel Process. Technol. 2000, 63, 197–213. [Google Scholar] [CrossRef]
- Frandsen, F.; Dam-Johansen, K.; Rasmussen, P. Trace elements from combustion and gasification of coal—An equilibrium approach. Prog. Energ. Combust. 1994, 20, 115–138. [Google Scholar] [CrossRef]
- Chen, L.; Duan, Y.; Zhuo, Y.; Yang, L.G.; Zhang, L.; Yang, X.H.; Yao, Q.; Jiang, Y.M.; Xu, X.C. Mercury transformation across particulate control devices in six power plants of China: The co-effect of chlorine and ash composition. Fuel 2007, 86, 603–610. [Google Scholar]
- Ito, S.; Yokoyama, T.; Asakura, K. Emissions of mercury and other trace elements from coal-fired power plants in Japan. Sci. Total Environ. 2006, 368, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Pavlish, J.H.; Holmes, M.J.; Benson, S.A.; Crocker, C.R.; Galbreath, K.C. Application of sorbents for mercury control for utilities burning lignite coal. Fuel Process. Technol. 2004, 85, 563–576. [Google Scholar] [CrossRef]
- Laudal, D.L.; Brown, T.D.; Nott, B.R. Effects of flue gas constituents on mercury speciation. Fuel Process. Technol. 2000, 65, 157–165. [Google Scholar] [CrossRef]
- Wang, J.S.; Clements, B.; Zanganeh, K. An interpretation of flue-gas mercury speciation data from a kinetic point of view. Fuel 2003, 82, 1009–1011. [Google Scholar] [CrossRef]
- Jiao, Y.; Dibble, T.S. First kinetic study of the atmospherically important reactions BrHg+NO2 and BrHg+HOO. Phys. Chem. Chem. Phys. 2017, 19, 1826–1838. [Google Scholar] [CrossRef]
- Sliger, R.N.; Kramlich, J.C.; Marinov, N.M. Towards the development of a chemical kinetic model for the homogeneous oxidation of mercury by chlorine species. Fuel Process. Technol. 2000, 65, 423–438. [Google Scholar] [CrossRef]
- Galbreath, K.C.; Zygarlicke, C.J.; Tibbetts, J.E.; Schulz, R.L.; Dunham, G.E. Effects of NOx, α-Fe2O3, γ-Fe2O3, and HCl on mercury transformations in a 7-kW coal combustion system. Fuel Process. Technol. 2004, 86, 429–448. [Google Scholar] [CrossRef]
- Wang, F.; Li, G.; Wang, S.; Wu, Q.; Zhang, L. Modeling the heterogeneous oxidation of elemental mercury by chlorine in flue gas. Fuel 2020, 262, 116506. [Google Scholar] [CrossRef]
- Lv, Q.; Wang, C.A.; He, Y.; Cai, M.; Che, D.F. Elemental mercury removal over CeO2/TiO2 catalyst prepared by sol–gel method. Appl. Sci. 2020, 10, 2706. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Wu, Y.W.; Han, J.; Wang, H.X.; Liu, D.J.; Lu, Q.; Yang, Y.P. Density functional theory study on mechanism of mercury removal by CeO2 modified activated carbon. Energies 2018, 11, 2872. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Chen, X.; Liu, J.; Fang, Q.; Zhang, C.; Li, Y.; Mao, R.; Ren, L.; Zhang, P.; Chen, G. Insights into the causes and controlling strategies of gas temperature deviation in a 660 MW tangentially fired tower-type boiler. Appl. Therm. Eng. 2021, 196, 117297. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Tan, H.; Mo, Q.; Wang, X.; Wang, Y. Numerical and experimental study on co-firing of low volatile coal in a 330 MW tangentially fired boiler. J. Energy Inst. 2021, 96, 242–250. [Google Scholar] [CrossRef]
- Sankar, G.; Chandra, S.D.; Santhosh, K.D.; Balasubramanian, K.R. Numerical simulation of the heat transfer and NOx emissions in a 660 MW tangentially fired pulverised-coal supercritical boiler. Heat Mass Transfer 2020, 56, 2693–2709. [Google Scholar]
- Jiang, Y.; Lee, B.-H.; Oh, D.-H.; Jeon, C.-H. Optimization of operating conditions to achieve combustion stability and reduce NOx emission at half-load for a 550-MW tangentially fired pulverized coal boiler. Fuel 2021, 306, 121727. [Google Scholar] [CrossRef]
- Che, D. Boilers-Theory, Design and Operation, 1st ed.; Xi’an Jiaotong University Press: Xi’an, China, 2008; p. 245. [Google Scholar]
- Vuthaluru, R.; Vuthaluru, H.B. Modelling of a wall fired furnace for different operating conditions using FLUENT. Fuel Process. Technol. 2006, 87, 633–639. [Google Scholar] [CrossRef]
- Hurt, R.; Sun, J.K.; Lunden, M. A kinetic model of carbon burnout in pulverized coal combustion. Combust. Flame 1998, 113, 181–197. [Google Scholar] [CrossRef]
- Haas, J.; Tamura, M.; Weber, R. Characterization of coal blends for pulverized fuel combustion. Fuel 2001, 80, 1317–1323. [Google Scholar] [CrossRef]
- Vasquez, S.A.; Ivanov, V.A. A Phase Coupled Method for Solving Multiphase Problems on Unstructured Meshes. In Proceedings of the ASME 2000 Fluids Engineering Division Summer Meeting, Boston, MA, USA, 11–15 June 2000. [Google Scholar]
- Hill, S.C.; Smoot, L.D. Modeling of nitrogen oxides formation and destruction in combustion systems. Prog. Energ. Combust. 2000, 26, 417–458. [Google Scholar] [CrossRef]
- Magnussen, B.F. On the Structure of Turbulence and a Generalized Eddy Dissipation Concept for Chemical Reaction in Turbulent Flow. In Proceedings of the 19th Aerospace Sciences Meeting, St. Louis, MO, USA, 12–15 January 1981. [Google Scholar]
- Gran, I.R.; Magnussen, B.F. A numerical study of a bluff-body stabilized diffusion flame. Part 2. Influence of combustion modeling and finite-rate chemistry. Combust. Sci. Technol. 1996, 119, 191. [Google Scholar] [CrossRef]
- Mayrhofer, M.; Koller, M.; Seemann, P.; Prieler, R.; Hochenauer, C. Evaluation of flamelet-based combustion models for the use in a flameless burner under different operating conditions. Appl. Therm. Eng. 2021, 183, 116190. [Google Scholar] [CrossRef]
- Sorrentino, G.; Ceriello, G.; Cavaliere, A.; de Joannon, M.; Ragucci, R. Thermo-chemical manifold reduction for tabulated chemistry modeling. Temperature and dilution constraints for smooth combustion reactors. Proc. Combust. Inst. 2021, 38, 5393–5402. [Google Scholar] [CrossRef]
- Wen, X.; Luo, Y.; Wang, H.; Luo, K.; Jin, H.; Fan, J. A three mixture fraction flamelet model for multi-stream laminar pulverized coal combustion. Proc. Combust. Inst. 2018, 37, 2901–2910. [Google Scholar] [CrossRef]
- Pan, H.Y.; Minet, R.G.; Benson, S.W.; Tsotsis, T.T. Process for converting hydrogen-chloride to chlorine. Ind. Eng. Chem. Res. 1994, 33, 2996–3003. [Google Scholar] [CrossRef]
- Xu, M.H.; Qiao, Y.; Zheng, C.G.; Li, L.C.; Liu, J. Modeling of homogeneous mercury speciation using detailed chemical kinetics. Combust. Flame 2003, 132, 208–218. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Yi, G.; Li, N.; Che, D. Effects of air staging conditions on the combustion and NOx emission characteristics in a 600 MW wall fired utility boiler using lean coal. Energ. Fuel. 2013, 27, 5831–5840. [Google Scholar] [CrossRef]
- Brown, T.D.; Smith, D.N.; O’Dowd, W.J.; Hargis Jr, R.A. Control of mercury emissions from coal-fired power plants: A preliminary cost assessment and the next steps for accurately assessing control costs. Fuel Process. Technol. 2000, 65, 311–341. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, M.; Wu, H.; Yang, H. Numerical simulation on mercury emission and transformation of occurrence state in a 410 t/h coal-fired boiler. Proc. CSEE 2012, 32, 65–70. [Google Scholar]
- Kellie, S.; Cao, Y.; Duan, Y.; Li, L.; Chu, P.; Mehta, A.; Carty, R.; Riley, J.T.; Pan, W.-P. Factors affecting mercury speciation in a 100 MW coal-fired boiler with low-NOx burners. Energy Fuels 2005, 19, 800–806. [Google Scholar] [CrossRef]
- Zhuang, Y.; Thompson, J.S.; Zygarlicke, C.J.; Pavlish, J.H. Impact of calcium chloride addition on mercury transformations and control in coal flue gas. Fuel 2007, 86, 2351–2359. [Google Scholar] [CrossRef]











| Parameters | Unit | Value |
|---|---|---|
| coal feeding rate | kg·s−1 | 60.0833 |
| theoretical air requirement | kg·kg−1 | 7.6401 |
| total excess air ratio | - | 1.2 |
| excess air ratio in the primary zone | - | 0.8 |
| total air flow rate | kg·s−1 | 550.8512 |
| PA rate | - | 0.29 |
| SA rate | - | 0.51 |
| SOFA rate | - | 0.40 |
| PA temperature | K | 353 |
| SA temperature | K | 612 |
| PA density | kg·m−3 | 1.0000 |
| SA density | kg·m−3 | 0.5768 |
| Length of PA nozzles | mm | 516 |
| Height of UFA nozzles | mm | 218 |
| Height of CCOFA nozzles | mm | 254 |
| Height of SOFA nozzles | mm | 490 |
| Case | Hg0 | HgCl | HgCl2 | HgO |
|---|---|---|---|---|
| A | 99.039 | 0.008 | 0.922 | 0.031 |
| B | 95.196 | 0.041 | 4.746 | 0.017 |
| C | 84.145 | 0.596 | 15.239 | 0.020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, Q.; Wang, C.; Liu, X.; Che, D. Numerical Study on the Homogeneous Reactions of Mercury in a 600 MW Coal-Fired Utility Boiler. Energies 2022, 15, 446. https://doi.org/10.3390/en15020446
Lyu Q, Wang C, Liu X, Che D. Numerical Study on the Homogeneous Reactions of Mercury in a 600 MW Coal-Fired Utility Boiler. Energies. 2022; 15(2):446. https://doi.org/10.3390/en15020446
Chicago/Turabian StyleLyu, Qiang, Chang’an Wang, Xuan Liu, and Defu Che. 2022. "Numerical Study on the Homogeneous Reactions of Mercury in a 600 MW Coal-Fired Utility Boiler" Energies 15, no. 2: 446. https://doi.org/10.3390/en15020446
APA StyleLyu, Q., Wang, C., Liu, X., & Che, D. (2022). Numerical Study on the Homogeneous Reactions of Mercury in a 600 MW Coal-Fired Utility Boiler. Energies, 15(2), 446. https://doi.org/10.3390/en15020446






