Thermodynamics and Kinetic Modeling of the ZnSO4·H2O Thermal Decomposition in the Presence of a Pd/Al2O3 Catalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Thermodynamic Study
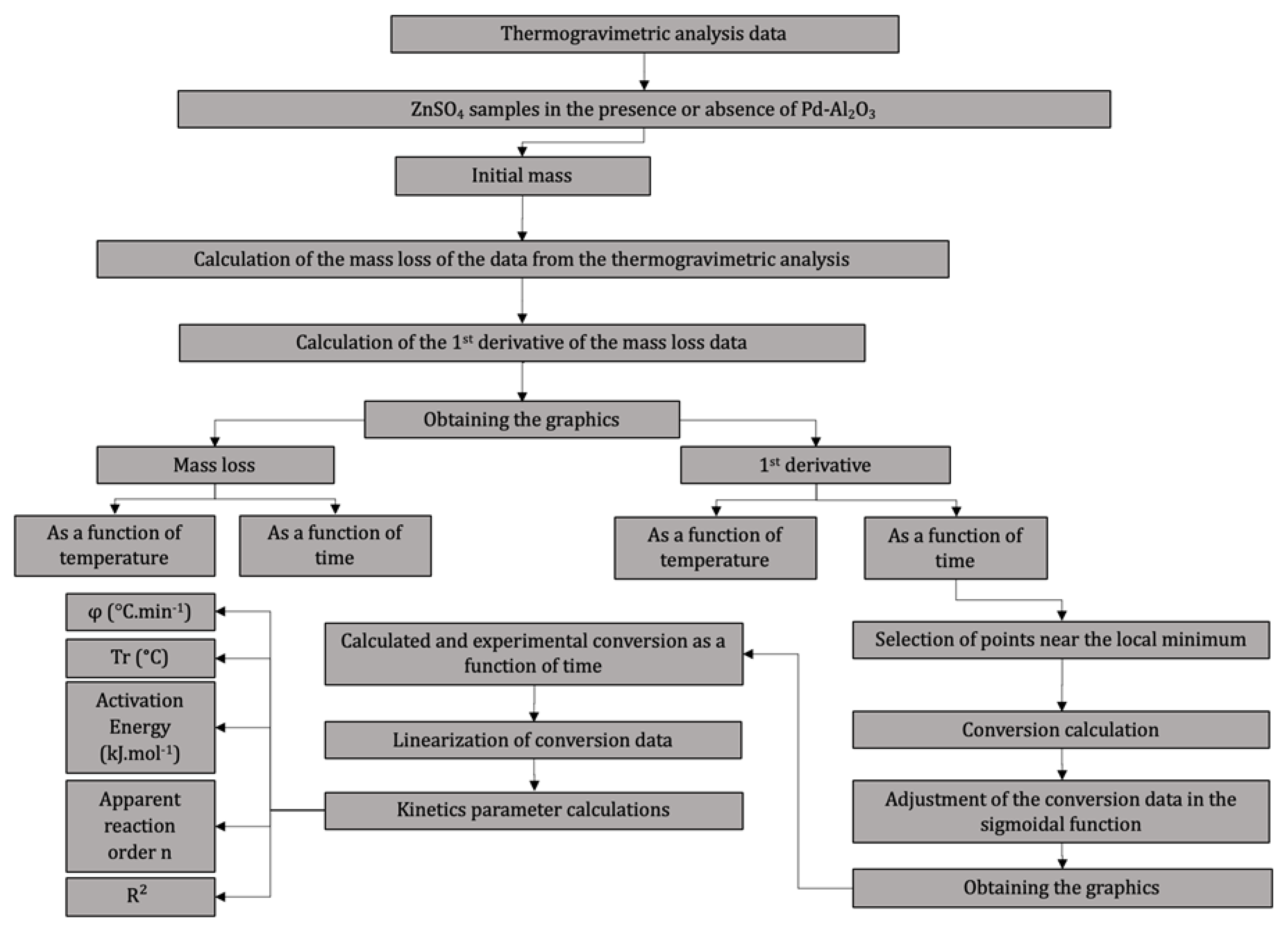
2.2. Thermogravimetric Analysis and Kinetic Modeling
3. Results
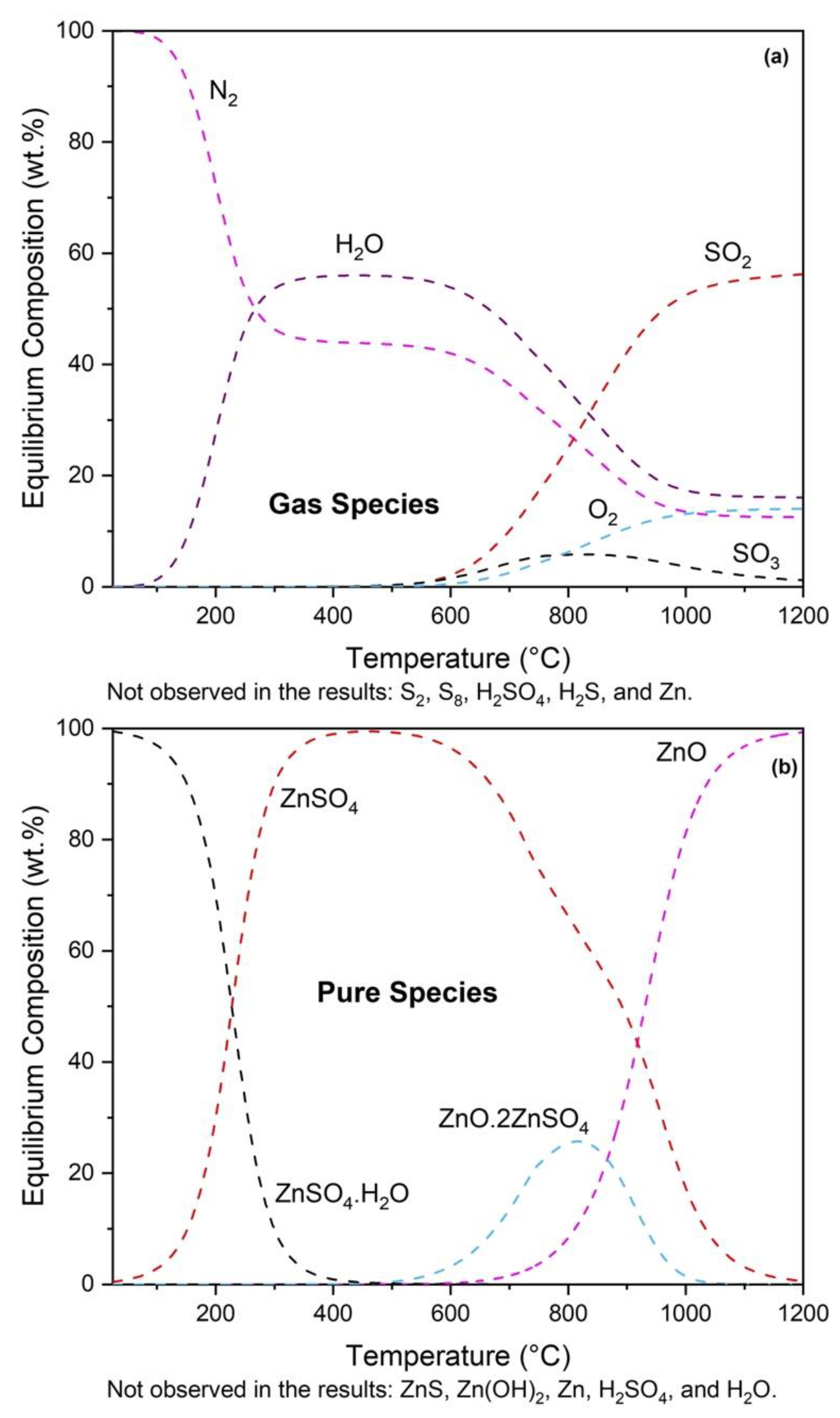
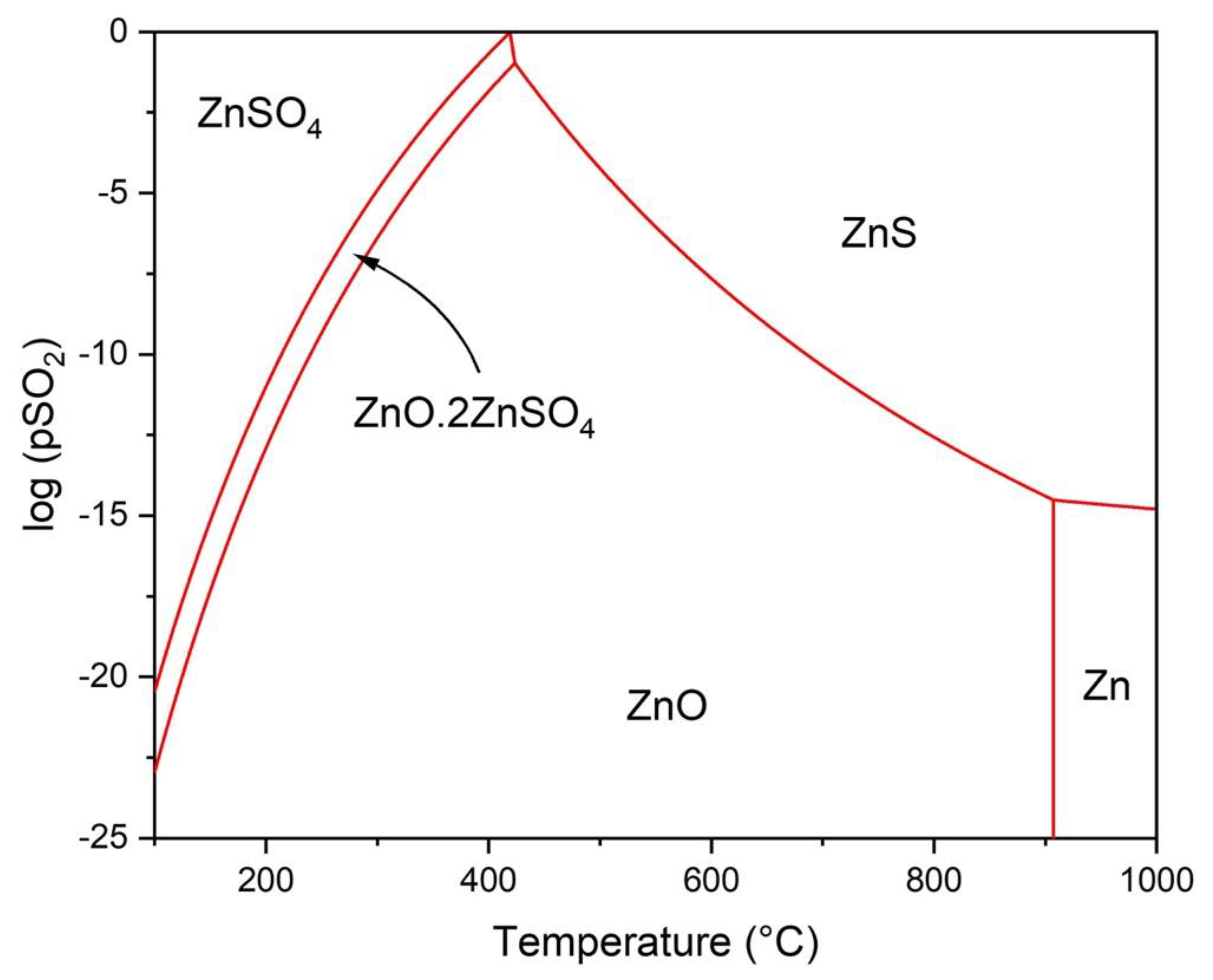
3.1. Thermodynamic Modeling
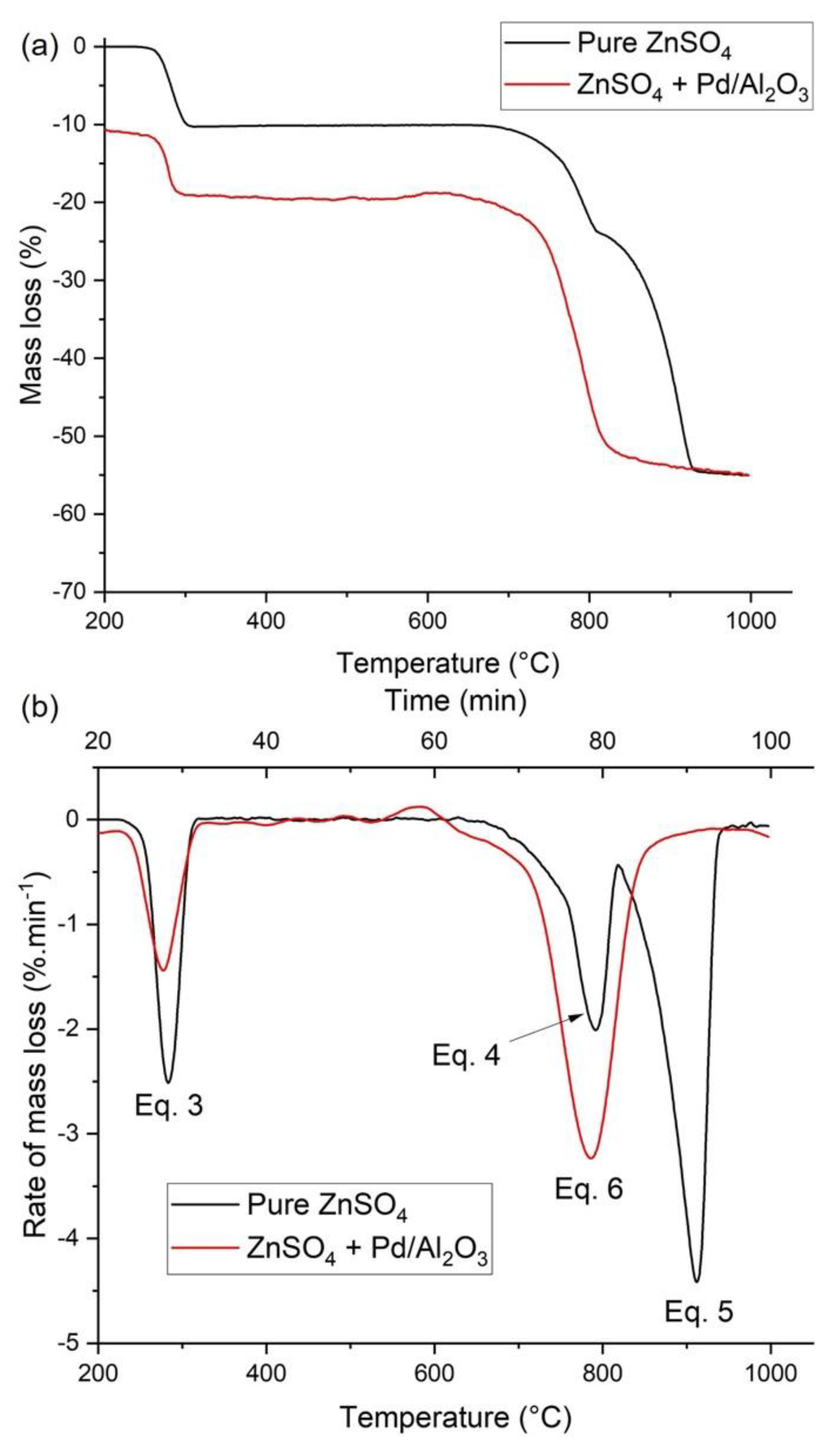
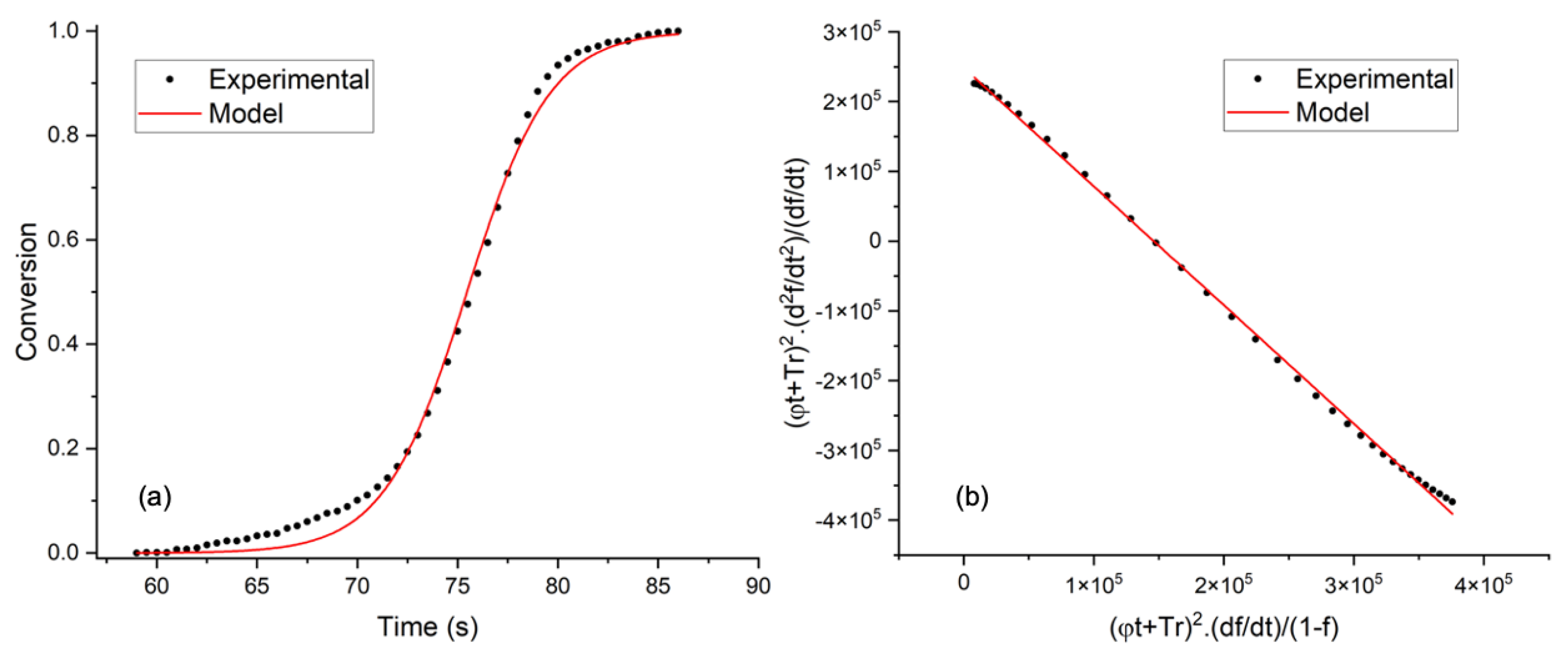
3.2. Thermogravimetric Analysis and Kinetic Modeling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Report of the Conference of the Parties on Its Twenty-First Session, Held in Paris from 30 November to 11 December 2015. Part One: Proceedings; United Nations: Paris, France, 2016; p. 42.
- Sencar, M.; Pozeb, V.; Krope, T. Development of EU (European Union) Energy Market Agenda and Security of Supply. Energy 2014, 77, 117–124. [Google Scholar] [CrossRef]
- Hanley, E.S.; Deane, J.; Gallachóir, B.Ó. The Role of Hydrogen in Low Carbon Energy Futures–A Review of Existing Perspectives. Renew. Sustain. Energy Rev. 2018, 82, 3027–3045. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen Energy, Economy and Storage: Review and Recommendation. Int. J. Hydrog. Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Cavaliere, P.D.; Perrone, A.; Silvello, A. Water Electrolysis for the Production of Hydrogen to Be Employed in the Ironmaking and Steelmaking Industry. Metals 2021, 11, 1816. [Google Scholar] [CrossRef]
- Wang, B.; Peng, Y.; Yao, S. Oxidative Reforming of N-Heptane in Gliding Arc Plasma Reformer for Hydrogen Production. Int. J. Hydrog. Energy 2019, 44, 22831–22840. [Google Scholar] [CrossRef]
- Ismail, A.A.; Bahnemann, D.W. Photochemical Splitting of Water for Hydrogen Production by Photocatalysis: A Review. Sol. Energy Mater. Sol. Cells 2014, 128, 85–101. [Google Scholar] [CrossRef]
- Safari, F.; Dincer, I. A Review and Comparative Evaluation of Thermochemical Water Splitting Cycles for Hydrogen Production. Energy Convers. Manag. 2020, 205, 112182. [Google Scholar] [CrossRef]
- Nouruzi, N.; Dinari, M.; Mokhtari, N.; Farajzadeh, M.; Gholipour, B.; Rostamnia, S. Selective Catalytic Generation of Hydrogen over Covalent Organic Polymer Supported Pd Nanoparticles (COP-Pd). Mol. Catal. 2020, 493, 111057. [Google Scholar] [CrossRef]
- Alamgholiloo, H.; Rostamnia, S.; Hassankhani, A.; Liu, X.; Eftekhari, A.; Hasanzadeh, A.; Zhang, K.; Karimi-Maleh, H.; Khaksar, S.; Varma, R.S.; et al. Formation and Stabilization of Colloidal Ultra-Small Palladium Nanoparticles on Diamine-Modified Cr-MIL-101: Synergic Boost to Hydrogen Production from Formic Acid. J. Colloid Interface Sci. 2020, 567, 126–135. [Google Scholar] [CrossRef]
- Doustkhah, E.; Mohtasham, H.; Farajzadeh, M.; Rostamnia, S.; Wang, Y.; Arandiyan, H.; Assadi, M.H.N. Organosiloxane Tunability in Mesoporous Organosilica and Punctuated Pd Nanoparticles Growth; Theory and Experiment. Microporous Mesoporous Mater. 2020, 293, 109832. [Google Scholar] [CrossRef]
- Abbas, H.F.; Wan Daud, W.M.A. Hydrogen Production by Methane Decomposition: A Review. Int. J. Hydrog. Energy 2010, 35, 1160–1190. [Google Scholar] [CrossRef]
- Ashik, U.P.M.; Wan Daud, W.M.A.; Abbas, H.F. Production of Greenhouse Gas Free Hydrogen by Thermocatalytic Decomposition of Methane—A Review. Renew. Sustain. Energy Rev. 2015, 44, 221–256. [Google Scholar] [CrossRef] [Green Version]
- Syed Muhammad, A.F.; Awad, A.; Saidur, R.; Masiran, N.; Salam, A.; Abdullah, B. Recent Advances in Cleaner Hydrogen Productions via Thermo-Catalytic Decomposition of Methane: Admixture with Hydrocarbon. Int. J. Hydrog. Energy 2018, 43, 18713–18734. [Google Scholar] [CrossRef] [Green Version]
- Onuki, K.; Kubo, S.; Terada, A.; Sakaba, N.; Hino, R. Thermochemical Water-Splitting Cycle Using Iodine and Sulfur. Energy Environ. Sci. 2009, 2, 491–497. [Google Scholar] [CrossRef]
- Norman, J.; Mysels, K.; Sharp, R.; Williamson, D. Studies of the Sulfur-Iodine Thermochemical Water-Splitting Cycle. Int. J. Hydrog. Energy 1982, 7, 545–556. [Google Scholar] [CrossRef]
- Barbarossa, V.; Brutti, S.; Diamanti, M.; Sau, S.; Demaria, G. Catalytic Thermal Decomposition of Sulphuric Acid in Sulphur–Iodine Cycle for Hydrogen Production. Int. J. Hydrog. Energy 2006, 31, 883–890. [Google Scholar] [CrossRef]
- Brown, L.; Besenbruch, G.; Lentsch, R.; Schultz, K.; Funk, J.; Pickard, P.; Marshall, A.; Showalter, S. High Efficiency Generation of Hydrogen Fuels Using Nuclear Power; General Atomics: San Diego, CA, USA, 2003; p. 814014. [Google Scholar]
- O’Keefe, D.; Allen, C.; Besenbruch, G.; Brown, L.; Norman, J.; Sharp, R.; McCorkle, K. Preliminary Results from Bench-Scale Testing of a Sulfur-Iodine Thermochemical Water-Splitting Cycle. Int. J. Hydrog. Energy 1982, 7, 381–392. [Google Scholar] [CrossRef] [Green Version]
- Cerri, G.; Salvini, C.; Corgnale, C.; Giovannelli, A.; De Lorenzo Manzano, D.; Martinez, A.O.; Le Duigou, A.; Borgard, J.-M.; Mansilla, C. Sulfur–Iodine Plant for Large Scale Hydrogen Production by Nuclear Power. Int. J. Hydrog. Energy 2010, 35, 4002–4014. [Google Scholar] [CrossRef]
- Duigou, A.; Borgard, J.; Larousse, B.; Doizi, D.; Allen, R.; Ewan, B.; Hpriestman, G.; Elder, R.; Devonshire, R.; Ramos, V. HYTHEC: An EC Funded Search for a Long Term Massive Hydrogen Production Route Using Solar and Nuclear Technologies. Int. J. Hydrog. Energy 2007, 32, 1516–1529. [Google Scholar] [CrossRef]
- Murphy, J.E.; O’Connell, J.P. Process Simulations of HI Decomposition via Reactive Distillation in the Sulfur–Iodine Cycle for Hydrogen Manufacture. Int. J. Hydrog. Energy 2012, 37, 4002–4011. [Google Scholar] [CrossRef]
- Cho, W.-C.; Park, C.-S.; Kang, K.-S.; Kim, C.-H.; Bae, K.-K. Conceptual Design of Sulfur–Iodine Hydrogen Production Cycle of Korea Institute of Energy Research. Nucl. Eng. Des. 2009, 239, 501–507. [Google Scholar] [CrossRef]
- Leybros, J.; Gilardi, T.; Saturnin, A.; Mansilla, C.; Carles, P. Plant Sizing and Evaluation of Hydrogen Production Costs from Advanced Processes Coupled to a Nuclear Heat Source. Part I: Sulphur–Iodine Cycle. Int. J. Hydrog. Energy 2010, 35, 1008–1018. [Google Scholar] [CrossRef]
- Kaur, H.; Wang, M.; Gorensek, M.B.; Chen, C.-C. Thermodynamic Modeling of the Hybrid Sulfur (HyS) Cycle for Hydrogen Production. Fluid Phase Equilibria 2018, 460, 175–188. [Google Scholar] [CrossRef]
- Bhosale, R.; Kumar, A.; AlMomani, F.; Gupta, R.B. Solar Thermochemical ZnO/ZnSO4 Water Splitting Cycle for Hydrogen Production. Int. J. Hydrog. Energy 2017, 42, 23474–23483. [Google Scholar] [CrossRef]
- Soto-Díaz, O.; Orozco-Mena, R.E.; Román-Aguirre, M.; Romero-Paredes, H.; Camacho-Dávila, A.A.; Ramos-Sánchez, V.H. Metal Sulfate Decomposition Using Green Pd-Based Catalysts Supported on ΓAl2O3 and SiC: A Common Step in Sulfur-Family Thermochemical Cycles. Int. J. Hydrog. Energy 2019, 44, 12309–12314. [Google Scholar] [CrossRef]
- Ginosar, D.M.; Rollins, H.W.; Petkovic, L.M.; Burch, K.C.; Rush, M.J. High-Temperature Sulfuric Acid Decomposition over Complex Metal Oxide Catalysts. Int. J. Hydrog. Energy 2009, 34, 4065–4073. [Google Scholar] [CrossRef]
- Petkovic, L.M.; Ginosar, D.M.; Rollins, H.W.; Burch, K.C.; Pinhero, P.J.; Farrell, H.H. Pt/TiO2 (Rutile) Catalysts for Sulfuric Acid Decomposition in Sulfur-Based Thermochemical Water-Splitting Cycles. Appl. Catal. A Gen. 2008, 338, 27–36. [Google Scholar] [CrossRef]
- Karagiannakis, G.; Agrafiotis, C.C.; Zygogianni, A.; Pagkoura, C.; Konstandopoulos, A.G. Hydrogen Production via Sulfur-Based Thermochemical Cycles: Part 1: Synthesis and Evaluation of Metal Oxide-Based Candidate Catalyst Powders for the Sulfuric Acid Decomposition Step. Int. J. Hydrog. Energy 2011, 36, 2831–2844. [Google Scholar] [CrossRef]
- Ginosar, D.M.; Petkovic, L.M.; Glenn, A.W.; Burch, K.C. Stability of Supported Platinum Sulfuric Acid Decomposition Catalysts for Use in Thermochemical Water Splitting Cycles. Int. J. Hydrog. Energy 2007, 32, 482–488. [Google Scholar] [CrossRef]
- Prosini, P.; Cento, C.; Giaconia, A.; Caputo, G.; Sau, S. A Modified Sulphur–Iodine Cycle for Efficient Solar Hydrogen Production. Int. J. Hydrog. Energy 2009, 34, 1218–1225. [Google Scholar] [CrossRef]
- Tizzoni, A.C.; Corsaro, N.; D’Ottavi, C.; Licoccia, S.; Sau, S.; Tarquini, P. Oxygen Production by Intermediate Metal Sulphates in Sulphur Based Thermochemical Water Splitting Cycles. Int. J. Hydrog. Energy 2015, 40, 4065–4083. [Google Scholar] [CrossRef]
- Bhosale, R.R.; Kumar, A.; van den Broeke, L.J.P.; Gharbia, S.; Dardor, D.; Jilani, M.; Folady, J.; Al-Fakih, M.S.; Tarsad, M.A. Solar Hydrogen Production via Thermochemical Iron Oxide–Iron Sulfate Water Splitting Cycle. Int. J. Hydrog. Energy 2015, 40, 1639–1650. [Google Scholar] [CrossRef]
- Narayan, R.; Tabatabaie-Raissi, A.; Antal, M.J. A Study of Zinc Sulfate Decomposition at Low Heating Rates. Ind. Eng. Chem. Res. 1988, 27, 1050–1058. [Google Scholar] [CrossRef]
- Ibanez, J.; Wentworth, W.E.; Batten, C.F.; Chen, E. Kinetics of the Thermal Decomposition of Zinc Sulfate. Rev. Int. Des Hautes Temp. Des Refract. 1984, 21, 113–124. [Google Scholar]
- Ducarroir, M.; Romeroparedes, H.; Steinmetz, D.; Sibieude, F.; Tmar, M. On the Kinetics of the Thermal Decomposition of Sulfates Related with Hydrogen Water Splitting Cycles. Int. J. Hydrog. Energy 1984, 9, 579–585. [Google Scholar] [CrossRef]
- Mu, J.; Perlmutter, D.D. Thermal Decomposition of Inorganic Sulfates and Their Hydrates. Ind. Eng. Chem. Proc. Des. Dev. 1981, 20, 640–646. [Google Scholar] [CrossRef]
- Kolta, G.A.; Askar, M.H. Thermal Decomposition of Some Metal Sulphates. Thermochim. Acta 1975, 11, 65–72. [Google Scholar] [CrossRef]
- Mello, N.M.; Rego, A.S.C.; Brocchi, E.A.; de Campos, J.B.; Moura, F.J.; Souza, R.F.M. Effect of an Alumina Supported Palladium Catalyst on the Magnesium Sulfate Decomposition Kinetics. Mat. Res. 2020, 23, e20200344. [Google Scholar] [CrossRef]
- Roine, A. HSC Chemistry 9; Metso-Outotec: Pori, Finland, 2019. [Google Scholar]
- Kurban, G.V.T. Decomposição Térmica Do Sulfato de Zinco Na Presença de Agentes Modificadores Do Mecanismo Reacional. Master’s Thesis, Department of Chemical and Materials Engineering—Pontifical Catholic University of Rio de Janeiro, Rio de Janeiro, Brazil, 2019. [Google Scholar]
- Mello, N.M. Estudo Cinético Da Reação de Redução Do NO Pelo CO Em Catalisador de Paládio Suportado Em Alumina. Master’s Thesis, Chemistry Institute—State University of Rio de Janeiro, Rio de Janeiro, Brazil, 2017. [Google Scholar]
- Speyer, R.F. Thermal Analysis of Materials; Materials Engineering; Marcel Dekker: New York, NY, USA, 1994; ISBN 978-0-8247-8963-3. [Google Scholar]
- Vachuška, J.; Vobořil, M. Kinetic Data Computation from Non-Isothermal Thermogravimetric Curves of Non-Uniform Heating Rate. Thermochim. Acta 1971, 2, 379–392. [Google Scholar] [CrossRef]
- Rego, A.S.C.; Marprates, C.V.B.; Silva, T.S.X.; Neto, J.G.; Navarro, R.C.S.; Souza, R.F.M.; Brocchi, E.A. KAl(SO4)2 Thermal Decomposition Kinetics Modeling through Graphical and PSO Methods. J. Mater. Res. Technol. 2021, 14, 1975–1987. [Google Scholar] [CrossRef]
- Straszko, J.; Olszak-Humienik, M.; Możejko, J. Kinetics of Thermal Decomposition of ZnSO4·7H2O. Thermochim. Acta 1997, 292, 145–150. [Google Scholar] [CrossRef]
- Rehman, A.U.; Hayat, A.; Munis, A.; Zhao, T.; Israr, M.; Zheng, M. Characterisation of Magnesium, Zinc and Iron Sulfates for Thermochemical Storage. Proc. Inst. Civ. Eng. Energy 2020, 173, 60–67. [Google Scholar] [CrossRef]
- Ingraham, T.R.; Marier, P. Kinetics of the Thermal Decomposition of ZnSO4 and ZnO2ZnSO4. Can. Metall. Q. 1967, 6, 249–261. [Google Scholar] [CrossRef]
- Tagawa, H.; Saijo, H. Kinetics of the Thermal Decomposition of Some Transition Metal Sulfates. Thermochim. Acta 1985, 91, 67–77. [Google Scholar] [CrossRef]
- Ingraham, T.R.; Kellogg, H.H. Thermodynamic Properties of Zinc Sulfate, Zinc Basic Sulfate, and the System Zn-S-O; Reprint; Department of Mines and Technical Surveys: Ottawa, ON, Canada, 1963; p. R122. [Google Scholar]
- Krikorian, O.; Shell, P. The Utilization of ZnSO4 Decomposition in Thermochemical Hydrogen Cycles. Int. J. Hydrog. Energy 1982, 7, 463–469. [Google Scholar] [CrossRef]
- Gimzewski, E.; Wyatt, P.A.H. The Isothermal Decomposition of Metal Sulphate Powders in High Vacuum. Thermochim. Acta 1981, 44, 125–130. [Google Scholar] [CrossRef]
- O’keefe, D.R.; Norman, J.H.; Williamson, D.G. Catalysis Research in Thermochemical Water-Splitting Processes. Catal. Rev. 1980, 22, 325–369. [Google Scholar] [CrossRef]

| Sample | Step | n | Ea (kJ·mol−1) | R2 |
|---|---|---|---|---|
| ZnSO4 | 1 | 1.76 | 238 | 0.979 |
| 2 | 1.79 | 368 | 0.989 | |
| ZnSO4 + Pd/Al2O3 | 1 | 1.69 | 204 | 0.997 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurban, G.V.T.; Rego, A.S.C.; Mello, N.M.; Brocchi, E.A.; Navarro, R.C.S.; Souza, R.F.M. Thermodynamics and Kinetic Modeling of the ZnSO4·H2O Thermal Decomposition in the Presence of a Pd/Al2O3 Catalyst. Energies 2022, 15, 548. https://doi.org/10.3390/en15020548
Kurban GVT, Rego ASC, Mello NM, Brocchi EA, Navarro RCS, Souza RFM. Thermodynamics and Kinetic Modeling of the ZnSO4·H2O Thermal Decomposition in the Presence of a Pd/Al2O3 Catalyst. Energies. 2022; 15(2):548. https://doi.org/10.3390/en15020548
Chicago/Turabian StyleKurban, Gabriela V. T., Artur S. C. Rego, Nathalli M. Mello, Eduardo A. Brocchi, Rogério C. S. Navarro, and Rodrigo F. M. Souza. 2022. "Thermodynamics and Kinetic Modeling of the ZnSO4·H2O Thermal Decomposition in the Presence of a Pd/Al2O3 Catalyst" Energies 15, no. 2: 548. https://doi.org/10.3390/en15020548
APA StyleKurban, G. V. T., Rego, A. S. C., Mello, N. M., Brocchi, E. A., Navarro, R. C. S., & Souza, R. F. M. (2022). Thermodynamics and Kinetic Modeling of the ZnSO4·H2O Thermal Decomposition in the Presence of a Pd/Al2O3 Catalyst. Energies, 15(2), 548. https://doi.org/10.3390/en15020548








