Medium-Temperature Phosphate Glass Composite Material as a Matrix for the Immobilization of High-Level Waste Containing Volatile Radionuclides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Procedures
2.2. Methods
3. Results and Discussion
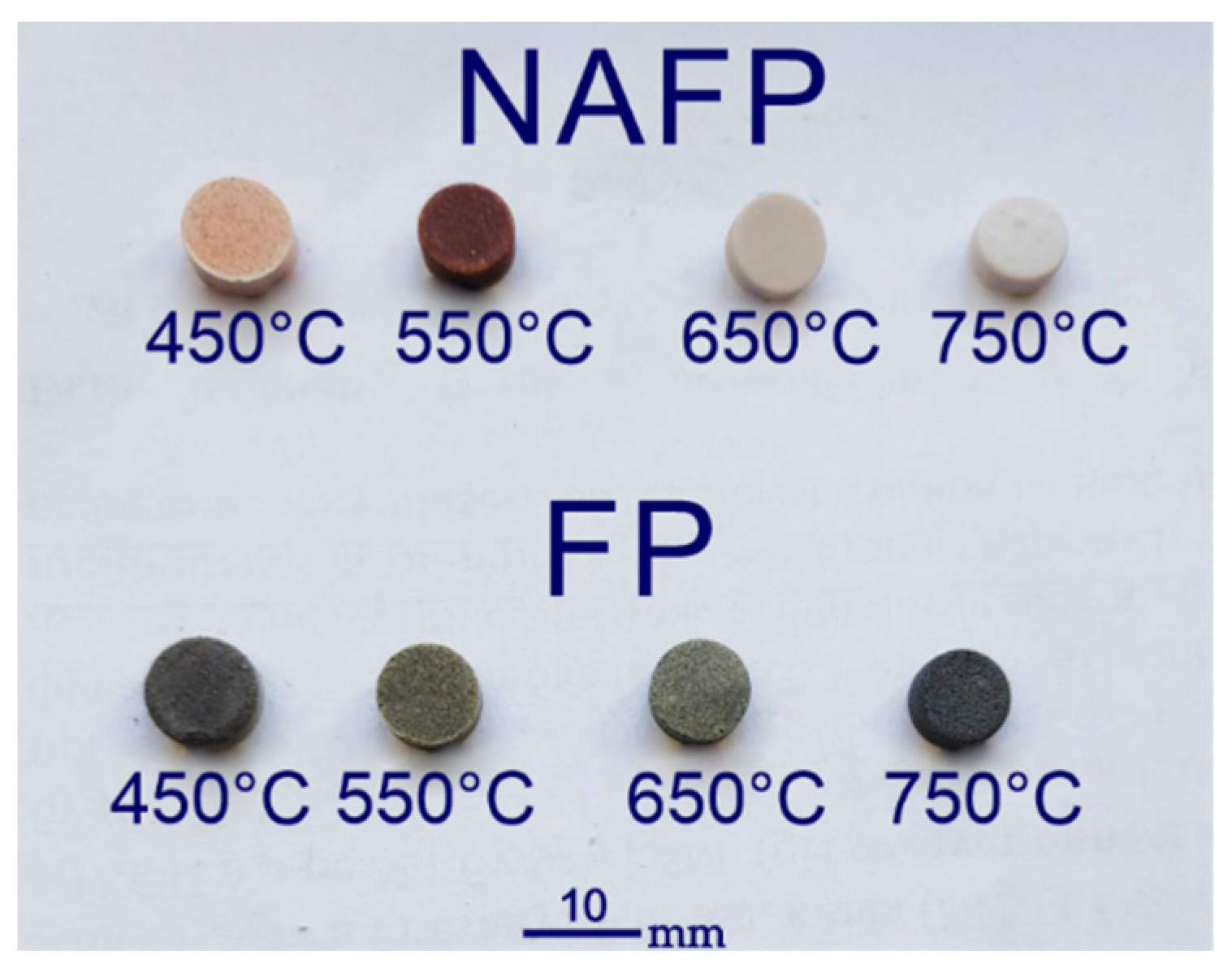
3.1. Geometric Parameters and Density of Glass Composite Material Tablets
3.2. Characterization of the Synthesized Compounds by XRD and SEM/EDS
3.3. Hydrothermal Resistance of Glass Composite Matrices in PCT Standard Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fedorov, Y.S.; Zil’berman, B.Y.; Aloi, A.S.; Puzikov, E.A.; Shadrin, A.Y.; Alyapyshev, M.Y. Problems of Modernization of Spent Nuclear Fuel Extraction Processing. Russ. J. Gen. Chem. 2011, 81, 1932–1948. [Google Scholar] [CrossRef]
- Alyapyshev, M.Y.; Babain, V.A.; Ustynyuk, Y.A. Recovery of Minor Actinides from High-Level Wastes: Modern Trends. Russ. Chem. Rev. 2016, 85, 943–961. [Google Scholar] [CrossRef]
- Lee, H. Oxide Electroreduction and Other Processes for Pyrochemical Processing of Spent Nuclear Fuels. In Reprocessing and Recycling of Spent Nuclear Fuel; Elsevier: Amsterdam, The Netherlands, 2015; pp. 415–436. [Google Scholar]
- Ma, B.; Charlet, L.; Fernandez-Martinez, A.; Kang, M.; Madé, B. A Review of the Retention Mechanisms of Redox-Sensitive Radionuclides in Multi-Barrier Systems. Appl. Geochem. 2019, 100, 414–431. [Google Scholar] [CrossRef]
- Vuori, S. The Environmental and Ethical Basis of the Geological Disposal of Long-Lived Radioactive Waste. ATS Ydintekniikka 1995, 24, 16–17. [Google Scholar]
- Watson, L.C.; Aikin, A.M.; Bancroft, A.I. The Permanent Disposal of Highly Radioactive Wastes by Incorporation into Glass. In Disposal of Radioactive Wastes. Vol. I. Proceedings of the Scientific Conference on the Disposal of Radioactive Wastes; International Atomic Energy Agency (IAEA): Vienna, Austria, 1960. [Google Scholar]
- Gregg, D.J.; Farzana, R.; Dayal, P.; Holmes, R.; Triani, G. Synroc Technology: Perspectives and Current Status (Review). J. Am. Ceram. Soc. 2020, 103, 5424–5441. [Google Scholar] [CrossRef]
- Vance, E.R.; Chavara, D.T.; Gregg, D.J. Synroc Development—Past and Present Applications. MRS Energy Sustain. 2017, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Deokattey, S.; Bhaskar, N.; Kalyane, V.L.; Kumar, V.; Jahagirdar, P.B. Borosilicate Glass and Synroc R&D for Radioactive Waste Immobilization: An International Perspective. JOM 2003, 55, 48–51. [Google Scholar] [CrossRef]
- Ringwood, E.; Kelly, P.M. Immobilization of High-Level Waste in Ceramic Waste Forms. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1986, 319, 63–82. [Google Scholar] [CrossRef]
- Ewing, R.C. Ceramic Matrices for Plutonium Disposition. Prog. Nucl. Energy 2007, 49, 635–643. [Google Scholar] [CrossRef]
- Arinicheva, Y.; Bukaemskiy, A.; Neumeier, S.; Modolo, G.; Bosbach, D. Studies on Thermal and Mechanical Properties of Monazite-Type Ceramics for the Conditioning of Minor Actinides. Prog. Nucl. Energy 2014, 72, 144–148. [Google Scholar] [CrossRef]
- Zhang, Y.; Vance, E.R. Plutonium in Monazite and Brabantite: Diffuse Reflectance Spectroscopy Study. J. Nucl. Mater. 2008, 375, 311–314. [Google Scholar] [CrossRef]
- Crum, J.; Maio, V.; McCloy, J.; Scott, C.; Riley, B.; Benefiel, B.; Vienna, J.; Archibald, K.; Rodriguez, C.; Rutledge, V.; et al. Cold Crucible Induction Melter Studies for Making Glass Ceramic Waste Forms: A Feasibility Assessment. J. Nucl. Mater. 2014, 444, 481–492. [Google Scholar] [CrossRef]
- McCloy, J.S.; Schuller, S. Vitrification of Wastes: From Unwanted to Controlled Crystallization, a Review. Comptes Rendus Géoscience 2022, 354, 1–40. [Google Scholar] [CrossRef]
- Li, H.; Wu, L.; Wang, X.; Xu, D.; Teng, Y.; Li, Y. Crystallization Behavior and Microstructure of Barium Borosilicate Glass–Ceramics. Ceram. Int. 2015, 41, 15202–15207. [Google Scholar] [CrossRef]
- Richerson, D.W.; Lee, W.E. Modern Ceramic Engineering; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9780429488245. [Google Scholar]
- Choi, J.; Um, W.; Choung, S. Development of Iron Phosphate Ceramic Waste Form to Immobilize Radioactive Waste Solution. J. Nucl. Mater. 2014, 452, 16–23. [Google Scholar] [CrossRef]
- Poluektov, P.P.; Schmidt, O.V.; Kascheev, V.A.; Ojovan, M.I. Modelling Aqueous Corrosion of Nuclear Waste Phosphate Glass. J. Nucl. Mater. 2017, 484, 357–366. [Google Scholar] [CrossRef]
- Lee, W.E.; Ojovan, M.I.; Stennett, M.C.; Hyatt, N.C. Immobilisation of Radioactive Waste in Glasses, Glass Composite Materials and Ceramics. Adv. Appl. Ceram. 2006, 105, 3–12. [Google Scholar] [CrossRef]
- Soderquist, C.Z.; Schweiger, M.J.; Kim, D.-S.; Lukens, W.W.; McCloy, J.S. Redox-Dependent Solubility of Technetium in Low Activity Waste Glass. J. Nucl. Mater. 2014, 449, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Donald, I.W.; Metcalfe, B.L.; Fong, S.K.; Gerrard, L.A.; Strachan, D.M.; Scheele, R.D. A Glass-Encapsulated Calcium Phosphate Wasteform for the Immobilization of Actinide-, Fluoride-, and Chloride-Containing Radioactive Wastes from the Pyrochemical Reprocessing of Plutonium Metal. J. Nucl. Mater. 2007, 361, 78–93. [Google Scholar] [CrossRef]
- Shadrin, A.Y.; Dvoeglazov, K.N.; Maslennikov, A.G.; Kashcheev, V.A.; Tret’yakova, S.G.; Shmidt, O.V.; Vidanov, V.L.; Ustinov, O.A.; Volk, V.I.; Veselov, S.N.; et al. PH Process as a Technology for Reprocessing Mixed Uranium–Plutonium Fuel from BREST-OD-300 Reactor. Radiochemistry 2016, 58, 271–279. [Google Scholar] [CrossRef]
- Simpson, M.F.; Sachdev, P. Development of Electrorefiner Waste Salt Disposal Process for The Ebr- Ii Spent Fuel Treatment Project. Nucl. Eng. Technol. 2008, 40, 175–182. [Google Scholar] [CrossRef]
- Pereira, C.; Hash, M.; Lewis, M.; Richmann, M. Ceramic-Composite Waste Forms from the Electrometallurgical Treatment of Spent Nuclear Fuel. JOM 1997, 49, 34–40. [Google Scholar] [CrossRef]
- Priebe, S.; Bateman, K. The Ceramic Waste Form Process at Idaho National Laboratory. Nucl. Technol. 2008, 162, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Jena, H.; Maji, B.K.; Asuvathraman, R.; Govindan Kutty, K.V. Synthesis and Thermal Characterization of Glass Bonded Ca-Chloroapatite Matrices for Pyrochemical Chloride Waste Immobilization. J. Non-Cryst. Solids 2012, 358, 1681–1686. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Petrov, V.A.; Yudintsev, S.V. Glass Crystalline Materials as Advanced Nuclear Wasteforms. Sustainability 2021, 13, 4117. [Google Scholar] [CrossRef]
- Stoch, P.; Ciecinska, M. Thermochemistry of Phosphate Glasses for Immobilization of Dangerous Waste. J. Therm. Anal. Calorim. 2012, 108, 705–709. [Google Scholar] [CrossRef] [Green Version]
- Michie, E.M.; Grimes, R.W.; Boccaccini, A.R. Hot-Pressed Phosphate Glass–Ceramic Matrix Composites Containing Calcium Phosphate Particles for Nuclear Waste Encapsulation. J. Mater. Sci. 2008, 43, 4152–4156. [Google Scholar] [CrossRef]
- Danilov, S.S.; Frolova, A.V.; Kulikova, S.A.; Vinokurov, S.E.; Maslakov, K.I.; Teterin, A.Y.; Teterin, Y.A.; Myasoedov, B.F. Immobilization of Rhenium as a Technetium Surrogate in Aluminum Iron Phosphate Glass. Radiochemistry 2021, 63, 99–106. [Google Scholar] [CrossRef]
- Kulikova, S.A.; Belova, K.Y.; Tyupina, E.A.; Vinokurov, S.E. Conditioning of Spent Electrolyte Surrogate LiCl-KCl-CsCl Using Magnesium Potassium Phosphate Compound. Energies 2020, 13, 1963. [Google Scholar] [CrossRef]
- Kulikova, S.A.; Danilov, S.S.; Matveenko, A.V.; Frolova, A.V.; Belova, K.Y.; Petrov, V.G.; Vinokurov, S.E.; Myasoedov, B.F. Perspective Compounds for Immobilization of Spent Electrolyte from Pyrochemical Processing of Spent Nuclear Fuel. Appl. Sci. 2021, 11, 11180. [Google Scholar] [CrossRef]
- Day, D.E.; Marasinghe, K.; Ray, C.S. An Alternative Host Matrix Based on Iron Phosphate Glasses for the Vitrification of Specialized Nuclear Waste Forms; Annual Progress Report, September 15, 1996–September 14, 1997. 1997. Available online: https://inis.iaea.org/search/search.aspx?orig_q=RN:31003327 (accessed on 8 October 2022).
- Brow, R.K. Review: The Structure of Simple Phosphate Glasses. J. Non-Cryst. Solids 2000, 263–264, 1–28. [Google Scholar] [CrossRef]
- Ray, C.S.; Fang, X.; Karabulut, M.; Marasinghe, G.K.; Day, D.E. Effect of Melting Temperature and Time on Iron Valence and Crystallization of Iron Phosphate Glasses. J. Non-Cryst. Solids 1999, 249, 1–16. [Google Scholar] [CrossRef]
- Kalmykov, S.N.; Presniakov, I.A.; Stefanovskaya, O.I.; Stefanovsky, S.V.; Myasoedov, B.F.; Glazkova, Y.S.; Sobolev, A.V.; Vinokurov, S.E. Oxidation state and coordination of iron in sodium–aluminum–iron phosphate glasses and their hydrolytic stability. Dokl. Phys. Chem. 2015, 463, 145–149. [Google Scholar] [CrossRef]
- Danilov, S.S.; Stefanovsky, S.V.; Stefanovskaya, O.I.; Vinokurov, S.E.; Myasoedov, B.F.; Teterin, Y.A. Aluminum (iron) phosphate glasses containing rare earth and transuranium elements: Phase composition, oxidation state of Np and Pu, and hydrolytic durability. Radiochemistry 2018, 60, 434–439. [Google Scholar] [CrossRef]
- Day, D.E.; Wu, Z.; Ray, C.S.; Hrma, P. Chemically Durable Iron Phosphate Glass Wasteforms. J. Non-Cryst. Solids 1998, 241, 1–12. [Google Scholar] [CrossRef]
- Ren, M.; Cai, S.; Xu, G.; Ye, X.; Dou, Y.; Huang, K.; Wang, X. Influence of Heat Treatment on Crystallization and Corrosion Behavior of Calcium Phosphate Glass Coated AZ31 Magnesium Alloy by Sol–Gel Method. J. Non-Cryst. Solids 2013, 369, 69–75. [Google Scholar] [CrossRef]
- C 1285-14; Standard Test Methods for Determining Chemical Durability of Nuclear, Hazardous, and Mixed Waste Glasses and Multiphase Glass Ceramics: The Product Consistency Test (PCT). ASTM International: West Conshohocken, PA, USA, 2014.





| Element | FP | NAFP |
|---|---|---|
| P | 24.9 | 23.0 |
| Fe | 30.0 | 10.4 |
| Na | - | 17.1 |
| Al | - | 5.0 |
| O | 45.1 | 44.6 |
| Sample | Parameters | before Synthesis | 450 °C | 550 °C | 650 °C | 750 °C |
|---|---|---|---|---|---|---|
| FP | d | 10.10 | 10.10 | 9.45 | 9.38 | 9.20 |
| h | 3.60 | 3.60 | 3.50 | 3.45 | 3.40 | |
| ρ | 2.12 | 2.12 | 2.44 | 2.52 | 2.52 | |
| NAFP | d | 10.10 | 10.10 | 10.10 | 9.15 | 9.15 |
| h | 3.80 | 3.80 | 3.80 | 3.55 | 3.50 | |
| ρ | 1.97 | 1.97 | 1.97 | 2.57 | 2.62 |
| Element | Phase #1 | Phase #2 |
|---|---|---|
| P | 24.5 ± 2.4 | 23.5 ± 2.3 |
| Fe | 30.7 ± 3.1 | 30.8 ± 3.1 |
| O | 44.8 ± 4.5 | 44.3 ± 4.4 |
| Mo | - | 1.5 ± 0.2 |
| Element | Phase #1 | Phase #2 | Phase #3 | Phase #4 |
|---|---|---|---|---|
| P | 22.3 ± 2.2 | 23.8 ± 2.4 | 24.5 ± 2.4 | 20.8 ± 2.1 |
| Fe | 12.1 ± 1.2 | 16.3 ± 1.6 | 9.4 ± 0.9 | 20.5 ± 2.1 |
| Na | 16.0 ± 1.6 | 9.5 ± 0.9 | 14.1 ± 1.4 | 14.0 ± 1.4 |
| Al | 5.9 ± 0.8 | 5.1 ± 0.7 | 6.1 ± 0.8 | 2.2 ± 0.3 |
| O | 43.4 ± 4.3 | 44.4 ± 4.4 | 45.9 ± 4.6 | 42.5 ± 4.3 |
| Si | 0.3 ± 0.1 | 1.0 ± 0.2 | - | 0.24 ± 0.1 |
| Sample | Temperature Synthesis | P | Fe | Na | Al |
|---|---|---|---|---|---|
| FP | 450 | 1.0 × 10−6 | 1.1 × 10−7 | - | - |
| 550 | 6.5 × 10−7 | 1.1 × 10−7 | - | - | |
| 650 | 5.7 × 10−7 | 5.7 × 10−8 | - | - | |
| 750 | 3.9 × 10−7 | 7.9 × 10−9 | - | - | |
| NAFP | 450 | 1.1 × 10−6 | 5.0 × 10−6 | 1.7 × 10−5 | 6.8 × 10−6 |
| 550 | 8.5 × 10−5 | 8.6 × 10−6 | 1.5 × 10−4 | 6.2 × 10−6 | |
| 650 | 6.1 × 10−5 | 1.2 × 10−5 | 8.1 × 10−5 | 2.0 × 10−5 | |
| 750 | 1.1 × 10−4 | 9.0 × 10−6 | 1.7 × 10−4 | 1.6 × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frolova, A.V.; Vinokurov, S.E.; Gromyak, I.N.; Danilov, S.S. Medium-Temperature Phosphate Glass Composite Material as a Matrix for the Immobilization of High-Level Waste Containing Volatile Radionuclides. Energies 2022, 15, 7506. https://doi.org/10.3390/en15207506
Frolova AV, Vinokurov SE, Gromyak IN, Danilov SS. Medium-Temperature Phosphate Glass Composite Material as a Matrix for the Immobilization of High-Level Waste Containing Volatile Radionuclides. Energies. 2022; 15(20):7506. https://doi.org/10.3390/en15207506
Chicago/Turabian StyleFrolova, Anna V., Sergey E. Vinokurov, Irina N. Gromyak, and Sergey S. Danilov. 2022. "Medium-Temperature Phosphate Glass Composite Material as a Matrix for the Immobilization of High-Level Waste Containing Volatile Radionuclides" Energies 15, no. 20: 7506. https://doi.org/10.3390/en15207506
APA StyleFrolova, A. V., Vinokurov, S. E., Gromyak, I. N., & Danilov, S. S. (2022). Medium-Temperature Phosphate Glass Composite Material as a Matrix for the Immobilization of High-Level Waste Containing Volatile Radionuclides. Energies, 15(20), 7506. https://doi.org/10.3390/en15207506







