Screening of Nickel and Platinum Catalysts for Glycerol Conversion to Gas Products in Hydrothermal Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
2.2.1. Characterisation of Feedstock and Catalysts
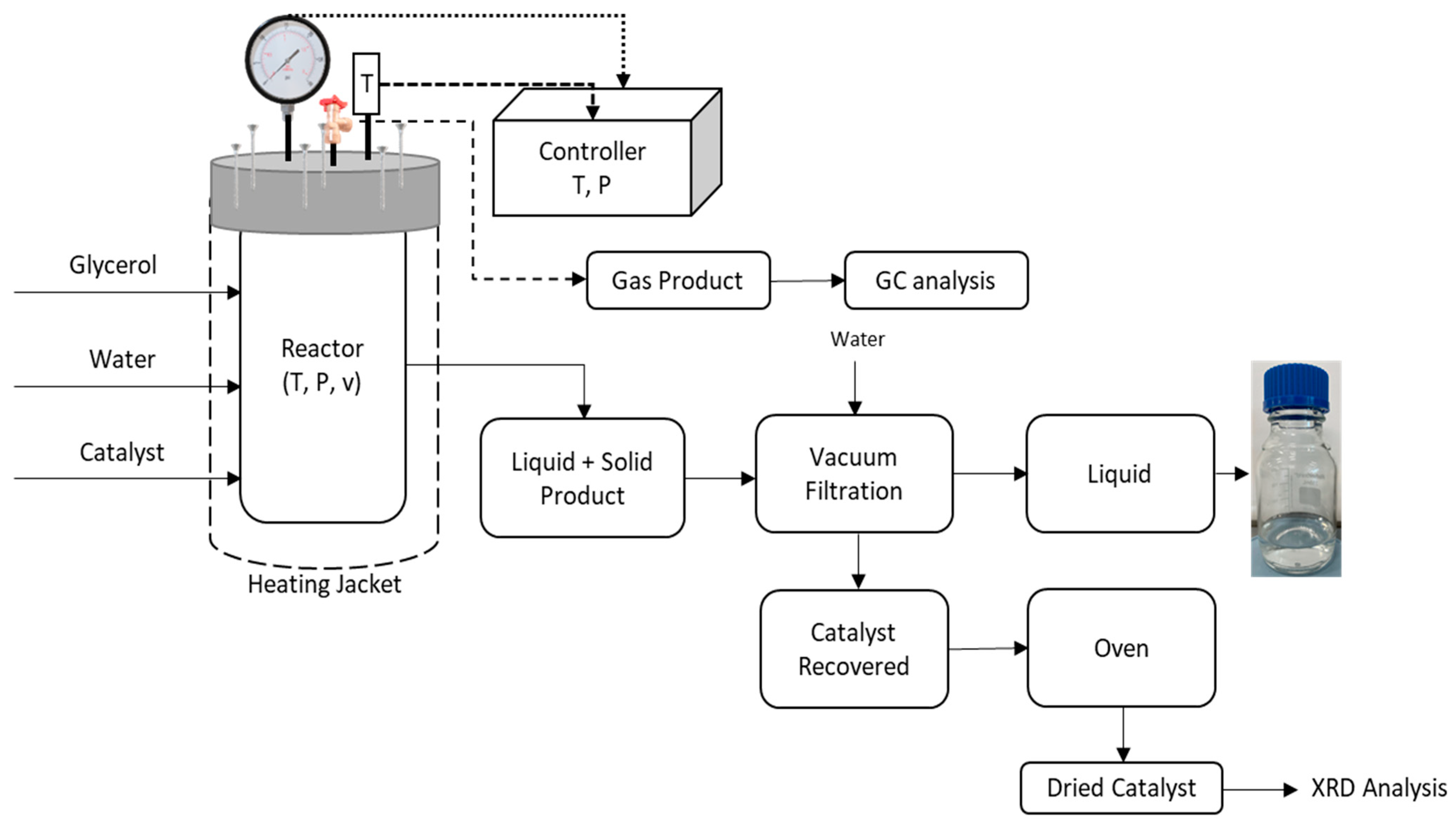
2.2.2. Experimental Set-Up
2.2.3. Analysis of Products
Gas Product Analysis
2.3. Carbon and Hydrogen Gasification Efficiencies (CGE and HGE)
3. Results and Discussions
3.1. Conversion of Glycerol in Relation to Catalyst and Reaction Conditions
3.2. Effect of Temperature
3.3. Structural Stability of Used Catalysts in Hydrothermal Media
3.3.1. NiFe2O4
3.3.2. Physical Mixtures of NiFe2O4 and Pt Catalysts
3.3.3. Ni-Cu/Al2O3
3.3.4. Ni-Cu/Al2O3-Pt/SiO2
3.3.5. Ni-Cu/Al2O3-Pt/Al2O3
3.3.6. Ni-Cu/Al2O3-Pt/C
3.3.7. Catalyst Reuse
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, K.; Peng, X.; Kong, L.; Wu, W.; Chen, Y.; Maravelias, C.T. Greenhouse Gas Emission Mitigation Potential of Chemicals Produced from Biomass. ACS Sust. Chem. Eng. 2021, 9, 14480–14487. [Google Scholar] [CrossRef]
- Wang, K.; Zuo, Y.; Pei, P.; Xie, X.; Wei, M.; Xiong, J.; Zhang, P. Highly efficient hydrogen production via a zinc carbon @ nickel system. Int. J. Hydrog. Energy 2022, 47, 5354–5360. [Google Scholar] [CrossRef]
- Ballantyne, A.P.; Alden, C.B.; Miller, J.B.; Tans, P.P.; White, J.W.C. Increase in observed net carbon dioxide uptake by land and oceans during the past 50 years. Nature 2012, 488, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Hermann, B.G.; Blok, K.; Patel, M.K. Producing Bio-Based Bulk Chemicals Using Industrial Biotechnology Saves Energy and Combats Climate Change. Environ. Sci. Technol. 2007, 41, 7915–7921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ardi, M.S.; Aroua, M.K.; Hashim, A.N. Progress, prospect and challenges in glycerol purification process: A review. Renew. Sust. Energy Rev. 2015, 42, 1164–1173. [Google Scholar] [CrossRef] [Green Version]
- Yahya, M.; Dutta, A.; Bouri, E.; Wadström, C.; Uddin, G.S. Dependence structure between the international crude oil market and the European markets of biodiesel and rapeseed oil. Renew. Energy 2022, 197, 594–605. [Google Scholar] [CrossRef]
- Sittijunda, S.; Reungsang, A. Valorization of crude glycerol into hydrogen, 1,3-propanediol, and ethanol in an up-flow anaerobic sludge blanket (UASB) reactor under thermophilic conditions. Renew. Energy 2020, 161, 361–372. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Muldoon, V.L.; Deng, S. Crude glycerol and glycerol as fuels and fuel additives in combustion applications. Renew. Sust. Energy Rev. 2022, 159, 112206. [Google Scholar] [CrossRef]
- Fasolini, A.; Cespi, D.; Tabanelli, T.; Cucciniello, R.; Cavani, F. Hydrogen from Renewables: A Case Study of Glycerol Reforming. Catalysts 2019, 9, 722. [Google Scholar] [CrossRef] [Green Version]
- Sahraei, A.; Desgagnés, O.; Larachi, A.F.; Iliuta, M.C. Ni-Fe catalyst derived from mixed oxides Fe/Mg-bearing metallurgical waste for hydrogen production by steam reforming of biodiesel by-product: Investigation of catalyst synthesis parameters and temperature dependency of the reaction network. Appl. Catal. B Environ. 2020, 279, 119330. [Google Scholar] [CrossRef]
- Yun, S.; Zhang, Y.; Zhang, L.; Liu, Z.; Deng, Y. Ni and Fe nanoparticles, alloy and Ni/Fe-Nx coordination co-boost the catalytic activity of the carbon-based catalyst for triiodide reduction and hydrogen evolution reaction. J. Coll. Int. Sci. 2022, 615, 501–516. [Google Scholar] [CrossRef] [PubMed]
- Chakinala, A.G.; Chinthaginjala, J.K.; Seshanb, K.; van Swaaij, W.P.M.; Kersten, S.R.A.; Brilman, D.W.F. Catalyst screening for the hydrothermal gasification of aqueous phase of bio-oil. Catal. Today 2012, 195, 83–92. [Google Scholar] [CrossRef]
- Seretis, A.; Tsiakaras, P. A thermodynamic analysis of hydrogen production via aqueous phase reforming of glycerol. Fuel Process. Technol. 2015, 134, 107–115. [Google Scholar] [CrossRef]
- Shabaker, J.W.; Huber, G.W.; Davda, R.R.; Cortright, R.D.; Dumesic, J.A. Aqueous phase reforming of ethylene glycol over supported platinum catalysts. Catal. Lett. 2003, 88, 1–8. [Google Scholar] [CrossRef]
- Liguras, D.K.; Kondarides, D.I.; Verykios, X.E. Production of hydrogen for fuel cells by steam reforming of ethanol over supported noble metal catalysts. Appl. Catal. B Environ. 2003, 43, 345–354. [Google Scholar] [CrossRef]
- Roslan, N.A.; Abidin, S.Z.; Osazuwa, O.U.; Chin, S.Y.; Taufiq-Yap, Y.H. Enhanced syngas production from glycerol dry reforming over Ru promoted -Ni catalyst supported on extracted Al2O3. Fuel 2022, 314, 123050. [Google Scholar] [CrossRef]
- Roslan, N.A.; Abidin, S.Z.; Ideris, A.; Vo, D.V.N. A review on glycerol reforming processes over Ni-based catalyst for hydrogen and syngas productions. Int. J. Hydrog. Energy 2020, 45, 18466–18489. [Google Scholar] [CrossRef]
- Demsash, H.D.; Kondamudi, K.V.K.; Upadhyayula, S.; Mohan, R. Ruthenium doped nickel-alumina-ceria catalyst in glycerol steam reforming. Fuel Proc. Technol. 2018, 169, 150–156. [Google Scholar] [CrossRef]
- Suffredini, D.F.P.; Thyssen, V.V.; de Almeida, P.M.M.; Gomes, R.S.; Borges, M.C.; de Farias, A.M.D.; Assaf, E.M.; Fraga, M.A.; Brandão, S.T. Renewable hydrogen from glycerol reforming over nickel aluminate-based catalysts. Catal. Today 2017, 289, 96–104. [Google Scholar] [CrossRef]
- Yang, T.; Wang, P.; Li, Q.; Xia, C.; Yin, F.; Liang, C.; Zhang, Y. Hydrogen absorption and desorption behavior of Ni catalyzed MgeYeCeNi nanocomposites. Energy 2018, 165, 709–719. [Google Scholar] [CrossRef]
- Jiao, Y.; He, Z.; Wang, J.; Chen, Y. n-decane steam reforming for hydrogen production over mono- and bi-metallic Co-Ni/Ce-Al2O3 catalysts: Structure-activity correlations. Energy Conv. Manag. 2017, 148, 954–962. [Google Scholar] [CrossRef]
- Touri, A.E.; Taghizadeh, M. Hydrogen Production via Glycerol Reforming over Pt/SiO2 Nanocatalyst in a Spiral-Shaped Microchannel Reactor. Int. J. Chem. Reactor Eng. 2016, 14, 1059–1068. [Google Scholar] [CrossRef]
- Chakinala, N.; Chakinala, A.G. Catalytic reforming of glycerol in hot compressed water: Role of metal and support. J. Sup. Fluids 2022, 180, 105459. [Google Scholar] [CrossRef]
- Boga, D.A.; Liu, F.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Aqueous-phase reforming of crude glycerol: Effect of impurities on hydrogen production. Catal. Sci. Technol. 2016, 6, 134–143. [Google Scholar] [CrossRef] [Green Version]
- Ciftci, A.; Ligthart, M.D.A.J.; Arno, A.O.S.; van Hoof, J.F.; Friedrich, H.; Emiel Hensen, J.M. Pt-Re synergy in aqueous-phase reforming of glycerol and the water–gas shift reaction, Pt-Re synergy in aqueous-phase reforming of glycerol and the water–gas shift reaction. J. Catal. 2014, 311, 88–101. [Google Scholar] [CrossRef]
- Imai, H.; Yamawaki, M.; Li, X. Direct Synthesis of Methane from Glycerol by Using Silica-modified Nickel Catalyst. J. Jpn. Petrol. 2017, 60, 311–321. [Google Scholar] [CrossRef] [Green Version]
- Borges, A.C.P.; Onwudili, J.A.; Andrade, H.; Alves, C.; Ingram, A.; Vieira de Melo, S.; Torres, E. Catalytic Properties and Recycling of NiFe2O4 Catalyst for Hydrogen Production by Supercritical Water Gasification of Eucalyptus Wood Chips. Energies 2020, 13, 4553. [Google Scholar] [CrossRef]
- Gao, N.; Salisu, J.; Quan, C.; Williams, P. Modified nickel-based catalysts for improved steam reforming of biomass tar: A critical review. Renew. Sust. Energy Rev. 2021, 145, 111023. [Google Scholar] [CrossRef]
- Luo, M.F.; Lin, W.R.; Wen, W.H.; Chang, B.W. Methanol electro-oxidation and induced sintering on Pt nanoclusters supported on thin-film Al2O3/NiAl(1 0 0). Surf. Sci. 2008, 602, 3258–3265. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, X.; Azmat, M.U.; Xu, W.; Ren, J.; Wang, Y.; Lu, G. Hydrogen production by aqueous-phase reforming of glycerol over Ni-B catalysts. Int. J. Hydrog. Energy 2012, 37, 227–234. [Google Scholar] [CrossRef]
- Koichumanova, K.; Vikla, A.K.K.; de Vlieger, D.J.M.; Seshan, K.; Mojet, B.L.; Lefferts, L. Towards stable catalysts for aqueous phase conversion of ethylene glycol for renewable hydrogen. Chem. Sus. Chem. 2013, 6, 1717–1723. [Google Scholar] [CrossRef]
- Kim, T.; Song, H.; Heechul, K.; Jong, Y.; Chung, S. Steam reforming of n-dodecane over K2Ti2O5-added Ni-alumina and Ni-zirconia (YSZ) catalysts. Int. J. Hydrog. Energy 2016, 41, 17922–17932. [Google Scholar] [CrossRef]
- Razaq, I.; Simons, K.E.; Onwudili, J.A. Parametric Study of Pt/C-Catalysed Hydrothermal Decarboxylation of Butyric Acid as a Potential Route for Biopropane Production. Energies 2021, 14, 3316. [Google Scholar] [CrossRef]
- Tribalis, A.; Tsilomekis, G.; Boghosian, S. Molecular structure and reactivity of titania-supported transition metal oxide catalysts synthesized by equilibrium deposition filtration for the oxidative dehydrogenation of ethane. Comptes Rendus Chimie 2016, 19, 1226–1236. [Google Scholar] [CrossRef]
- Torres, A.; Roy, D.; Subramaniam, B.; Chaudhari, R.V. Kinetic Modeling of Aqueous-Phase Glycerol Hydrogenolysis in a Batch Slurry Reactor. Ind. Eng. Chem. Res. 2010, 49, 10826–10835. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Ighalo, J.O. Hydrogen production by the steam reforming of waste lubricating oil. Ind. Chem Eng. 2019, 61, 403–414. [Google Scholar] [CrossRef]
- Vasiliadou, E.S.; Eggenhuisen, T.M.; Munnik, P.; de Jongh, P.E.; de Jong, K.P.; Lemonidou, A.A. Synthesis and performance of highly dispersed Cu/SiO2 catalysts for the hydrogenolysis of glycerol. Appl. Catal. B Environ. 2014, 145, 108–119. [Google Scholar] [CrossRef]
- Schwengber, C.A.; Silva, F.A.; Schaffner, R.A.; Fernandes-Machado, N.R.C.; Ferracin, R.J.F.; Bach, V.R.; Alves, H.J. Methane dry reforming using Ni/Al2O3 catalysts: Evaluation of the effects of temperature, space velocity and reaction time. J. Environ. Chem. Eng. 2016, 4, 3688–3695. [Google Scholar] [CrossRef]
- Wu, H.; Guo, Y.Y.; Jiang, X.; Qi, Y.; Sun, B.; Li, H.; Zheng, J.; Li, X. A rare earth hydride supported ruthenium catalyst for the hydrogenation of N-heterocycles: Boosting the activity via a new hydrogen transfer path and controlling the stereoselectivity. Chem. Sci. 2019, 10, 10459–10465. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Zhou, Z.; Shishido, T. Behavior of active species on Pt-Sn/SiO2 catalyst during the dehydrogenation of propane and regeneration. Appl. Catal. A Gen. 2020, 606, 117826. [Google Scholar] [CrossRef]
- Pompeo, F.; Santori, G.; Nichio, N.N. Hydrogen and/or syngas from steam reforming of glycerol. Study of platinum catalysts. Int. J. Hydrog. Energy 2010, 35, 8912–8920. [Google Scholar] [CrossRef]













| Sample | C (wt%) | H (%) | N (%) | S (%) | O (%) | HHV (MJ/kg) | Density at 20 °C (kg/m3) | Ash (wt%) | Moisture (wt%) |
|---|---|---|---|---|---|---|---|---|---|
| Glycerol | 40.8 ± 0.43 | 9.86 ± 0.20 | 0.12 ± 0.01 | nd | 49.3 ± 0.62 | 19.1 ± 0.54 | 1261.3 ± 0.00 | nd | 0.11 ± 0.03 |
| Catalyst | m Glycerol (g) | m Water (g) | m Catalyst (g) | Time (min) | Temperature (°C) | Maximum Pressure (bar) | Conversion (%) |
|---|---|---|---|---|---|---|---|
| No Catalyst | 2.07 ± 0.01 | 20 | 0.00 | 60 | 250 | 36.7 ± 0.0 | 0.48 ± 0.01 |
| 2.08 ± 0.01 | 20 | 0.00 | 60 | 300 | 82.5 ± 0.1 | 0.94 ± 0.01 | |
| 2.05 ± 0.02 | 20 | 0.00 | 60 | 350 | 129.3 ± 0.1 | 41.90 ± 0.01 | |
| NiFe2O4 | 2.08 ± 0.03 | 20 | 1.01 | 60 | 250 | 37.3 ± 0.0 | 0.70 ± 0.00 |
| 2.00 ± 0.03 | 20 | 1.02 | 60 | 300 | 86.8 ± 0.1 | 28.61 ± 0.01 | |
| 2.10 ± 0.00 | 20 | 1.02 | 60 | 350 | 130.5 ± 0.0 | 53.11 ± 0.02 | |
| Ni-Cu/Al2O3 | 2.05 ± 0.01 | 20 | 1.02 | 60 | 250 | 36.9 ± 0.1 | 1.10 ± 0.02 |
| 2.10 ± 0.01 | 20 | 1.01 | 60 | 300 | 86.5 ± 0.3 | 14.24 ± 0.05 | |
| 1.98 ± 0.01 | 20 | 1.04 | 60 | 350 | 161.0 ± 0.0 | 63.45 ± 0.03 | |
| 5 wt% Pt/SiO2 | 2.08 ± 0.01 | 20 | 1.01 | 60 | 350 | 160.6 ± 0.1 | 32.53 ± 0.02 |
| 5 wt% Pt/Al2O3 | 2.08 ± 0.01 | 20 | 1.01 | 60 | 350 | 163.3 ± 0.1 | 99.84 ± 0.01 |
| 5 wt% Pt/C | 2.07 ± 0.02 | 20 | 1.02 | 60 | 350 | 175.70 ± 0.1 | 97.67 ± 0.05 |
| NiFe2O4 and 5 wt% Pt/SiO2 | 2.09 ± 0.00 | 20 | 1.00 | 60 | 350 | 171.0 ± 0.1 | 27.51 ± 0.01 |
| NiFe2O4 and 5 wt% Pt/Al2O3 | 2.04 ± 0.01 | 20 | 1.03 | 60 | 350 | 172.5 ± 0.3 | 32.92 ± 0.03 |
| NiFe2O4 and 5 wt% Pt/C | 2.08 ± 0.01 | 20 | 1.01 | 60 | 350 | 164.9 ± 0.2 | 99.84 ± 0.03 |
| Ni-Cu/Al2O3 and 5 wt% Pt/SiO2 | 2.04 ± 0.02 | 20 | 1.05 | 60 | 250 | 37.7 ± 0.0 | 3.52 ± 0.03 |
| 2.05 ± 0.01 | 20 | 1.00 | 60 | 300 | 84.6 ± 0.2 | 8.62 ± 0.03 | |
| 2.04 ± 0.02 | 20 | 1.01 | 60 | 350 | 173.4 ± 0.1 | 35.83 ± 0.00 | |
| Ni-Cu/Al2O3 and 5 wt% Pt/Al2O3 | 2.10 ± 0.03 | 20 | 1.07 | 60 | 250 | 51.6 ± 0.1 | 23.52 ± 0.03 |
| 2.05 ± 0.01 | 20 | 1.02 | 60 | 300 | 88.9 ± 0.0 | 29.44 ± 0.05 | |
| 2.04 ± 0.01 | 20 | 1.02 | 60 | 350 | 165.9 ± 0.1 | 99.67 ± 0.04 | |
| Ni-Cu/Al2O3 and 5 wt% Pt/C | 2.03 ± 0.02 | 20 | 1.02 | 60 | 250 | 37.0 ± 0.0 | 21.22 ± 0.02 |
| 2.05 ± 0.01 | 20 | 1.01 | 60 | 300 | 87.1 ± 0.1 | 42.59 ± 0.00 | |
| 2.05 ± 0.01 | 20 | 1.01 | 60 | 350 | 165.9 ± 0.1 | 99.73 ± 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, C.T.; Onwudili, J.A. Screening of Nickel and Platinum Catalysts for Glycerol Conversion to Gas Products in Hydrothermal Media. Energies 2022, 15, 7571. https://doi.org/10.3390/en15207571
Alves CT, Onwudili JA. Screening of Nickel and Platinum Catalysts for Glycerol Conversion to Gas Products in Hydrothermal Media. Energies. 2022; 15(20):7571. https://doi.org/10.3390/en15207571
Chicago/Turabian StyleAlves, Carine T., and Jude A. Onwudili. 2022. "Screening of Nickel and Platinum Catalysts for Glycerol Conversion to Gas Products in Hydrothermal Media" Energies 15, no. 20: 7571. https://doi.org/10.3390/en15207571
APA StyleAlves, C. T., & Onwudili, J. A. (2022). Screening of Nickel and Platinum Catalysts for Glycerol Conversion to Gas Products in Hydrothermal Media. Energies, 15(20), 7571. https://doi.org/10.3390/en15207571







