Application of Ionic Liquids for the Recycling and Recovery of Technologically Critical and Valuable Metals
Abstract
:1. Introduction
1.1. Metal Recovery by Ionic Liquid
1.2. Functionalized Ionic Liquids
1.3. Reaction Mechanism
1.4. Salting Effect
1.5. Stripping Agents
2. Precious Metals and Copper
2.1. Gold
2.2. Copper
2.3. Palladium
3. Battery Metals
3.1. Lithium
3.2. Cobalt
3.3. Nickel and Manganese
4. Metals for Electronic Devices and Optics
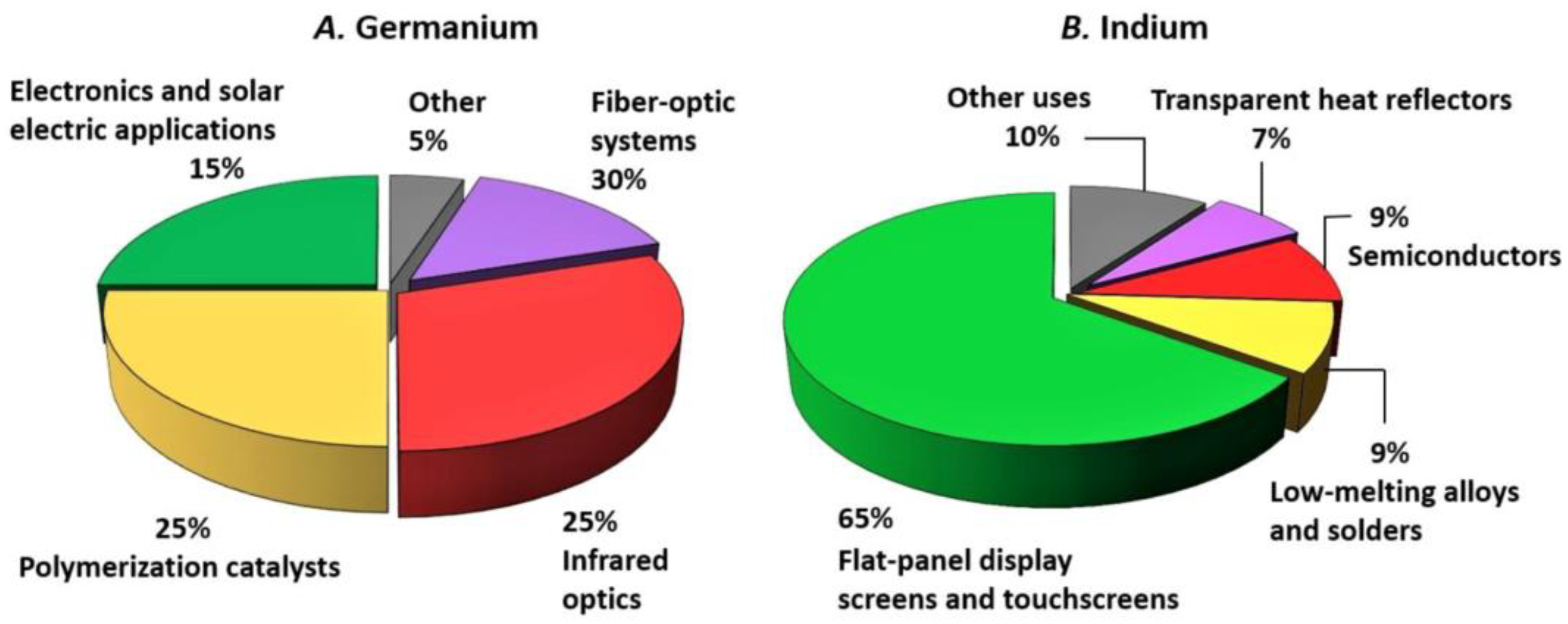
4.1. Indium
4.2. Germanium
4.3. Gallium
5. Rare Earth Elements (REE)
5.1. Rare Earths from Urban Waste
5.1.1. Nd-Fe-B Magnets
5.1.2. Samarium-Cobalt Magnets
5.1.3. Fluorescent Lamps
5.2. Lab-Test Separations of Rare Earths
6. Actinides
- PUREX (Plutonium/Uranium extraction), TBP.
- TRUEX (Transuranic extraction) CMPO and TBP.
- DIAMEX (Diamide extraction) DGA.
- SANEX (Selective Actinide Extraction), N-heterocyclic ligands.
- TALSPEAK (Trivalent Actinide Lanthanide Separations by Phosphorus-reagent Extraction from Aqueous Complexes), HDEHP, DTPA.
- AIROX (Atomics International Reduction Oxidation).
- OREOX (Oxidation and Reduction of Oxide fuel).
6.1. Major Actinide Partitioning
6.2. Minor Actinide Separation
7. Variation of Ionic Liquids: Deep Eutectic Solvents (DESs)
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- King, A.H. Our elemental footprint. Nat. Mater. 2019, 18, 408–409. [Google Scholar] [CrossRef]
- US DOE. Critical Materials Rare Earths Supply Chain: A Situational White Paper. Available online: https://www.energy.gov/eere/amo/downloads/critical-materials-supply-chain-white-paper-april-2020 (accessed on 29 November 2021).
- Herrington, R. Mining our green future. Nat. Rev. Mater. 2021, 6, 456–458. [Google Scholar] [CrossRef]
- Kalantzakos, S. The Race for Critical Minerals in an Era of Geopolitical Realignments. Int. Spect. 2020, 55, 1–16. [Google Scholar] [CrossRef]
- Rogers, R.D.; Seddon, K.R. Ionic Liquids—Solvents of the Future? Science 2003, 302, 792–793. Available online: https://www.science.org/doi/full/10.1126/science.1090313 (accessed on 29 November 2021). [CrossRef] [PubMed]
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forsyth, S.A.; Pringle, J.M.; MacFarlane, D.R. Ionic liquids—An overview. Aust. J. Chem. 2004, 57, 113–119. [Google Scholar] [CrossRef]
- Cho, C.-W.; Pham, T.P.T.; Zhao, Y.; Stolte, S.; Yun, Y.-S. Review of the Toxic Effects of Ionic Liquids. Sci. Total Environ. 2021, 786, 147309. [Google Scholar] [CrossRef] [PubMed]
- Pleshkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef]
- Prodius, D.; Mudring, A.-V. Rare earth metal-containing ionic liquids. Coord. Chem. Rev. 2018, 363, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, P.S.; Branco, L.C.; Crespo, J.G.; Nunes, M.C.; Raymundo, A.; Afonso, C.A.M. Comparison of Physicochemical Properties of New Ionic Liquids Based on Imidazolium, Quaternary Ammonium, and Guanidinium Cations. Chem. Eur. J. 2007, 13, 8478–8488. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T. Solvometallurgy: An Emerging Branch of Extractive Metallurgy. J. Sustain. Metall. 2017, 3, 570–600. [Google Scholar] [CrossRef] [Green Version]
- Paiva, A.P.; Nogueira, C.A. Ionic Liquids in the Extraction and Recycling of Critical Metals from Urban Mines. Waste Biomass Valorization 2021, 12, 1725–1747. [Google Scholar] [CrossRef]
- Mai, N.L.; Ahn, K.; Koo, Y.-M. Methods for recovery of ionic liquids—A review. Process Biochem. 2014, 49, 872–881. [Google Scholar] [CrossRef]
- Zhou, J.; Sui, H.; Jia, Z.; Yang, Z.; He, L.; Li, X. Recovery and purification of ionic liquids from solutions: A review. RSC Adv. 2018, 8, 32832–32864. [Google Scholar] [CrossRef] [Green Version]
- Hajipour, A.R.; Rafiee, F. Recent Progress in Ionic Liquids and Their Applications in Organic Synthesis. Org. Prep. Proced. Int. 2015, 47, 249–308. [Google Scholar] [CrossRef]
- Nockemann, P.; van Deun, R.; Thijs, B.; Huys, D.; Vanecht, E.; van Hecke, K.; van Meervelt, L.; Binnemans, K. Uranyl Complexes of Carboxyl-Functionalized Ionic Liquids. Inorg. Chem. 2010, 49, 3351–3360. [Google Scholar] [CrossRef]
- Fan, F.L.; Qin, Z.; Cao, S.W.; Tan, C.M.; Huang, Q.G.; Chen, D.S.; Wang, J.R.; Yin, X.J.; Xu, C.; Feng, X.G. Highly Efficient and Selective Dissolution Separation of Fission Products by an Ionic Liquid [Hbet][Tf2N]: A New Approach to Spent Nuclear Fuel Recycling. Inorg. Chem. 2019, 58, 603–609. [Google Scholar] [CrossRef]
- Rout, A.; Kotlarska, J.; Dehaen, W.; Binnemans, K. Liquid-Liquid Extraction of Neodymium (III) by Dialkylphosphate Ionic Liquids from Acidic Medium: The Importance of the Ionic Liquid Cation. Phys. Chem. Chem. Phys. 2013, 15, 16533–16541. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Adidharma, H.; Radosz, M.; Wan, P.; Xu, X.; Russell, C.K.; Tian, H.; Fan, M.; Yu, J. Recovery of Rare Earth Elements with Ionic Liquids. Green Chem. 2017, 4469–4493. [Google Scholar] [CrossRef]
- Mohapatra, P.K. Actinide Ion Extraction Using Room Temperature Ionic Liquids: Opportunities and Challenges for Nuclear Fuel Cycle Applications. Dalton Trans. 2017, 46, 1730–1747. [Google Scholar] [CrossRef]
- Sun, P.; Huang, K.; Liu, H. The Nature of Salt Effect in Enhancing the Extraction of Rare Earths by Non-Functional Ionic Liquids: Synergism of Salt Anion Complexation and Hofmeister Bias. J. Colloid Interface Sci. 2019, 539, 214–222. [Google Scholar] [CrossRef]
- Lommelen, R.; Onghena, B.; Binnemans, K. Cation Effect of Chloride Salting Agents on Transition Metal Ion Hydration and Solvent Extraction by the Basic Extractant Methyltrioctylammonium Chloride. Inorg. Chem. 2020, 59, 13442–13452. [Google Scholar] [CrossRef]
- Fu, J.; Chen, Q.; Sun, T.; Shen, X. Extraction of Th (IV) from Aqueous Solution by Room-Temperature Ionic Liquids and Coupled with Supercritical Carbon Dioxide Stripping. Sep. Purif. Technol. 2013, 119, 66–71. [Google Scholar] [CrossRef]
- Sepúlveda, R.; Romero, J.; Sánchez, J. Copper Removal from Aqueous Solutions by Means of Ionic Liquids Containing a β-Diketone and the Recovery of Metal Complexes by Supercritical Fluid Extraction. J. Chem. Technol. Biotechnol. 2014, 89, 899–908. [Google Scholar] [CrossRef]
- Zhou, Y.; Boudesocque, S.; Mohamadou, A.; Dupont, L. Extraction of metal ions with task specific ionic liquids: Influence of a coordinating anion. Sep. Sci. Technol. 2015, 50, 38–44. [Google Scholar] [CrossRef]
- Platzer, S.; Kar, M.; Leyma, R.; Chib, S.; Roller, A.; Jirsa, F.; Krachler, R.; MacFarlane, D.R.; Kandioller, W.; Keppler, B.K. Task-specific thioglycolate ionic liquids for heavy metal extraction: Synthesis, extraction efficacies and recycling properties. J. Hazard. Mater. 2017, 324, 241–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhiman, S.; Gupta, B. Recovery of pure germanium oxide from Zener diodes using a recyclable ionic liquid Cyphos IL 104. J. Environ. Manag. 2020, 276, 111218. [Google Scholar] [CrossRef] [PubMed]
- Hagelüken, C.; Corti, C.W. Recycling of Gold from Electronics: Cost-Effective Use through ‘Design for Recycling’. Gold Bull. 2010, 43, 209–220. [Google Scholar] [CrossRef] [Green Version]
- van den Bossche, A.; de Witte, E.; Dehaen, W.; Binnemans, K. Trihalide Ionic Liquids as Non-Volatile Oxidizing Solvents for Metals. Green Chem. 2018, 20, 3327–3338. [Google Scholar] [CrossRef]
- Alguacil, F.J. Non-Dispersive Extraction of Gold (III) with Ionic Liquid Cyphos IL101. Sep. Purif. Technol. 2017, 179, 72–76. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Lee, J.C.; Jeong, J.; Kim, B.S.; Cote, G.; Chagnes, A. Extraction of Gold (III) from Acidic Chloride Media Using Phosphonium-Based Ionic Liquid as an Anion Exchanger. Ind. Eng. Chem. Res. 2015, 54, 1350–1358. [Google Scholar] [CrossRef]
- Wang, N.; Wang, Q.; Geng, Y.; Sun, X.; Wu, D.; Yang, Y. Recovery of Au (III) from Acidic Chloride Media by Homogenous Liquid-Liquid Extraction with UCST-Type Ionic Liquids. ACS Sustain. Chem. Eng. 2019, 7, 19975–19983. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Q.; Geng, Y.; Wang, N.; Yang, Y. Gold (III) Separation from Acidic Medium by Amine-Based Ionic Liquid. J. Mol. Liq. 2020, 304, 112735. [Google Scholar] [CrossRef]
- Boudesocque, S.; Mohamadou, A.; Conreux, A.; Marin, B.; Dupont, L. The Recovery and Selective Extraction of Gold and Platinum by Novel Ionic Liquids. Sep. Purif. Technol. 2019, 210, 824–834. [Google Scholar] [CrossRef]
- Guo, W.; Yang, F.; Zhao, Z.; Liao, Q.; Cai, C.; Zhang, Y.; Bai, R. Cellulose-Based Ionic Liquids as an Adsorbent for High Selective Recovery of Gold. Miner. Eng. 2018, 125, 271–278. [Google Scholar] [CrossRef]
- Copper—The World’s Most Reusable Resource. Available online: https://www.copper.org/environment/lifecycle/g_recycl.html (accessed on 29 November 2021).
- Chen, M.; Huang, J.; Ogunseitan, O.A.; Zhu, N.; Wang, Y.M. Comparative Study on Copper Leaching from Waste Printed Circuit Boards by Typical Ionic Liquid Acids. Waste Manag. 2015, 41, 142–147. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Yang, J.; Tariq, S.M.; Duan, C.; Zhao, Y. Comparative Investigation on Copper Leaching Efficiency from Waste Mobile Phones Using Various Types of Ionic Liquids. J. Clean. Prod. 2020, 256, 120368. [Google Scholar] [CrossRef]
- Janssen, C.H.C.; Macías-Ruvalcaba, N.A.; Aguilar-Martínez, M.; Kobrak, M.N. Copper Extraction Using Protic Ionic Liquids: Evidence of the Hofmeister Effect. Sep. Purif. Technol. 2016, 168, 275–283. [Google Scholar] [CrossRef]
- Diabate, P.D.; Boudesocque, S.; Mohamadou, A.; Dupont, L. Separation of Cobalt, Nickel and Copper with Task-Specific Amido Functionalized Glycine-Betaine-Based Ionic Liquids. Sep. Purif. Technol. 2020, 244, 116782. [Google Scholar] [CrossRef]
- Damilano, G.; Laitinen, A.; Willberg-Keyriläinen, P.; Lavonen, T.; Häkkinen, R.; Dehaen, W.; Binnemans, K.; Kuutti, L. Effects of Thiol Substitution in Deep-Eutectic Solvents (DESs) as Solvents for Metal Oxides. RSC Adv. 2020, 10, 23484–23490. [Google Scholar] [CrossRef]
- PGMs Retain their Sheen. Available online: https://www.recyclingtoday.com/article/platinum-group-metals-commodity-focus/ (accessed on 29 November 2021).
- Regel-Rosocka, M.; Rzelewska, M.; Baczynska, M.; Janus, M.; Wisniewski, M. Removal of Palladium (II) from Aqueous Chloride Solutions with Cyphos Phosphonium Ionic Liquids as Metal Ion Carriers for Liquid-Liquid Extraction and Transport across Polymer Inclusion Membranes. Physicochem. Probl. Miner. Proc. 2015, 51, 621–631. [Google Scholar] [CrossRef]
- Katsuta, S.; Tamura, J. Extraction of Palladium (II) and Platinum (IV) from Hydrochloric Acid Solutions with Trioctylammonium Nitrate Ionic Liquid without Dilution. J. Solut. Chem. 2018, 47, 1293–1308. [Google Scholar] [CrossRef]
- Mokhodoeva, O.; Shkinev, V.; Maksimova, V.; Dzhenloda, R.; Spivakov, B. Recovery of Platinum Group Metals Using Magnetic Nanoparticles Modified with Ionic Liquids. Sep. Purif. Technol. 2020, 248, 117049. [Google Scholar] [CrossRef]
- Funaki, K.; Ma, S.; Kawamura, S.; Miyazaki, A.; Sugie, A.; Mori, A.; Muramatsu, A.; Kanie, K. Metal-Selective Deprotection-Mediated Palladium (II) Extraction by Ionic Liquids with Tetrahydropyran-2H-Yl-Protected Thiol Moieties. Chem. Lett. 2017, 46, 434–437. [Google Scholar] [CrossRef] [Green Version]
- Olivetti, E.A.; Ceder, G.; Gaustad, G.G.; Fu, X. Lithium-Ion Battery Supply Chain Considerations: Analysis of Potential Bottlenecks in Critical Metals. Joule 2017, 1, 229–243. [Google Scholar] [CrossRef] [Green Version]
- Abergel, T.; Bunsen, T.; Gorner, M.; Leduc, P. Global EV Outlook 2020: Entering the Decade of Electric Drive? OECD Publishing: Paris, France, 2020. [Google Scholar] [CrossRef]
- Li, Z.; Mercken, J.; Li, X.; Riaño, S.; Binnemans, K. Efficient and Sustainable Removal of Magnesium from Brines for Lithium/Magnesium Separation Using Binary Extractants. ACS Sustain. Chem. Eng. 2019, 7, 19225–19234. [Google Scholar] [CrossRef]
- He, Q.; Williams, N.J.; Oh, J.H.; Lynch, V.M.; Kim, S.K.; Moyer, B.A.; Sessler, J.L. Selective Solid-Liquid and Liquid-Liquid Extraction of Lithium Chloride Using Strapped Calix[4]Pyrroles. Angew. Chem. 2018, 130, 12100–12104. [Google Scholar] [CrossRef]
- Nelson, J.J.M.; Schelter, E.J. Sustainable Inorganic Chemistry: Metal Separations for Recycling. Inorg. Chem. 2019, 58, 979–990. [Google Scholar] [CrossRef]
- Zante, G.; Boltoeva, M.; Masmoudi, A.; Barillon, R.; Trébouet, D. Lithium Extraction from Complex Aqueous Solutions Using Supported Ionic Liquid Membranes. J. Membr. Sci. 2019, 580, 62–76. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, Z.; He, L. Highly Selective Lithium Recovery from High Mg/Li Ratio Brines. Desalination 2020, 474, 114185. [Google Scholar] [CrossRef]
- Roldán-Ruiz, M.J.; Ferrer, M.L.; Gutiérrez, M.C.; del Monte, F. Highly Efficient P-Toluenesulfonic Acid-Based Deep-Eutectic Solvents for Cathode Recycling of Li-Ion Batteries. ACS Sustain. Chem. Eng. 2020, 8, 5437–5445. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Y.; Liu, Z.; Zhou, L.; Li, Z.; Jiang, J.; Wei, L.; Ren, P.; Mu, T. Efficient Dissolution of Lithium-Ion Batteries Cathode LiCoO 2 by Polyethylene Glycol-Based Deep Eutectic Solvents at Mild Temperature. ACS Sustain. Chem. Eng. 2020, 8, 11713–11720. [Google Scholar] [CrossRef]
- Recycling Cathode of Lithium-Ion Battery by Using Deep Eutectic Solvents to Extract Cobalt. Available online: https://www.diva-portal.org/smash/get/diva2:1448722/FULLTEXT01.pdf (accessed on 29 November 2021).
- Tran, M.K.; Rodrigues, M.-T.F.; Kato, K.; Babu, G.; Ajayan, P.M. Deep Eutectic Solvents for Cathode Recycling of Li-Ion Batteries. Nat. Energy 2019, 4, 339–345. [Google Scholar] [CrossRef]
- Peeters, N.; Binnemans, K.; Riaño, S. Solvometallurgical Recovery of Cobalt from Lithium-Ion Battery Cathode Materials Using Deep-Eutectic Solvents. Green Chem. 2020, 22, 4210–4221. [Google Scholar] [CrossRef]
- A New Process for Cobalt—Nickel Separation. Available online: https://www.teck.com/media/CESL-Publication-Nickel-New-Process-Cobalt-Nickel-Separation-2010.pdf (accessed on 29 November 2021).
- Wellens, S.; Thijs, B.; Möller, C.; Binnemans, K. Separation of Cobalt and Nickel by Solvent Extraction with Two Mutually Immiscible Ionic Liquids. Phys. Chem. Chem. Phys. 2013, 15, 9663–9669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Ji, Y.; Hu, F.; He, B.; Chen, J.; Li, D. The Inner Synergistic Effect of Bifunctional Ionic Liquid Extractant for Solvent Extraction. Talanta 2010, 81, 1877–1883. [Google Scholar] [CrossRef]
- Wellens, S.; Thijs, B.; Binnemans, K. An Environmentally Friendlier Approach to Hydrometallurgy: Highly Selective Separation of Cobalt from Nickel by Solvent Extraction with Undiluted Phosphonium Ionic Liquids. Green Chem. 2012, 14, 1657–1665. [Google Scholar] [CrossRef] [Green Version]
- Onghena, B.; Opsomer, T.; Binnemans, K. Separation of Cobalt and Nickel Using a Thermomorphic Ionic-Liquid-Based Aqueous Biphasic System. Chem. Commun. 2015, 51, 15932–15935. [Google Scholar] [CrossRef] [Green Version]
- Othman, E.A.; van der Ham, A.G.J.; Miedema, H.; Kersten, S.R.A. Recovery of Metals from Spent Lithium-Ion Batteries Using Ionic Liquid [P8888][Oleate]. Sep. Purif. Technol. 2020, 252, 117435. [Google Scholar] [CrossRef]
- Zante, G.; Braun, A.; Masmoudi, A.; Barillon, R.; Trébouet, D.; Boltoeva, M. Solvent Extraction Fractionation of Manganese, Cobalt, Nickel and Lithium Using Ionic Liquids and Deep Eutectic Solvents. Miner. Eng. 2020, 156, 106512. [Google Scholar] [CrossRef]
- Shanks, W.C.P., III; Kimball, B.E.; Tolcin, A.C.; Guberman, D.E. Germanium and Indium. In Critical Mineral Resources of the United States—Economic and Environmental Geology and Prospects for Future Supply; Schulz, K.J., DeYoung, J.H., Jr., Seal, R.R., II, Bradley, D.C., Eds.; U.S. Geological Survey Professional Paper; U.S. Geological Survey: Reston, VA, USA, 2017; pp. 1–27. [Google Scholar] [CrossRef] [Green Version]
- Deferm, C.; Onghena, B.; Nguyen, V.T.; Banerjee, D.; Fransaer, J.; Binnemans, K. Non-Aqueous Solvent Extraction of Indium from an Ethylene Glycol Feed Solution by the Ionic Liquid Cyphos IL 101: Speciation Study and Continuous Counter-Current Process in Mixer-Settlers. RSC Adv. 2020, 10, 24595–24612. [Google Scholar] [CrossRef]
- van Roosendael, S.; Regadío, M.; Roosen, J.; Binnemans, K. Selective Recovery of Indium from Iron-Rich Solutions Using an Aliquat 336 Iodide Supported Ionic Liquid Phase (SILP). Sep. Purif. Technol. 2019, 212, 843–853. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Escudero, E. Solvent Extraction of Indium (III) from HCl Solutions by the Ionic Liquid (A324H+) (Cl−) Dissolved in Solvesso 100. Hydrometallurgy 2019, 189, 105104. [Google Scholar] [CrossRef]
- Luo, D.; Zhu, N.; Li, Y.; Cui, J.; Wu, P.; Wang, J. Simultaneous Leaching and Extraction of Indium from Waste LCDs with Acidic Ionic Liquids. Hydrometallurgy 2019, 189, 105146. [Google Scholar] [CrossRef]
- Matsumiya, M.; Sumi, M.; Uchino, Y.; Yanagi, I. Recovery of Indium Based on the Combined Methods of Ionic Liquid Extraction and Electrodeposition. Sep. Purif. Technol. 2018, 201, 25–29. [Google Scholar] [CrossRef]
- Monnens, W.; Deferm, C.; Sniekers, J.; Fransaer, J.; Binnemans, K. Electrodeposition of Indium from Non-Aqueous Electrolytes. Chem. Commun. 2019, 55, 4789–4792. [Google Scholar] [CrossRef] [Green Version]
- van Roosendael, S.; Roosen, J.; Banerjee, D.; Binnemans, K. Selective Recovery of Germanium from Iron-Rich Solutions Using a Supported Ionic Liquid Phase (SILP). Sep. Purif. Technol. 2019, 221, 83–92. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Lee, M.S. A Review on Germanium Resources and Its Extraction by Hydrometallurgical Method. Miner. Process. Extr. Metall. Rev. 2020, 42, 406–426. [Google Scholar] [CrossRef]
- Kamran Haghighi, H.; Irannajad, M.; Fortuny, A.; Sastre, A.M. Recovery of Germanium from Leach Solutions of Fly Ash Using Solvent Extraction with Various Extractants. Hydrometallurgy 2018, 175, 164–169. [Google Scholar] [CrossRef] [Green Version]
- Kamran Haghighi, H.; Irannajad, M.; Fortuny, A.; Sastre, A.M. Selective Separation of Germanium (IV) from Simulated Industrial Leachates Containing Heavy Metals by Non-Dispersive Ionic Extraction. Miner. Eng. 2019, 137, 344–353. [Google Scholar] [CrossRef]
- Ruiz, A.G.; Sola, P.C.; Moreno Palmerola, N.M. Germanium: Current and Novel Recovery Processes. In Advanced Material and Device Applications with Germanium; Lee, S., Ed.; InTechOpen: London, UK, 2018; Available online: https://doi.org/10.5772/intechopen.77997 (accessed on 29 November 2021).
- Endres, F.; Zein El Abedin, S. Nanoscale Electrodeposition of Germanium on Au (111) from an Ionic Liquid: An in Situ STM Study of Phase Formation. Part II. Ge from GeCl4. Phys. Chem. Chem. Phys. 2002, 4, 1649–1657. [Google Scholar] [CrossRef]
- Yu, Z.; Meng, X.; Yin, M.; Sun, M.; Yuan, M.; Li, H. Pulsed Laser-Assisted Ionic Liquid Electrodeposition of Gallium Nanoparticles and Germanium Nanostructures for Energy Storage. Chem. Phys. Lett. 2018, 698, 181–186. [Google Scholar] [CrossRef]
- van den Bossche, A.; Vereycken, W.; vander Hoogerstraete, T.; Dehaen, W.; Binnemans, K. Recovery of Gallium, Indium, and Arsenic from Semiconductors Using Tribromide Ionic Liquids. ACS Sustain. Chem. Eng. 2019, 7, 14451–14459. [Google Scholar] [CrossRef]
- Raiguel, S.; Dehaen, W.; Binnemans, K. Extraction of Gallium from Simulated Bayer Process Liquor by Kelex 100 Dissolved in Ionic Liquids. Dalton Trans. 2020, 49, 3532–3544. [Google Scholar] [CrossRef] [PubMed]
- Kinsman, L.M.M.; Morrison, C.A.; Ngwenya, B.T.; Love, J.B. Reducing the Competition: A Dual-Purpose Ionic Liquid for the Extraction of Gallium from Iron Chloride Solutions. Molecules 2020, 25, 4047. [Google Scholar] [CrossRef] [PubMed]
- Nassar, N.T.; Fortier, S.M. Methodology and Technical Input for the 2021 Review and Revision of the U.S. Critical Minerals List. In U.S. Geological Survey Open-File Report 2021–1045; U.S. Geological Survey: Reston, VA, USA, 2021; 31p. Available online: https://pubs.er.usgs.gov/publication/ofr20211045 (accessed on 29 November 2021).
- Li, Z.; Diaz, L.A.; Yang, Z.; Jin, H.; Lister, T.E.; Vahidi, E.; Zhao, F. Comparative Life Cycle Analysis for Value Recovery of Precious Metals and Rare Earth Elements from Electronic Waste. Resour. Conserv. Recycl. 2019, 149, 20–30. [Google Scholar] [CrossRef]
- Zuo, Y.; Liu, Y.; Chen, J.; de Li, Q. The Separation of Cerium (IV) from Nitric Acid Solutions Containing Thorium (IV) and Lanthanides (III) Using Pure [C8mim]PF6 as Extracting Phase. Ind. Eng. Chem. Res. 2008, 47, 2349–2355. [Google Scholar] [CrossRef]
- Nakashima, K.; Kubota, F.; Maruyama, T.; Goto, M. Feasibility of Ionic Liquids as Alternative Separation Media for Industrial Solvent Extraction Processes. Ind. Eng. Chem. Res. 2005, 44, 4368–4372. [Google Scholar] [CrossRef]
- Rout, A.; Binnemans, K. Influence of the Ionic Liquid Cation on the Solvent Extraction of Trivalent Rare-Earth Ions by Mixtures of Cyanex 923 and Ionic Liquids. Dalton Trans. 2014, 44, 1379–1387. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Bell, J.R.; Luo, H.; Dai, S. Extraction Separation of Rare-Earth Ions via Competitive Ligand Complexations between Aqueous and Ionic-Liquid Phases. Dalton Trans. 2011, 40, 8019–8023. [Google Scholar] [CrossRef]
- Jensen, M.P.; Neuefeind, J.; Beitz, J.V.; Skanthakumar, S.; Soderholm, L. Mechanisms of Metal Ion Transfer into Room-Temperature Ionic Liquids: The Role of Anion Exchange. J. Am. Chem. Soc. 2003, 125, 15466–15473. [Google Scholar] [CrossRef] [PubMed]
- Kubota, F.; Koyanagi, Y.; Nakashima, K.; Shimojo, K.; Kamiya, N.; Goto, M. Extraction of Lanthanide Ions with an Organophosphorous Extractant into Ionic Liquids. Solvent Extr. Res. Dev. 2008, 15, 81–87. [Google Scholar]
- Kubota, F.; Shimobori, Y.; Baba, Y.; Koyanagi, Y.; Shimojo, K.; Kamiya, N.; Goto, M. Application of Ionic Liquids to Extraction Separation of Rare Earth Metals with an Effective Diglycol Amic Acid Extractant. J. Chem. Eng. Jpn. 2011, 44, 307–312. [Google Scholar] [CrossRef] [Green Version]
- Dupont, D.; Binnemans, K. Recycling of Rare Earths from NdFeB Magnets Using a Combined Leaching/Extraction System Based on the Acidity and Thermomorphism of the Ionic Liquid [Hbet][Tf2N]. Green Chem. 2015, 17, 2150–2163. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Yang, H.; Cui, H.; Zhang, D.; Liu, Y.; Chen, J. Application of Bifunctional Ionic Liquid Extractants [A336][CA-12] and [A336][CA-100] to the Lanthanum Extraction and Separation from Rare Earths in the Chloride Medium. Ind. Eng. Chem. Res. 2011, 50, 7534–7541. [Google Scholar] [CrossRef]
- Rout, A.; Binnemans, K. Solvent Extraction of Neodymium (III) by Functionalized Ionic Liquid Trioctylmethylammonium Dioctyl Diglycolamate in Fluorine-Free Ionic Liquid Diluent. Ind. Eng. Chem. Res. 2014, 53, 6500–6508. [Google Scholar] [CrossRef]
- Khodakarami, M.; Alagha, L. Separation and Recovery of Rare Earth Elements Using Novel Ammonium-Based Task-Specific Ionic Liquids with Bidentate and Tridentate O-Donor Functional Groups. Sep. Purif. Technol. 2020, 232, 115952. [Google Scholar] [CrossRef]
- Sun, X.; Waters, K.E. Development of Industrial Extractants into Functional Ionic Liquids for Environmentally Friendly Rare Earth Separation. ACS Sustain. Chem. Eng. 2014, 2, 1910–1917. [Google Scholar] [CrossRef]
- Sun, X.; Luo, H.; Dai, S. Mechanistic Investigation of Solvent Extraction Based on Anion-Functionalized Ionic Liquids for Selective Separation of Rare-Earth Ions. Dalton Trans. 2013, 42, 8270–8275. [Google Scholar] [CrossRef]
- Guo, X.; Yao, W.; Chen, Y.; Fan, J.; Zhao, Y.; Wang, J. PEG-Functionalized Ionic Liquids: A Class of Liquid Materials for Highly Efficient Extraction of Rare Earth Metals from Aqueous Solutions. J. Mol. Liq. 2017, 236, 308–313. [Google Scholar] [CrossRef]
- Önal, M.A.R.; Dewilde, S.; Degri, M.; Pickering, L.; Saje, B.; Riaño, S.; Walton, A.; Binnemans, K. Recycling of Bonded NdFeB Permanent Magnets Using Ionic Liquids. Green Chem. 2020, 22, 2821–2830. [Google Scholar] [CrossRef]
- Orefice, M.; van den Bulck, A.; Blanpain, B.; Binnemans, K. Selective Roasting of Nd–Fe–B Permanent Magnets as a Pretreatment Step for Intensified Leaching with an Ionic Liquid. J. Sustain. Met. 2020, 6, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Prodius, D.; Gandha, K.; Mudring, A.V.; Nlebedim, I.C. Sustainable Urban Mining of Critical Elements from Magnet and Electronic Wastes. ACS Sustain. Chem. Eng. 2020, 8, 1455–1463. [Google Scholar] [CrossRef]
- Riaño, S.; Sobekova Foltova, S.; Binnemans, K. Separation of Neodymium and Dysprosium by Solvent Extraction Using Ionic Liquids Combined with Neutral Extractants: Batch and Mixer-Settler Experiments. RSC Adv. 2019, 10, 307–316. [Google Scholar] [CrossRef] [Green Version]
- Prodius, D.; Klocke, M.; Smetana, V.; Alammar, T.; Perez Garcia, M.; Windus, T.L.; Nlebedim, I.C.; Mudring, A.-V. Rationally Designed Rare Earth Separation by Selective Oxalate Solubilization. Chem. Commun. 2020, 56, 11386–11389. [Google Scholar] [CrossRef]
- Sobekova Foltova, S.; vander Hoogerstraete, T.; Banerjee, D.; Binnemans, K. Samarium/Cobalt Separation by Solvent Extraction with Undiluted Quaternary Ammonium Ionic Liquids. Sep. Purif. Technol. 2019, 210, 209–218. [Google Scholar] [CrossRef]
- Orefice, M.; Audoor, H.; Li, Z.; Binnemans, K. Solvometallurgical Route for the Recovery of Sm, Co, Cu and Fe from SmCo Permanent Magnets. Sep. Purif. Technol. 2019, 219, 281–289. [Google Scholar] [CrossRef]
- Dupont, D.; Binnemans, K. Rare-Earth Recycling Using a Functionalized Ionic Liquid for the Selective Dissolution and Revalorization of Y2O3:Eu3+ from Lamp Phosphor Waste. Green Chem. 2015, 17, 856–868. [Google Scholar] [CrossRef] [Green Version]
- Pateli, I.M.; Abbott, A.P.; Binnemans, K.; Rodriguez Rodriguez, N. Recovery of Yttrium and Europium from Spent Fluorescent Lamps Using Pure Levulinic Acid and the Deep Eutectic Solvent Levulinic Acid-Choline Chloride. RSC Adv. 2020, 10, 28879–28890. [Google Scholar] [CrossRef]
- Sanchez-Cupido, L.; Pringle, J.M.; Siriwardana, A.L.; Unzurrunzaga, A.; Hilder, M.; Forsyth, M.; Pozo-Gonzalo, C. Water-Facilitated Electrodeposition of Neodymium in a Phosphonium-Based Ionic Liquid. J. Phys. Chem. Lett. 2019, 10, 289–294. [Google Scholar] [CrossRef]
- Entezari-Zarandi, A.; Larachi, F. Selective Dissolution of Rare-Earth Element Carbonates in Deep Eutectic Solvents. J. Rare Earths 2019, 37, 528–533. [Google Scholar] [CrossRef]
- Liu, C.; Yan, Q.; Zhang, X.; Lei, L.; Xiao, C. Efficient Recovery of End-of-Life NdFeB Permanent Magnets by Selective Leaching with Deep Eutectic Solvents. Environ. Sci. Technol. 2020, 54, 10370–10379. [Google Scholar] [CrossRef] [PubMed]
- Riaño, S.; Petranikova, M.; Onghena, B.; vander Hoogerstraete, T.; Banerjee, D.; Foreman, M.R.S.; Ekberg, C.; Binnemans, K. Separation of Rare Earths and Other Valuable Metals from Deep-Eutectic Solvents: A New Alternative for the Recycling of Used NdFeB Magnets. RSC Adv. 2017, 7, 32100–32113. [Google Scholar] [CrossRef] [Green Version]
- Avdibegović, D.; Regadío, M.; Binnemans, K. Efficient Separation of Rare Earths Recovered by a Supported Ionic Liquid from Bauxite Residue Leachate. RSC Adv. 2018, 8, 11886–11893. [Google Scholar] [CrossRef] [Green Version]
- Avdibegović, D.; Binnemans, K. Separation of Scandium from Hydrochloric Acid–Ethanol Leachate of Bauxite Residue by a Supported Ionic Liquid Phase. Ind. Eng. Chem. Res. 2020, 59, 15332–15342. [Google Scholar] [CrossRef]
- Avdibegović, D.; Yagmurlu, B.; Dittrich, C.; Regadío, M.; Friedrich, B.; Binnemans, K. Combined Multi-Step Precipitation and Supported Ionic Liquid Phase Chromatography for the Recovery of Rare Earths from Leach Solutions of Bauxite Residues. Hydrometallurgy 2018, 180, 229–235. [Google Scholar] [CrossRef]
- Li, Z.; Onghena, B.; Li, X.; Zhang, Z.; Binnemans, K. Enhancing Metal Separations Using Hydrophilic Ionic Liquids and Analogues as Complexing Agents in the More Polar Phase of Liquid-Liquid Extraction Systems. Ind. Eng. Chem. Res. 2019, 58, 15628–15636. [Google Scholar] [CrossRef] [Green Version]
- Panigrahi, M.; Grabda, M.; Kozak, D.; Dorai, A.; Shibata, E.; Kawamura, J.; Nakamura, T. Liquid–Liquid Extraction of Neodymium Ions from Aqueous Solutions of NdCl3 by Phosphonium-Based Ionic Liquids. Sep. Purif. Technol. 2016, 171, 263–269. [Google Scholar] [CrossRef]
- Li, F.; Zeng, J.; Sun, X. Functionalized Ionic Liquids Based on Vegetable Oils for Rare Earth Elements Recovery. RSC Adv. 2020, 10, 26671–26674. [Google Scholar] [CrossRef]
- Iqbal, M.; Waheed, K.; Rahat, S.B.; Mehmood, T.; Lee, M.S. An overview of molecular extractants in room temperature ionic liquids and task specifc ionic liquids for the partitioning of actinides/lanthanides. J. Radioanal. Nucl. Chem. 2020, 325, 1–31. [Google Scholar] [CrossRef]
- Taebi, B.; Kloosterman, J.L. To Recycle or Not to Recycle? An Intergenerational Approach to Nuclear Fuel Cycles. Sci. Eng. Ethics 2008, 14, 177–200. [Google Scholar] [CrossRef] [Green Version]
- Leoncini, A.; Huskens, J.; Verboom, W. Ligands for F-Element Extraction Used in the Nuclear Fuel Cycle. Chem. Soc. Rev. 2017, 46, 7229–7273. [Google Scholar] [CrossRef]
- Zarrougui, R.; Mdimagh, R.; Raouafi, N. Highly Efficient Extraction and Selective Separation of Uranium (VI) from Transition Metals Using New Class of Undiluted Ionic Liquids Based on H-Phosphonate Anions. J. Hazard. Mater. 2018, 342, 464–476. [Google Scholar] [CrossRef]
- Irish, E.R.; Reas, W.H. The PUREX Process—A Solvent Extraction Reprocessing Method for Irradiated Uranium; HW-49483-A; General Electric Company Hanford Atomic Products Operation: Washington, DC, USA, 1957; Available online: https://doi.org/10.2172/4341712 (accessed on 29 November 2021).
- Sun, X.; Luo, H.; Dai, S. Ionic Liquids-Based Extraction: A Promising Strategy for the Advanced Nuclear Fuel Cycle. Chem. Rev. 2012, 112, 2100–2128. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.J.; Venkatesan, K.A.; Nagarajan, K.; Srinivasan, T.G. Dissolution of Uranium Oxides and Electrochemical Behavior of U(VI) in Task Specific Ionic Liquid. Radiochim. Acta 2008, 96, 403–409. [Google Scholar] [CrossRef]
- Moyer, B.A.; Lumetta, G.J.; Mincher, B.J. Minor Actinide Separation in the Reprocessing of Spent Nuclear Fuels: Recent Advances in the United States. In Reprocessing and Recycling of Spent Nuclear Fuel; Taylor, R., Ed.; Woodhead Publishing: Oxford, UK, 2015; pp. 289–312. Available online: https://doi.org/10.1016/B978-1-78242-212-9.00011-3 (accessed on 29 November 2021).
- Sun, X.; Do-Thanh, C.L.; Luo, H.; Dai, S. The Optimization of an Ionic Liquid-Based TALSPEAK-like Process for Rare Earth Ions Separation. Chem. Eng. J. 2014, 239, 392–398. [Google Scholar] [CrossRef]
- Taylor, R.J.; Gregson, C.R.; Carrott, M.J.; Mason, C.; Sarsfield, M.J. Progress towards the Full Recovery of Neptunium in an Advanced PUREX Process. Solvent Extr. Ion Exch. 2013, 31, 442–462. [Google Scholar] [CrossRef]
- Zsabka, P.; van Hecke, K.; Wilden, A.; Modolo, G.; Verwerft, M.; Binnemans, K.; Cardinaels, T. Selective Extraction of Americium from Curium and the Lanthanides by the Lipophilic Ligand CyMe4BTPhen Dissolved in Aliquat-336 Nitrate Ionic Liquid. Solvent Extr. Ion Exch. 2020, 38, 194–211. [Google Scholar] [CrossRef]
- Zsabka, P.; Opsomer, T.; van Hecke, K.; Dehaen, W.; Wilden, A.; Modolo, G.; Verwerft, M.; Binnemans, K.; Cardinaels, T. Solvent Extraction Studies for the Separation of Trivalent Actinides from Lanthanides with a Triazole-Functionalized 1,10-Phenanthroline Extractant. Solvent Extr. Ion Exch. 2020, 38, 719–734. [Google Scholar] [CrossRef]
- An Americium-Fueled Gas Core Nuclear Rocket. Available online: https://doi.org/10.1063/1.43073 (accessed on 29 November 2021).
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. Available online: https://pubs.acs.org/doi/10.1021/acs.chemrev.0c00385 (accessed on 29 November 2021). [CrossRef] [PubMed]
- Ni, S.; Su, J.; Zhang, H.; Zeng, Z.; Zhi, H.; Sun, X. A cleaner strategy for comprehensive recovery of waste SmCo magnets based on deep eutectic solvents. Chem. Eng. J. 2021, 412, 128602. [Google Scholar] [CrossRef]
- Zürner, P.; Frisch, G. Leaching and Selective Extraction of Indium and Tin from Zinc Flue Dust Using an Oxalic Acid-Based Deep Eutectic Solvent. ACS Sustain. Chem. Eng. 2019, 7, 5300–5308. Available online: https://pubs.acs.org/doi/10.1021/acssuschemeng.8b06331 (accessed on 29 November 2021). [CrossRef]
- Yu, G.; Zhao, D.; Wen, L.; Yang, S.; Chen, X. Viscosity of Ionic Liquids: Database, Observation, and Quantitative Structure-Property Relationship Analysis. AIChE J. 2012, 58, 2885–2899. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, J.; Huang, Y.; Zhou, Q.; Zhang, X.; Zhang, S. Toxicity of Ionic Liquids: Database and Prediction via Quantitative Structure-Activity Relationship Method. J. Hazard. Mater. 2014, 278, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Hora, P.I.; Pati, S.G.; McNamara, P.J.; Arnold, W.A. Increased Use of Quaternary Ammonium Compounds during the SARS-CoV-2 Pandemic and Beyond: Consideration of Environmental Implications. Environ. Sci. Technol. Lett. 2020, 7, 622–631. [Google Scholar] [CrossRef]
- Ionic Liquids: Environmentally Sustainable Solvent, Energy Storage and Separation Processes. Available online: https://www.bccresearch.com/market-research/chemicals/ionic-liquids-market-research-report.html (accessed on 29 November 2021).
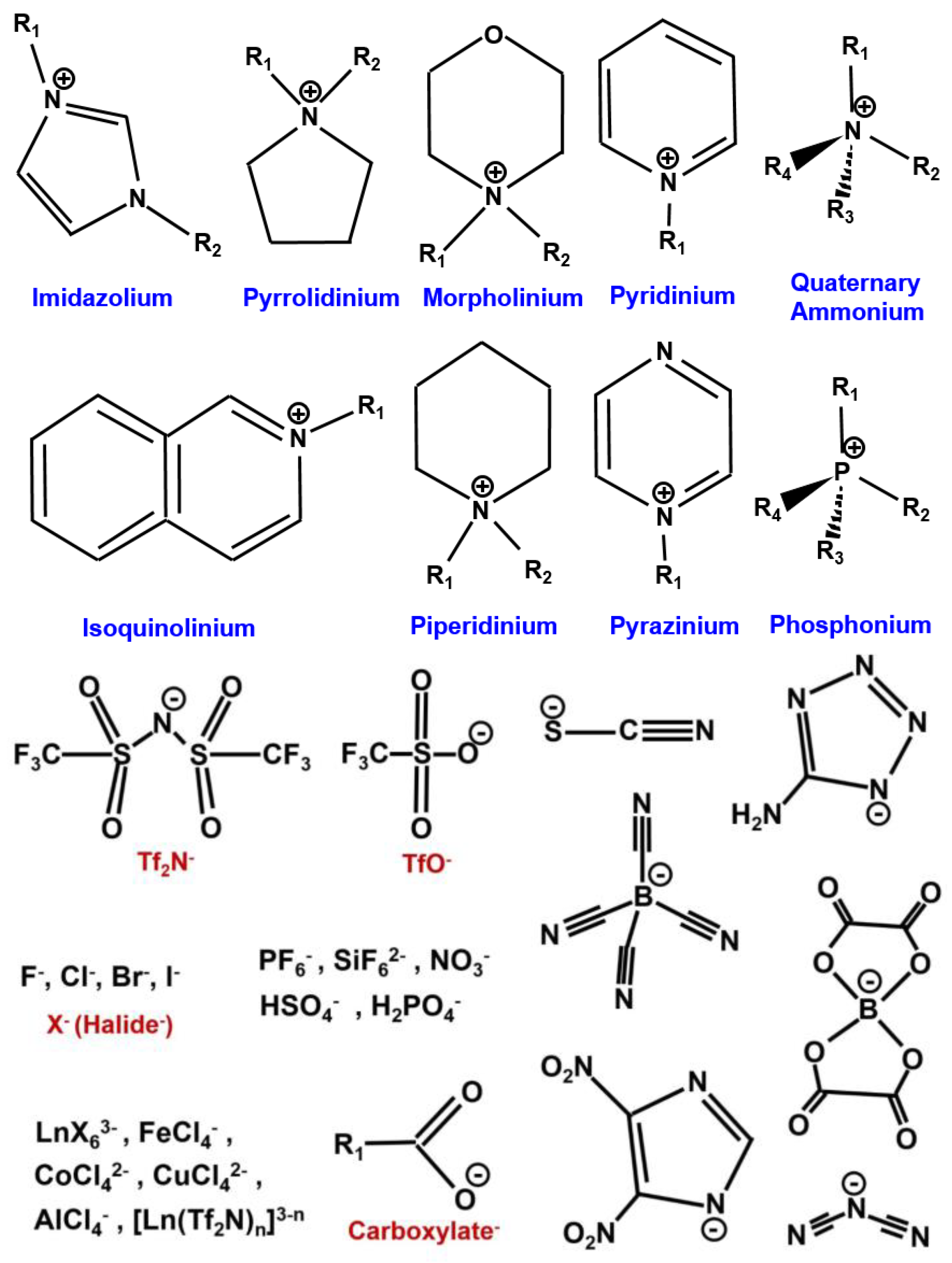

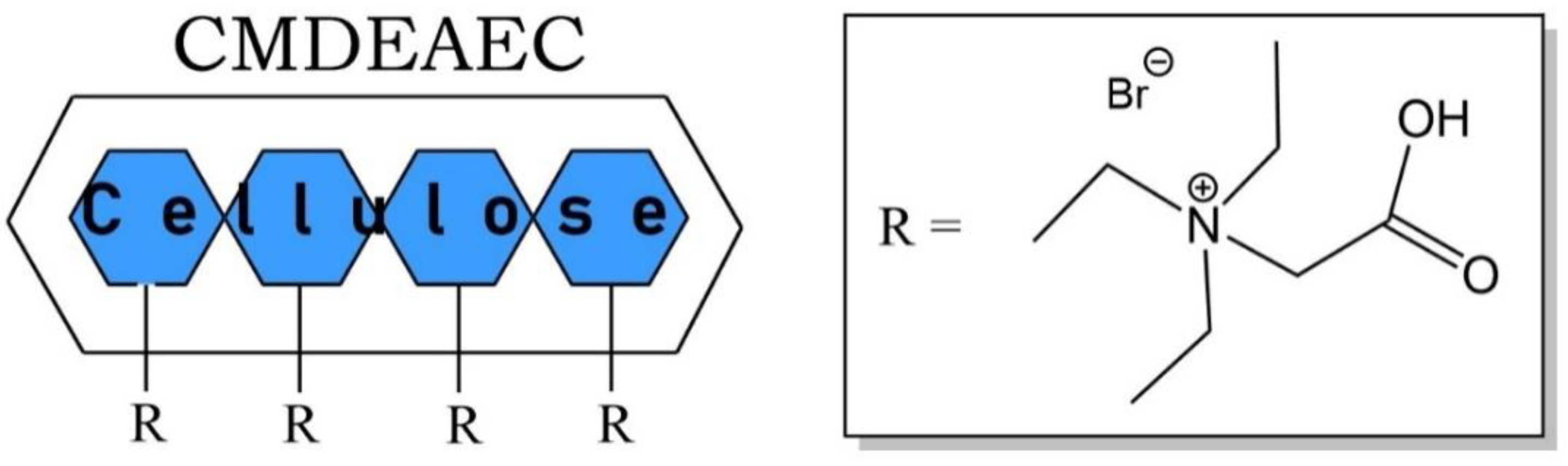
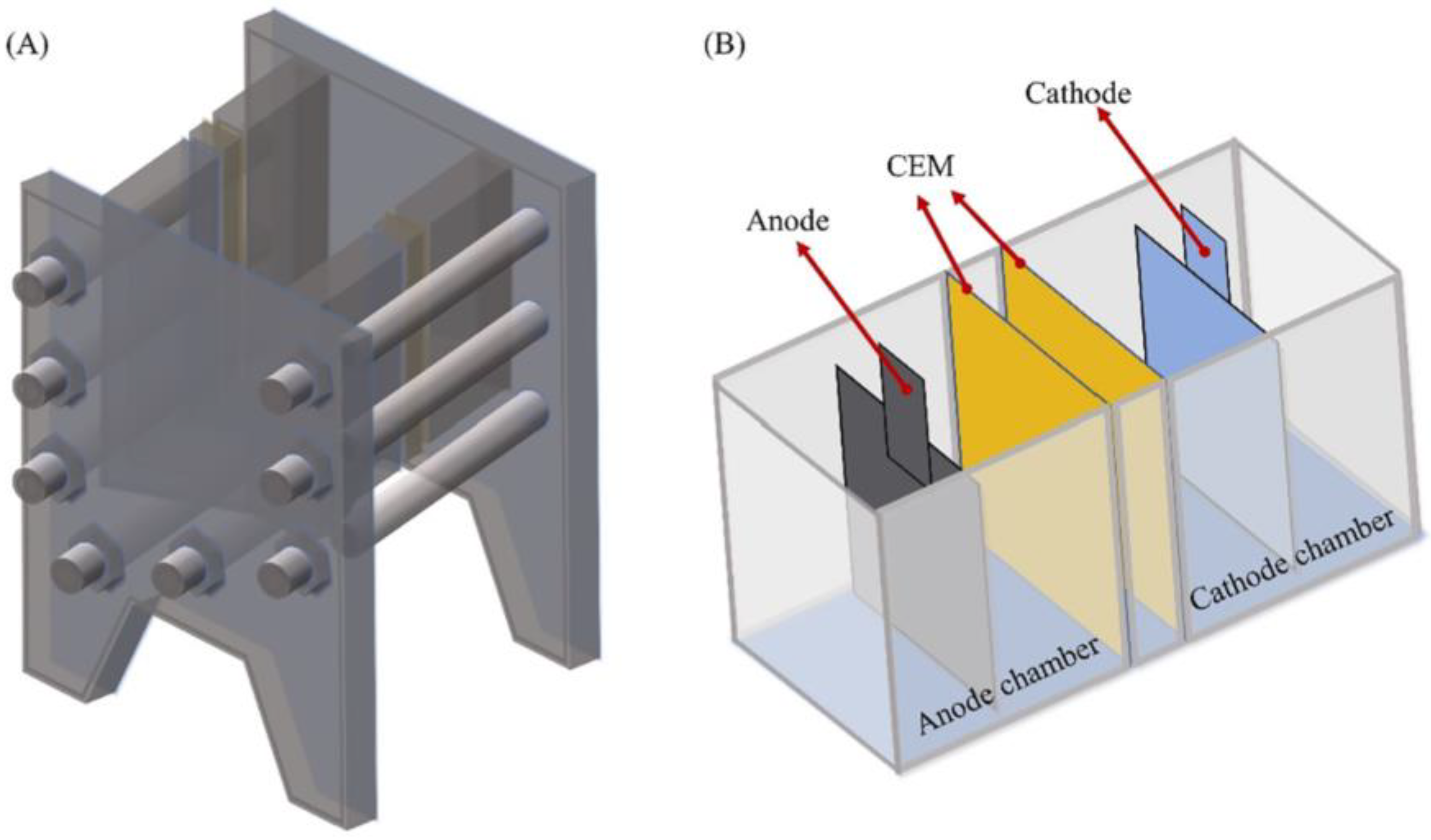
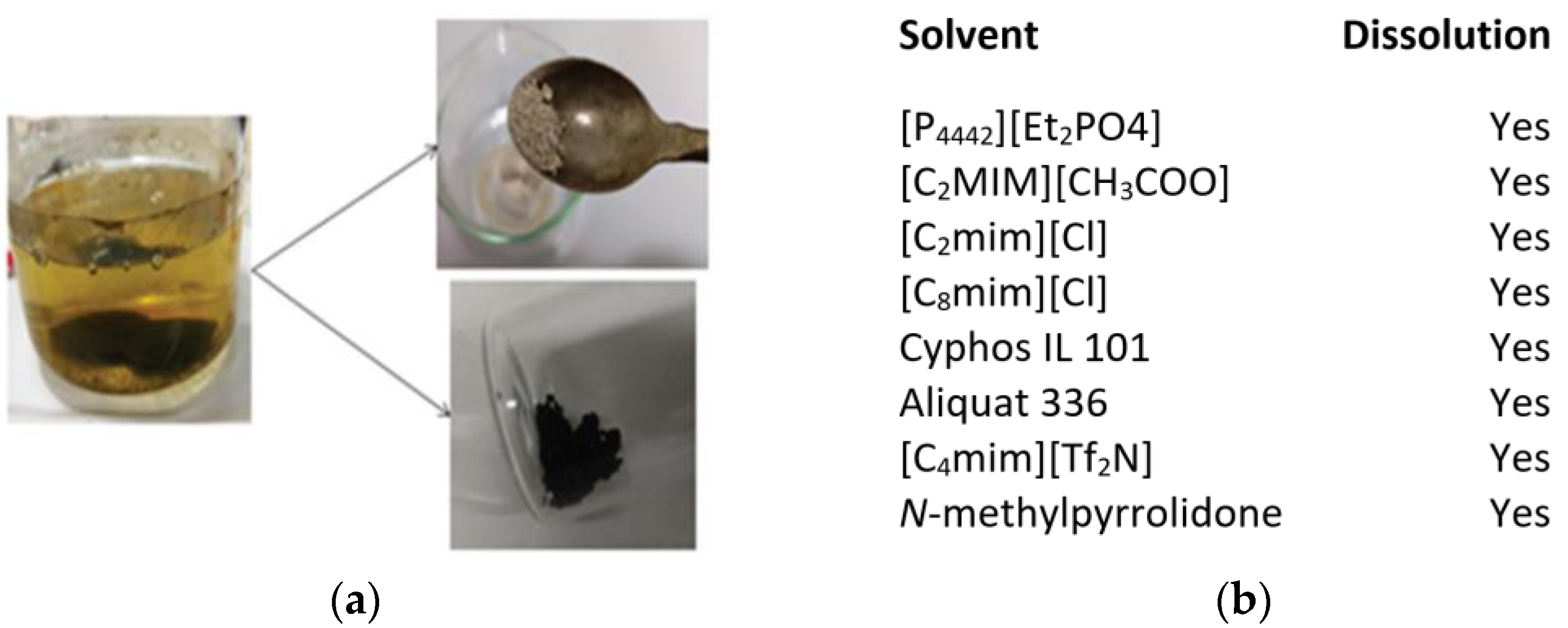
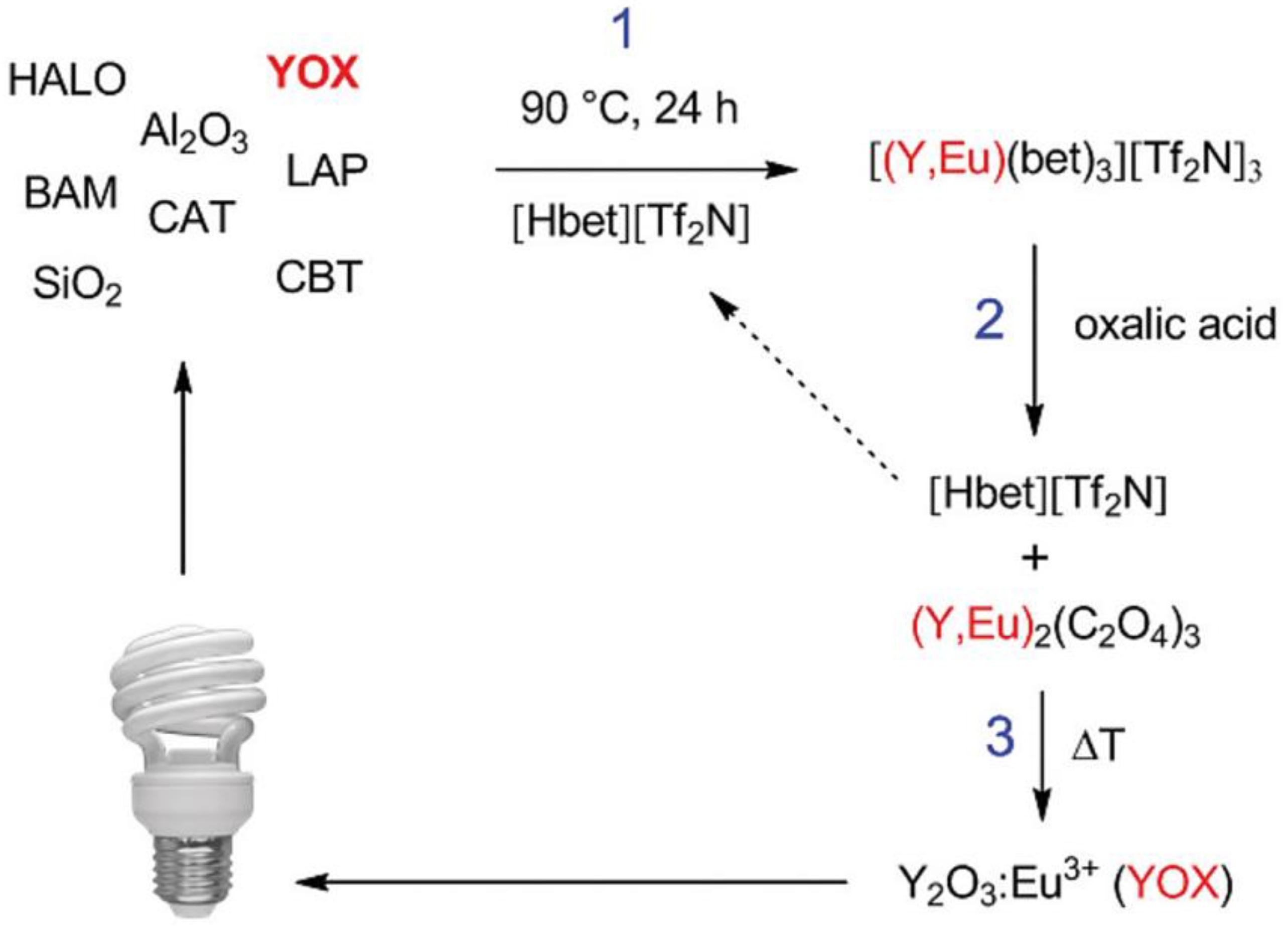
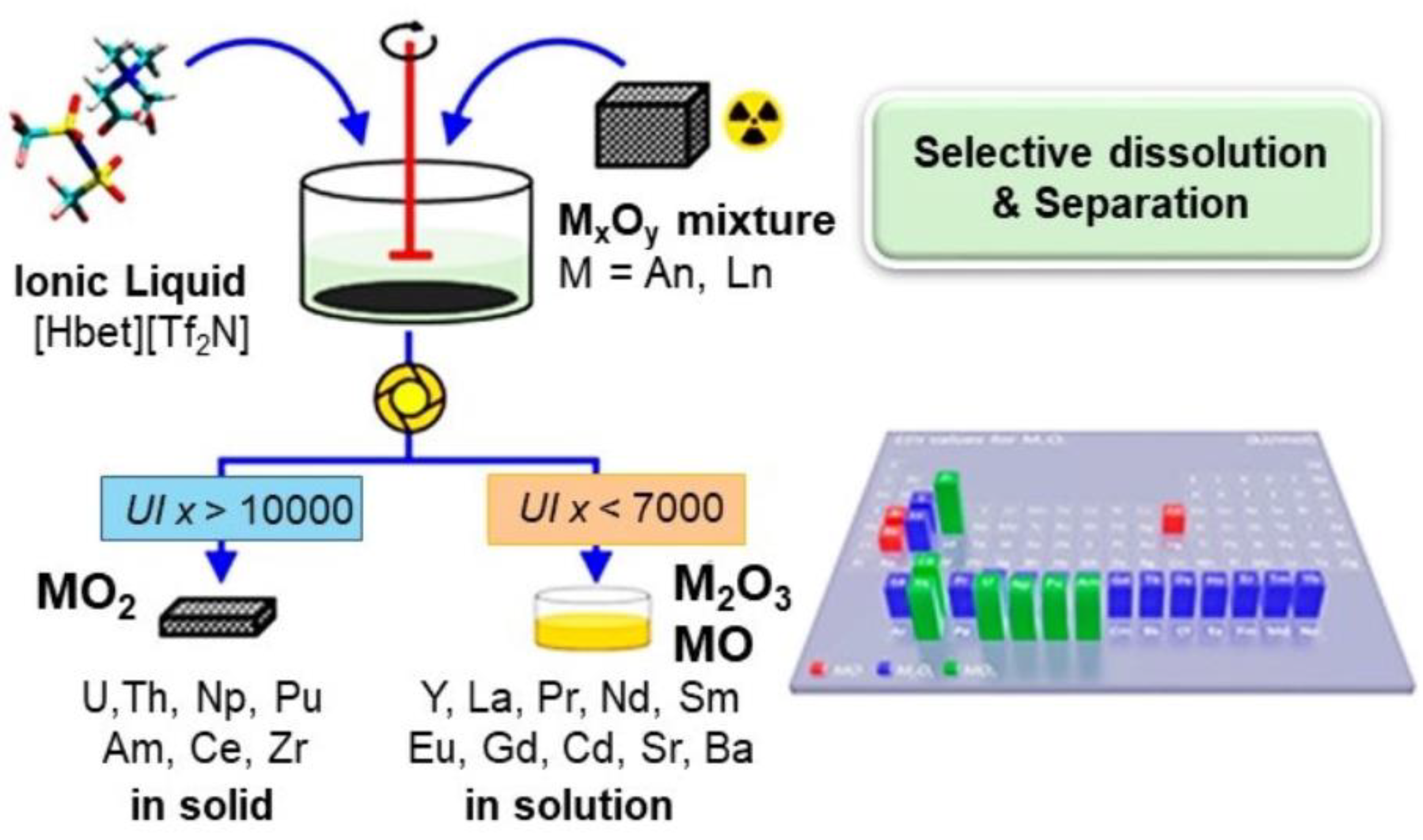
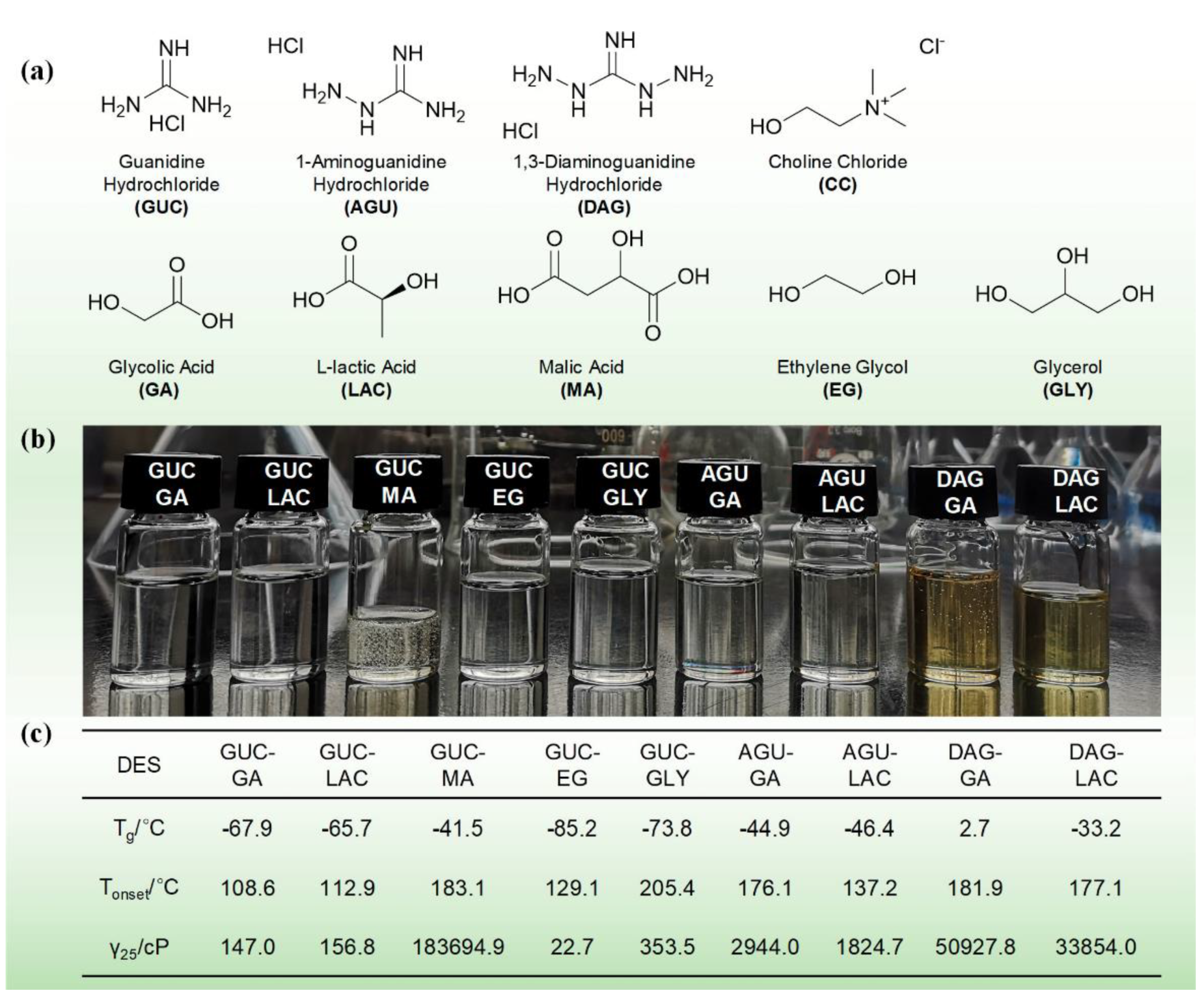
| Property | Organic Solvents | Ionic Liquids |
|---|---|---|
| Number of solvents | >1000 | >1,000,000 |
| Applicability | Single function- | Multifunction |
| Catalytic ability | Rare | Common and tunable |
| Chirality | Rare | Common and tunable |
| Vapor pressure | Obeys the Clausius-Clapeyron Equation | Negligible under normal conditions |
| Flammability | Usually flammable | Usually nonflammable |
| Solvation | Weakly solvating | Strongly solvating |
| Tunability | Limited range of solvents available | Unlimited range means ‘designer solvents’ |
| Polarity | Conventional polarity concepts apply | Polarity concept questionable |
| Cost | Normally inexpensive | 2 to 100 times the cost of organic solvents |
| Recyclability | Green imperative | Economic imperative |
| Viscosity/cP | 0.2–100 | 22–40,000 |
| Density/g cm−3 | 0.6–1.7 | 0.8–3.3 |
| Refractive index | 1.3–1.6 | 1.5–2.2 |
| Structure of Cation | Ionic Liquid | Viscosity, η (Pa·s) | Glass Transition, Tg (°C) | Melting Point, Tm (°C) | Decomposition, Td (°C) | Solubility: Miscible/Immiscible |
|---|---|---|---|---|---|---|
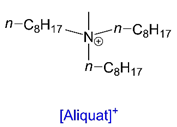 | [Aliquat][TSA] | - | - | 77.98 | 241 | E, EA/W, H |
| [Aliquat][DCA] | 0.300 | −89.94 | - | >300 | E/W, H | |
| [Aliquat][SAC] | 2.824 | −54.02 | - | 205 | E, EA/W | |
| [Aliquat][TFA] | 0.740 | −70.95 | - | 183 | E, H,EA/W | |
| [Aliquat][Tf2N] | 0.354 | −81.42 | - | >300 | E, EA/W, H | |
| [Aliquat][TfO] | - | - | - | >300 | E, EA/W, H | |
| [Aliquat][SCN] | 1.017 | - | - | - | E, EA/W, H | |
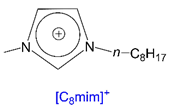 | [C8mim][TSA] | - | - | 89.48 | >300 | W, EA/H, E |
| [C8mim][DCA] | 0.034 | −92.93 | - | 250 | W, EA/H, E | |
| [C8mim][SAC] | 0.389 | −63.68 | - | 245 | EA/H, E | |
| [C8mim][TFA] | 0.054 | −88.90 | - | 190 | EA/H, E | |
| [C8mim][Tf2N] | 0.087 | −86.45 | - | >300 | EA/W, H,E | |
| [C8mim][TfO] | 0.100 | −92.30 | - | >300 | EA/W, H,E | |
| [C8mim][SCN] | 0.070 | - | - | 226 | W, EA/H, E |
| DES Used | Other Reagents | Temp (°C) | Time (h) | Leaching Efficiency Co, % | Recovery of Cobalt, % | Leaching Efficiency Li, % | Refs. |
|---|---|---|---|---|---|---|---|
| PTSA·nH2O·ChClDES | - | 90 | 0.25 | 100.0 | 94.0 | 100.0 | [55] |
| PEG200/thiourea (2:1) | - | 160 | 24 | 60.2 | - | - | [56] |
| Reline (Urea-ChCl) | 4% H2O | 160 | 24 | 43.0 | - | - | [57] |
| (ChCl:EG) | - | 180 | 24 | 29.6–32.0 | 74.0 | 71.0 | [58] |
| Choline-Chloride-citric acid | Al, Cu, 35 wt.% H2O | 40 | 1 | 99.6 | 81.0 | 93.0 | [59] |
| Year | Ionic Liquid | Aqueous Medium | Separation Factor | Mechanism | Refs. |
|---|---|---|---|---|---|
| 2010 | [A336][CA-12] in toluene | Sulfate | 25 | Ion association | [62] |
| 2012 | Cyphos® IL 101, aka P66614Cl saturated with water | Chloride | 50,000 | Anion exchange | [63] |
| 2013 | Cyphos IL 104 aka, [P66614][R2POO], R = 2,4,4-trimethylpentyl | [C2mim]Cl | 207 | Anion exchange | [61] |
| 2014 | [P44414][Cl]–NaCl–H2O | Chloride | <500 | Anion exchange | [64] |
| Extractant | Solvent/Diluent | Feed | Target Product | Mechanism | Refs. |
|---|---|---|---|---|---|
| [C8mim][PF6] | water | Nitric acid solution of lanthanides (III) and thorium (IV) | Cerium(IV) | Anion exchange KPF6 strippant | [86] |
| CMPO | [C4mim][PF6] | Nitric acid solution of mixed salts | Ce(III), Eu(III), Y(III) | Cation exchange | [87] |
| Cyanex 923 | [C4mim][Tf2N], [N1444][Tf2N], [C10mim][Tf2N], [P66614][Tf2N], [N1888][Tf2N] | Nd in nitric acids solution | Nd(III) | Cation exchange | [88] |
| HDEHP | [Cnmim][Tf2N] [Cnmim][BETI] [C4mPy][Tf2N] | Lanthanide ions in glycolic acid or citric acid | REE | Cation exchange | [89] |
| Htta | [C4mim][Tf2N] | Nd(III), Eu(III) | Anion exchange HTfN strip | [90] | |
| PC-88A | ([Cnmim][Tf2N], n = 8, 12) | La(III),Ce(III), Eu(III), and Y(III) | proton exchange | [91] | |
| DODGAA | [Cnmim][Tf2N] n = 4, 8,12) | Y3+, Eu3+, Zn2+ | Y(III), Eu(III) | Proton exchange | [92] |
| [Hbet][Tf2N] | water | Roasted NdFeB magnets | REOn | Release of protons by [Hbet]+ | [93] |
| [A366][CA-12] [A336][CA-100] | n-heptane | Chloride medium | La(III) | [94] | |
| [A336][DGA] | [A336][NO3] | Nitric acid medium | Nd(III) | [95] | |
| [A336][DHDGA] [OcGBOEt][Br] | Hexane, toluene, chloroform | Nitrate feed | La, Pr, Nd, Sm, Eu, Tb, Dy, Y, Er | Ion association | [96] |
| [N2222][EHEHP] [N2222][DEHP] [N4444][DEHP] [N6666][DEHP] [N8888][DEHP] | heptane | REE chloride solution | RE | [97] | |
| [TOMA][DEHP] | [Cnmim][Tf2N], n = 4, 6, 8, 10 [Cnmim][BETI], n = 4, 6, 8, 10) | RE solution | RE | [98] | |
| [PEGm(mim)2] [Tf2N]2 (m = 200, 400, 600) | water | Nitrate feed | Sc, Y, La-Nd, Sm, Gd, Dy, Ho, Yb, Lu | Ion pair association | [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inman, G.; Nlebedim, I.C.; Prodius, D. Application of Ionic Liquids for the Recycling and Recovery of Technologically Critical and Valuable Metals. Energies 2022, 15, 628. https://doi.org/10.3390/en15020628
Inman G, Nlebedim IC, Prodius D. Application of Ionic Liquids for the Recycling and Recovery of Technologically Critical and Valuable Metals. Energies. 2022; 15(2):628. https://doi.org/10.3390/en15020628
Chicago/Turabian StyleInman, Grace, Ikenna C. Nlebedim, and Denis Prodius. 2022. "Application of Ionic Liquids for the Recycling and Recovery of Technologically Critical and Valuable Metals" Energies 15, no. 2: 628. https://doi.org/10.3390/en15020628
APA StyleInman, G., Nlebedim, I. C., & Prodius, D. (2022). Application of Ionic Liquids for the Recycling and Recovery of Technologically Critical and Valuable Metals. Energies, 15(2), 628. https://doi.org/10.3390/en15020628








