Thermal Analysis and Optimization of the Phase Diagram of the Cu-Ag Sulfide System
Abstract
:1. Introduction
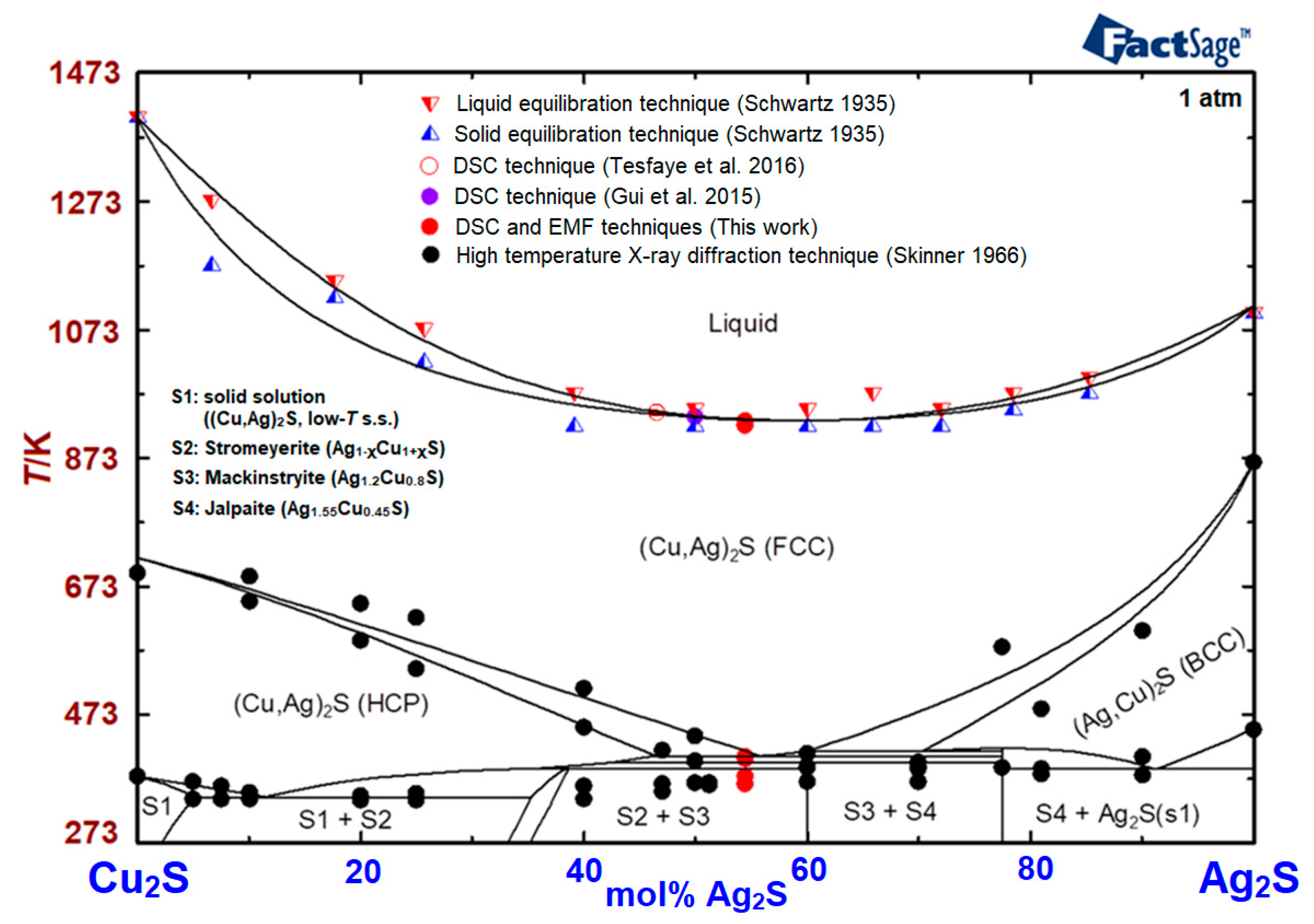
Literature Data on Phase Equilibria and Thermodynamics in the Ag2S-Cu2S System
2. Materials and Methods
2.1. Materials Preparation
2.2. Thermal Analysis
2.3. Electrochemical Examination
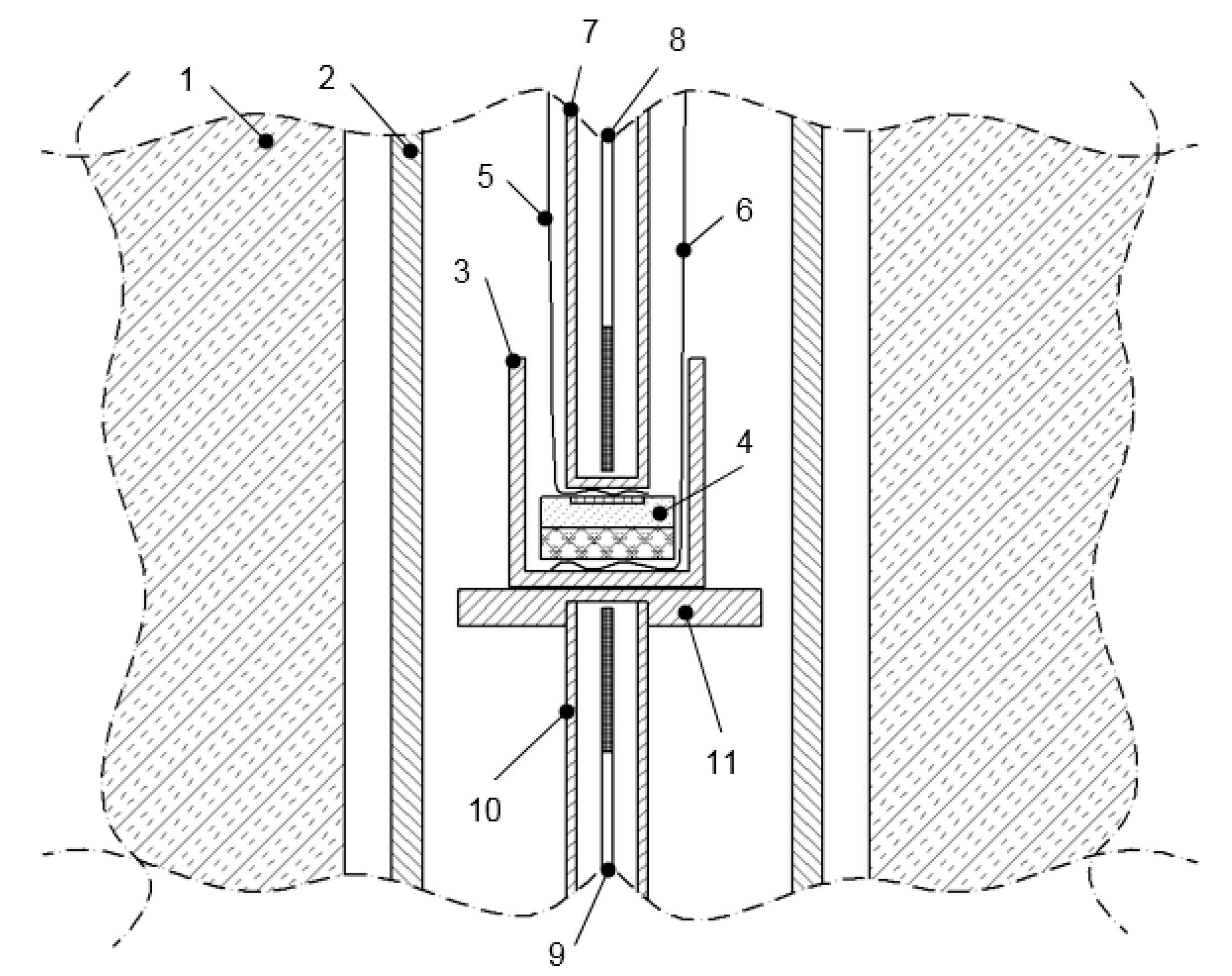
2.3.1. Galvanic Cell Formation and the Experimental Setup
2.3.2. Measuring Devices and the Procedure
3. Thermodynamic Optimization
4. Results and Discussion
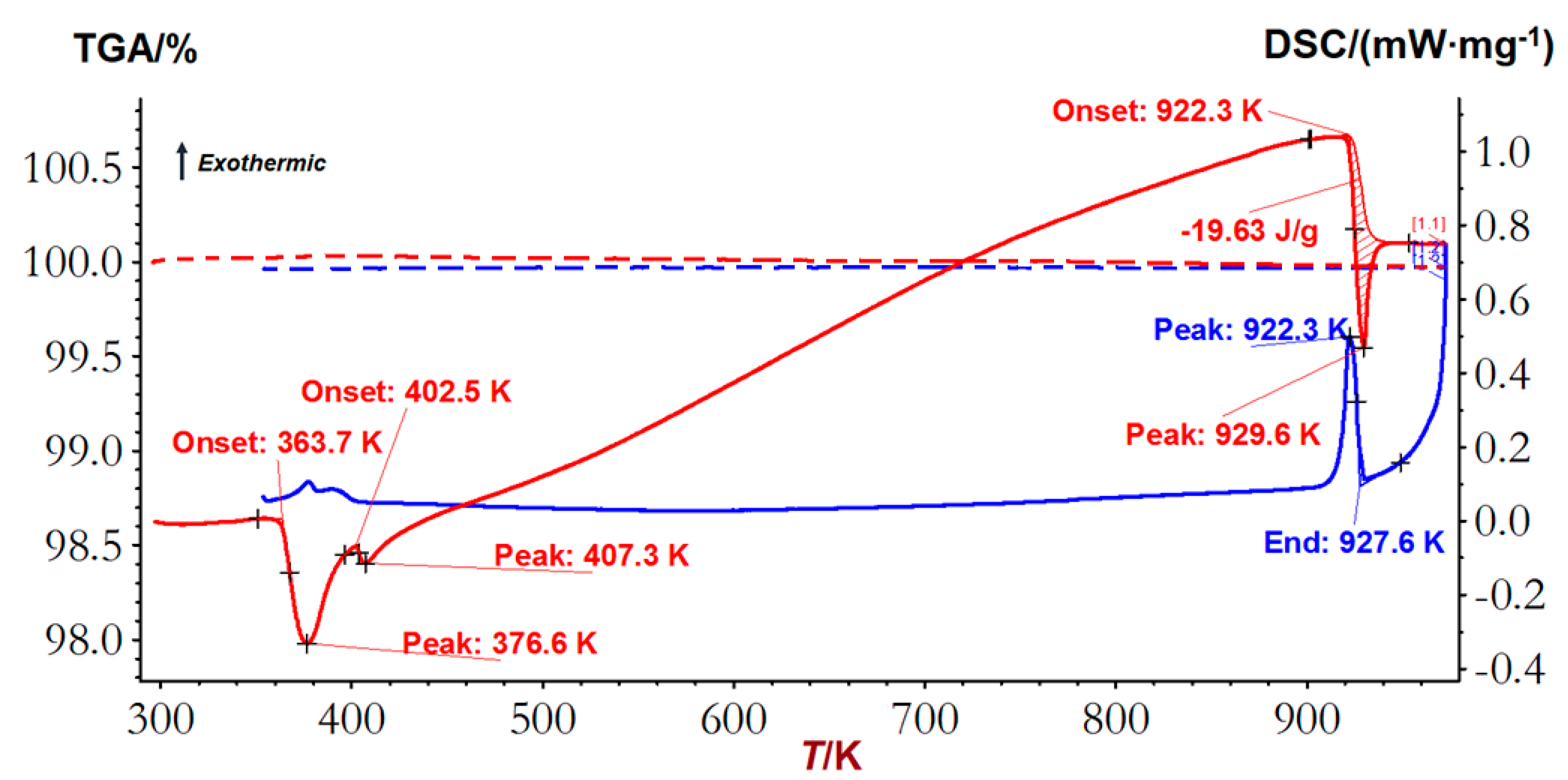
4.1. DSC Measurement Data
4.2. EMF Measurement Data Analysis
Gibbs Energies of Formation (ΔfG°)
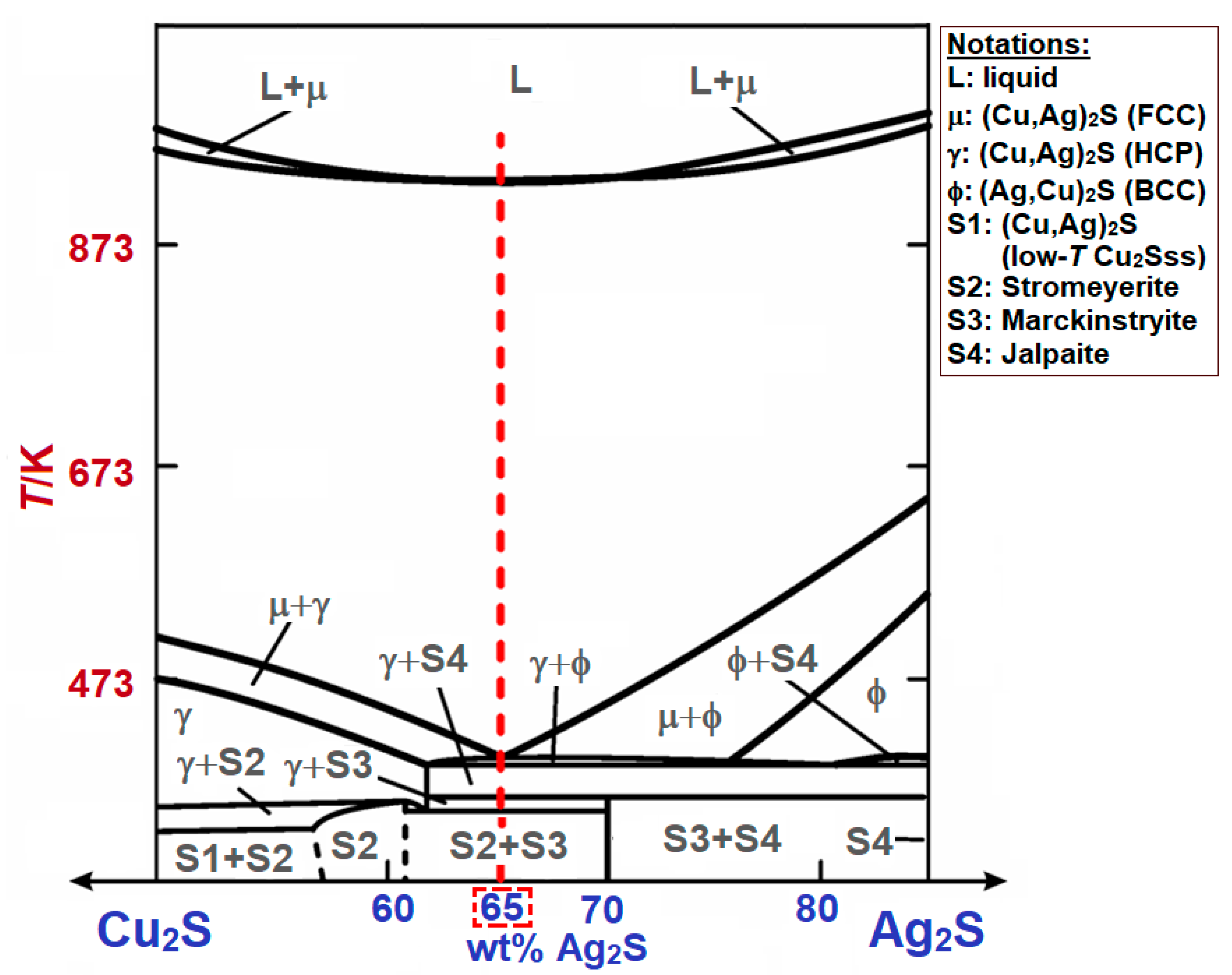
4.3. Results of Thermodynamic Calculations for Optimization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Najdenov, I.; Raić, K.T.; Kokeza, G. Aspects of energy reduction by autogenous copper production in the copper smelting plant Bor. Energy 2012, 43, 376–384. [Google Scholar] [CrossRef]
- Tesfaye, F.; Lindberg, D.; Hamuyuni, J. Valuable metals and energy recovery from electronic waste streams. In Energy Technology 2017 (The Minerals, Metals & Materials Series), 1st ed.; Zhang, L., Drelich, J.W., Neelameggham, N.R., Guillen, D.P., Haque, N., Zhu, J., Sun, Z., Wang, T., Howarter, J.A., Tesfaye, F., et al., Eds.; Springer: Cham, Switzerland, 2017; Volume 1, pp. 103–116. [Google Scholar]
- Craig, J.R.; Kullerud, G. The Cu-Zn-S System. Miner. Depos. 1973, 8, 81–91. [Google Scholar] [CrossRef]
- Tesfaye, F.; Lindberg, D.; Taskinen, P. Solid state electrochemical and calorimetric study of the equilibrium phase (Cu,Ag)2S. J. Chem. Thermodyn. 2016, 94, 101–109. [Google Scholar] [CrossRef]
- Feng, D.; Taskinen, P. Silver Selenide Thermodynamics for Copper Anode Slime Refining. In REWAS 2013; Kvithyld, A., Meskers, C., Kirchain, R., Krumdick, G., Mishra, B., Reuter, M., Wang, C., Schlesinger, M., Gaustad, G., Lados, D., et al., Eds.; Springer: Cham, Switzerland, 2013; pp. 133–139. [Google Scholar]
- Guin, S.N.; Biswas, K. Temperature driven p-n-p type conduction switching materials:current trends and future directions. Phys. Chem. Chem. Phys. 2015, 17, 10316–10325. [Google Scholar] [CrossRef]
- Keen, D.A. Disordering phenomena in superionic conductors. J. Phys. Condens. Matter 2002, 14, 19–57. [Google Scholar] [CrossRef]
- Guin, S.N.; Pan, J.; Bhowmik, A.; Sanyal, D.; Waghmare, U.V.; Biswas, K. Temperature dependent reversible p-n-p type conduction switching with colossal change in thermopower of semiconducting AgCuS. J. Am. Chem. Soc. 2014, 136, 12712–12720. [Google Scholar] [CrossRef]
- Lange, S.; Nilges, T. Ag10Te4Br3: A New Silver(I) (poly)-Chalcogenide Halide Solid Electrolyte. Chem. Mater. 2006, 18, 2538–2544. [Google Scholar] [CrossRef]
- Perez, S.D.; Garcia, M.A.; Garcia, M.D.; Domene, G.B.; Mühle, C.; Jansen, M. Structural phase transitions on AgCuS stromeyerite mineral under compression. Inorg. Chem. 2013, 52, 355–361. [Google Scholar] [CrossRef]
- Trots, M.; Senyshyn, A.; Mikhailova, D.A.; Knapp, M.; Baehtz, C.; Hoelzel, M.; Fuess, H. High-temperature thermal expansion and structural behaviour of stromeyerite, AgCuS. J. Phys. Condens. Matter 2007, 19, 136204. [Google Scholar] [CrossRef] [Green Version]
- Kadrgulov, R.F.; Yakshibaev, R.A.; Khasanov, M.A. Phase relations, ionic transport and diffusion in the alloys of Cu2S-Ag2S mixed conductors. Ionics 2001, 7, 156–160. [Google Scholar] [CrossRef]
- Zhu, H.; Lian, C.; Wu, D. Room-temperature synthesis of (Ag,Cu)2S hollow spheres by cation exchange and their optical properties. Mater. Chem. Phys. 2011, 127, 24–27. [Google Scholar]
- Dutta, M.; Sanyal, D.; Biswas, K. Tuning of p–n–p-Type Conduction in AgCuS through Cation Vacancy: Thermopower and Positron Annihilation Spectroscopy Investigations. Inorg. Chem. 2018, 57, 7481–7489. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Lee, S.; Kim, D.R.; Park, J.H.; Kim, Y.; Choi, W.-J.; Son, C.S.; Son, Y.G.; Hwang, D. Effect of RF Power on the Properties of Sputtered-CuS Thin Films for Photovoltaic Applications. Energies 2020, 13, 688. [Google Scholar] [CrossRef] [Green Version]
- Tesfaye, F.; Taskinen, P. Thermodynamic properties of equilibrium phases in the Ag-Cu-S system below 500 K: Experimental Study. In EPD Congress 2014; Yurko, J., Zhang, L., Allanore, A., Wang, C., Spangenberger, J.S., Kirchain, R.E., Downey, J.P., May, L.D., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 181–193. [Google Scholar]
- Fleet, M. Phase Equilibria at High Temperature. Rev. Mineral. Geochem. 2006, 61, 365–419. [Google Scholar] [CrossRef]
- Skinner, B.J. The System Cu-Ag-S. Econ. Geol. 1966, 6, 11–26. [Google Scholar] [CrossRef]
- Sharma, R.C.; Chang, Y.A. Ag–S (silver–sulfur). In Binary Alloy Phase Diagrams, 2nd ed.; Massalski, T.B., Okamoto, H., Subramanian, P.R., Kacprzak, L., Eds.; ASM International: Novelty, OH, USA, 1990; pp. 86–87. [Google Scholar]
- Chang, Y.A.; Neumann, J.P.; Choudary, U.V. Phase Diagrams and Thermodynamic Properties of Ternary Copper–Sulfur–Metal Systems; INCRA Monograph VII; The Metallurgy of Copper, NBS: Washington, DC, USA, 1979; pp. 191–193. [Google Scholar]
- Wu, D. Phase Relations in the System Ag2S-Cu2S-PbS and Ag2S-Cu2S-Bi2S3, and Their Mineral Assemblages. Chin. J. Geochem. 1987, 6, 225–233. [Google Scholar]
- Djurle, S. An X-Ray Study on the System Ag-Cu-S. Acta Chem. Stand. 1958, 12, 1427–1436. [Google Scholar] [CrossRef]
- Frueh, A.J. The crystal structure of stromeyerite, AgCuS: A possible defect structure. Jr. Z. Kristallogr. 1955, 106, 299–307. [Google Scholar] [CrossRef]
- Skomorokhov, A.N.; Trots, D.M.; Ovchinnikov, S.G.; Fuess, H. Lattice vibrations in an α- and β-AgCuS superionic conductor: Experimental time-of-flight inelastic neutron scattering studies. J. Phys. Condens. Matter 2007, 19, 186228. [Google Scholar] [CrossRef] [PubMed]
- Takuhara, Y.; Tezuka, K.; Shan, Y.J.; Imoto, H.J. Syntheses of complex sulfides AgCuS and Ag3CuS2 from the elements under hydrothermal conditions. Ceram. Soc. Jpn. 2009, 117, 359–362. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, J.A.; Sagua, A.E. Thermodynamic quantities for the ternary compound Stromeyerite: Cu1+δAg1-δS for 0 ≤ δ ≤ 0.1. J. Chem. Thermodyn. 1993, 25, 1453–1459. [Google Scholar] [CrossRef]
- Sack, R.O.; Ebel, D.S. Thermochemistry of sulfide mineral solutions. Rev. Mineral. Geochem. 2006, 61, 265–364. [Google Scholar] [CrossRef]
- Harlov, D.E.; Sack, R.O. Thermochemistry of Ag2S-Cu2S sulfide solutions: Constraints derived from coexisting Sb2S3- and As2S3-bearing sulfosalts. Geochim. Cosmochim. Acta 1995, 59, 4351–4365. [Google Scholar] [CrossRef]
- Schwartz, G.M. Relationship of Chalcocite–Stromeyerite-Argentite. Econ. Geo. 1935, 30, 128–146. [Google Scholar] [CrossRef]
- Perrot, P.; Jeannot, C. Relations activite-composition dans les solutions solides Ag2S-Cu2S. Rev. De Chim. Miner. 1971, 8, 87–97. [Google Scholar]
- Werner, A. Untersuchungen am System Kupfer-Silber-Schwefel. Z. Für. Phys. Chem. Neue Folge Bd. 1965, 47, 267–285. [Google Scholar] [CrossRef]
- Schmidt, J.A.; Sagua, A.E.; Bazan, J.C. Phase equilibrium of (CuS + Ag2S + Ag1.55Cu0.45S) and thermodynamic quantities for the ternary-phase Jalpaite from e.m.f. measurements in solid-state galvanic cells. J. Chem. Thermodyn. 1990, 22, 181–187. [Google Scholar] [CrossRef]
- Sagua, A.E.; Schmidt, J.A. Thermodynamic quantities for the ternary compound Jalpaita: Ag1.5 + xCu0.5-xS for 0 <= x <= 0.05. An. Asoc. Quim. Argent. 1997, 85, 197–201. [Google Scholar]
- Schmidt, J.A.; Sagua, A.E. Phase equilibrium of (Ag1.55Cu0.45S + Ag1.2Cu0.8S + CuS) and thermodynamic quantities for the ternary phase Ag1.2Cu0.8S. J. Chem. Thermodyn. 1990, 22, 1207–1212. [Google Scholar] [CrossRef]
- García, C.; Franco, J.; López Tonazzi, J.; Walsöe de Reca, N. Conductivity behavior of RbAg4I5. Solid State Ion. 1983, 9–10, 1233–1236. [Google Scholar] [CrossRef]
- Ipser, H.; Mikula, A.; Katayama, I. Overview: The emf method as a source of experimental thermodynamic data. Calphad 2010, 34, 271–278. [Google Scholar] [CrossRef]
- SGTE Substance Database. Scientific Group Thermodata Europe. Available online: https://www.sgte.net/en/neu (accessed on 5 May 2021).
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.-H.; Kang, Y.B.; Melançon, J.; et al. FactSage thermochemical software and databases, 2010–2016. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef] [Green Version]
- Tesfaye, F.; Lindberg, D.; Moroz, M.; Hupa, L. Investigation of the K-Mg-Ca Sulfate System as Part of Monitoring Problematic Phase Formations in Renewable-Energy Power Plants. Energies 2020, 13, 5366. [Google Scholar] [CrossRef]
- Mohr, P.J.M.; Newell, D.B.; Taylor, B.N. CODATA Recommended Values of the Fundamental Physical Constants: 2014; National Institute of Standards and Technology: Gaithersburg, ML, USA, 2015; pp. 1–11. [Google Scholar]
- Barin, I. Thermochemical Data of Pure Substances. Part I & II; VCH Verlagsgesellschaft: Weinheim, Germany; VCH Publishers: New York, NY, USA, 1989. [Google Scholar]
- Craig, J.R.; Barton, P.B. Thermochemical approximations for sulfosalts. Econ. Geol. 1973, 68, 493–506. [Google Scholar] [CrossRef]
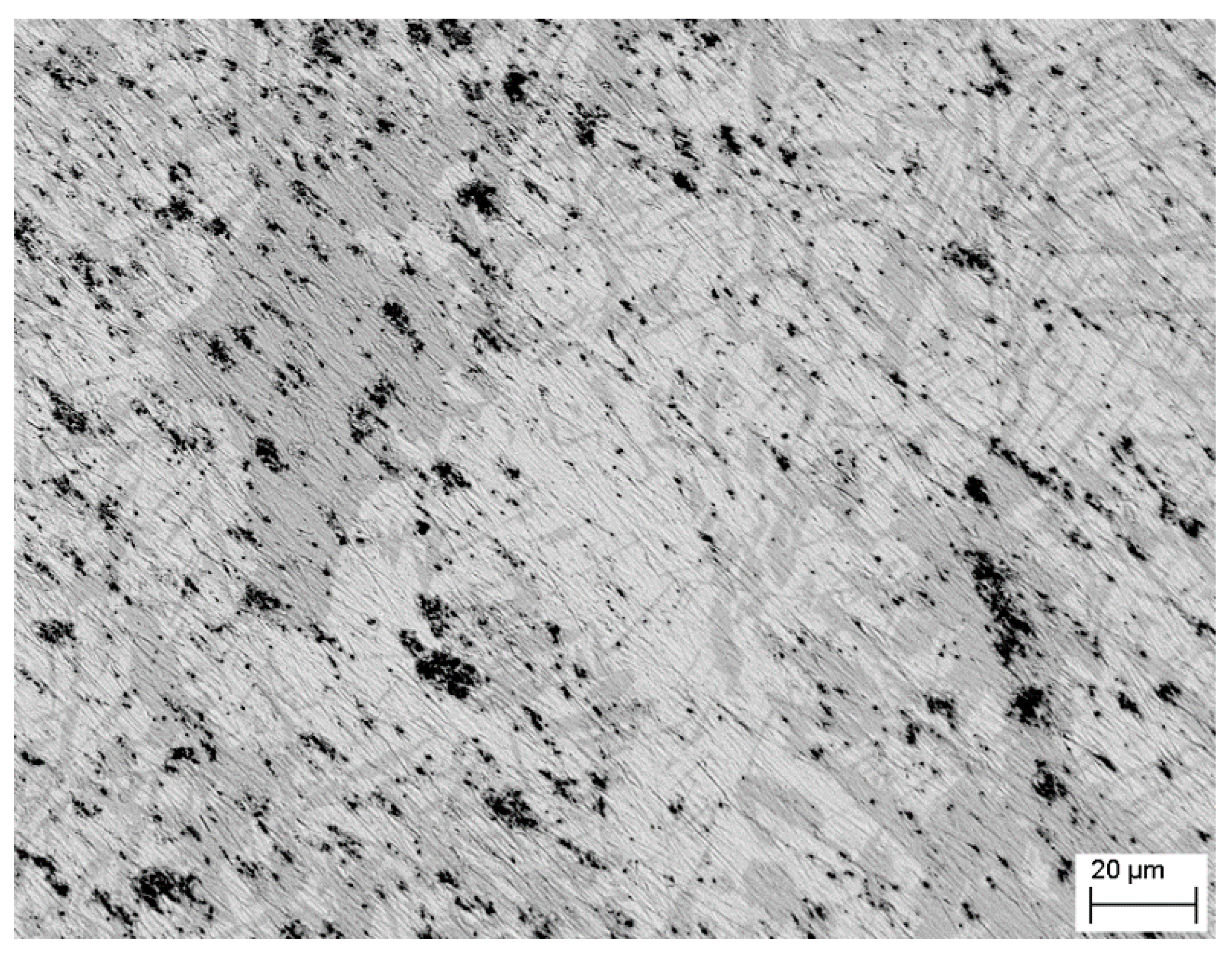
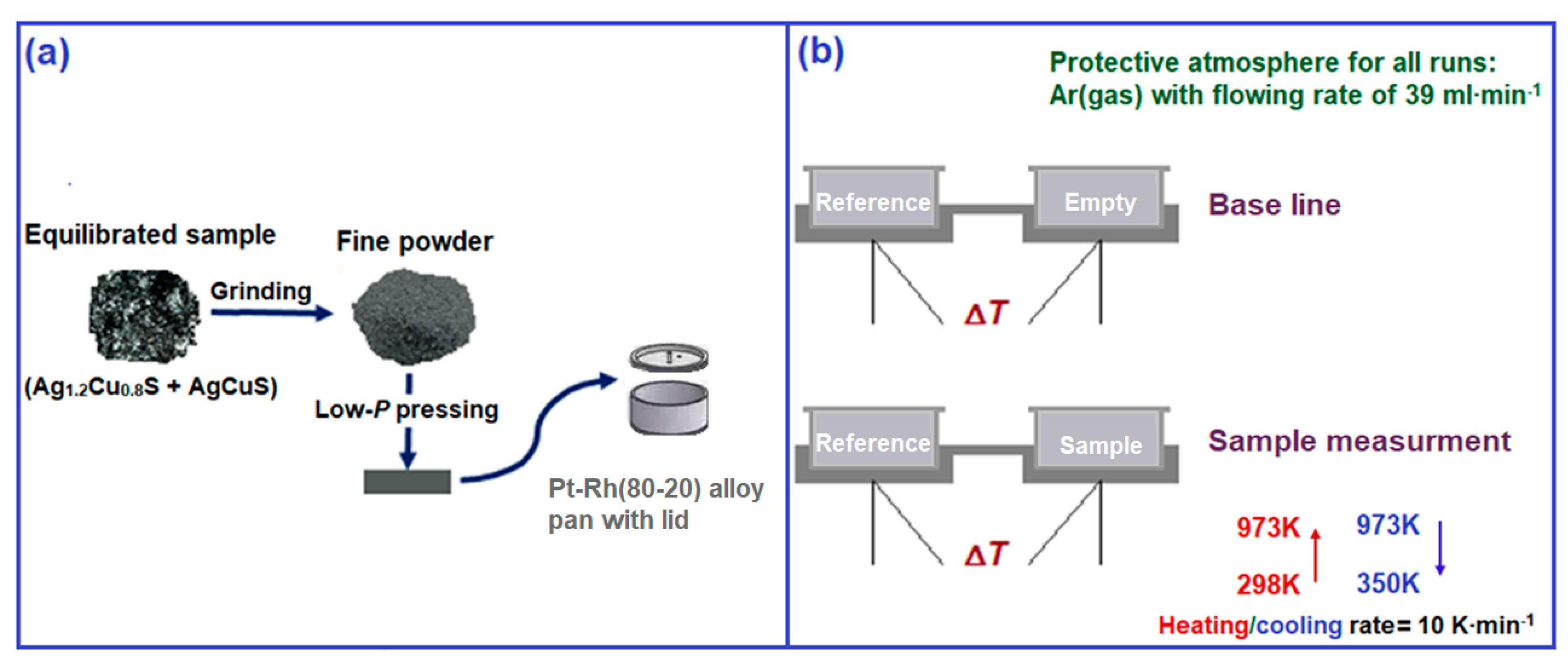
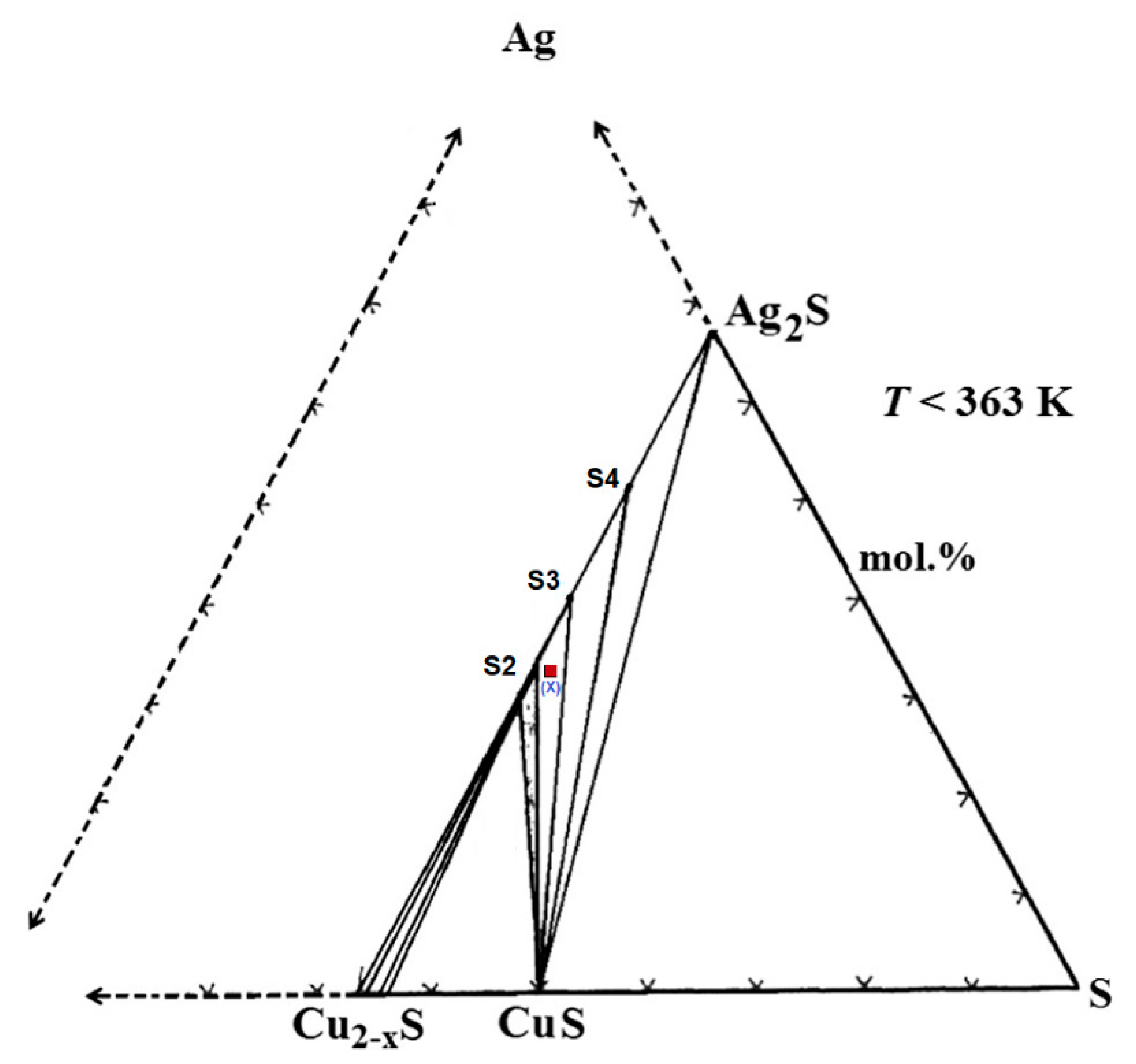
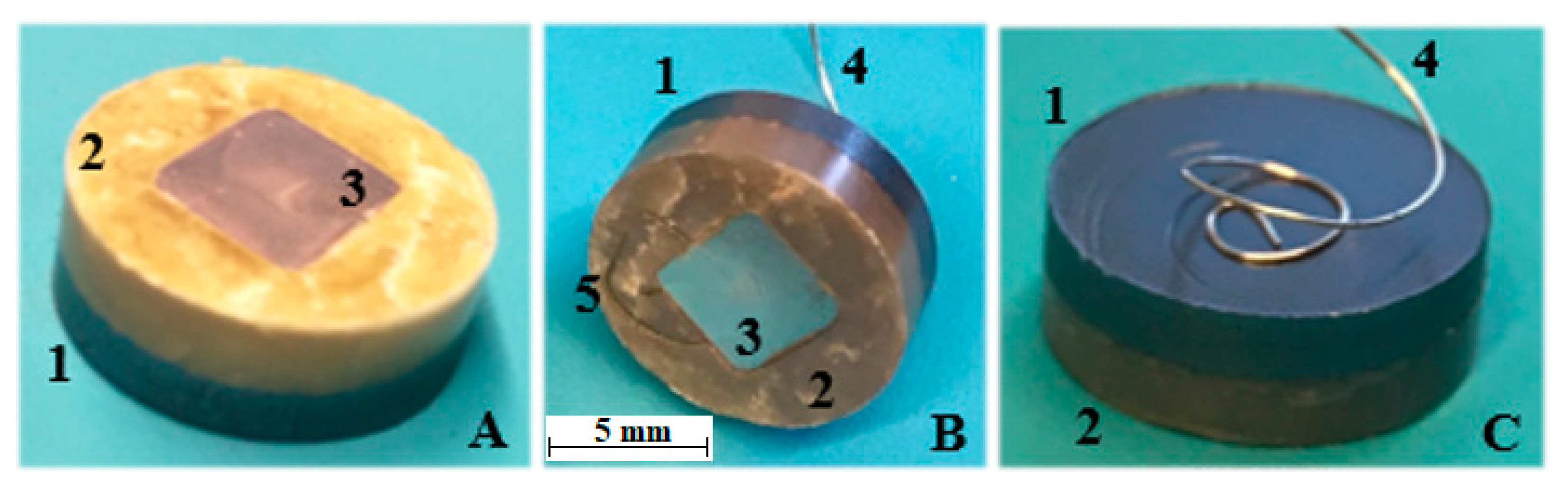

| Chemical | Purity | Source | CAS No. | Form |
|---|---|---|---|---|
| Ag | 99.9 wt% | Alfa Aesar, Merck (Darmstadt, Germany) | 7440-22-4 | Foil |
| AgI | 99.9 wt% | Alfa Aesar, Merck (Darmstadt, Germany) | 7783-96-2 | Powder |
| Ag2S | >99.9 wt% | Alfa Aesar, Merck (Darmstadt, Germany) | 21548-73-2 | Powder |
| Ar | 99.999 vol% | Oy AGA Ab (Espoo, Finland) | - | Compressed gas |
| CuS | 99.8 wt% | Alfa Aesar, Merck (Darmstadt, Germany) | 1317-40-4 | Powder |
| Cu2S | 99.5 wt% | Alfa Aesar, Merck (Darmstadt Germany) | 22205-45-4 | Powder |
| Pt | 99.99 wt% | Johnson Matthey (Royston, UK) | Wire | |
| RbI | 99.8 wt% | Alfa Aesar, Merck (Darmstadt, Germany) | 7790-29-6 | Powder |
| Ttr/melt/K | 65 wt% Ag2S [This work] | 55.4 wt% Ag2S [4] | 65 wt% Ag2S [20] | 61 wt% Ag2S [11] | 61 wt% Ag2S [6] | Reaction |
|---|---|---|---|---|---|---|
| Ttr | 364 ± 1 | 361 ± 1 | 353 | 361 | 365 | Ag0.93Cu1.07S (ortho) + Ag1.2Cu0.8S ⇄ (Ag,Cu)2S(HCP) + Ag1.2Cu0.8S |
| Tmelt | 922 ± 1 | 944 ± 1 | 937 | 936 | - | (Ag,Cu)2S(FCC) ⇄ L |
| ΔfG°/(kJ∙mol−1) | ΔfH°/(kJ∙mol−1) | ΔfS°/(J∙(K·mol)−1) | Reference |
|---|---|---|---|
| −55.9 ± 1 | −50.2 ± 1 | 19.1 ± 0.4 | This work |
| −55.5 | −46.7 | 29.67 | Schmidt and Sagua [34] 1 |
| −50.6 | −50.5 | 0.12 | Calculated by the Graig and Barton method [42] 2 |
| Reaction | Temperature (K) | Exp. Method | |
|---|---|---|---|
| Exp. | Opt. | ||
| AgCuS ⇄ (Ag,Cu)2S(HCP) 1 | 375 ± 1 | 387 | EMF |
| (Ag,Cu)2S(FCC) 2 ⇄ L | 922.3 ± 1 | 930 | DSC-TGA |
| (Ag,Cu)2S(HCP) 2 + Ag1.2Cu0.8S ⇄ Ag1.55Cu0.45S + (Ag,Cu)2S(HCP) 2 | 402.5 ± 1 | 398 | DSC-TGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tesfaye, F.; Lindberg, D.; Sukhomlinov, D.; Taskinen, P.; Hupa, L. Thermal Analysis and Optimization of the Phase Diagram of the Cu-Ag Sulfide System. Energies 2022, 15, 593. https://doi.org/10.3390/en15020593
Tesfaye F, Lindberg D, Sukhomlinov D, Taskinen P, Hupa L. Thermal Analysis and Optimization of the Phase Diagram of the Cu-Ag Sulfide System. Energies. 2022; 15(2):593. https://doi.org/10.3390/en15020593
Chicago/Turabian StyleTesfaye, Fiseha, Daniel Lindberg, Dmitry Sukhomlinov, Pekka Taskinen, and Leena Hupa. 2022. "Thermal Analysis and Optimization of the Phase Diagram of the Cu-Ag Sulfide System" Energies 15, no. 2: 593. https://doi.org/10.3390/en15020593







