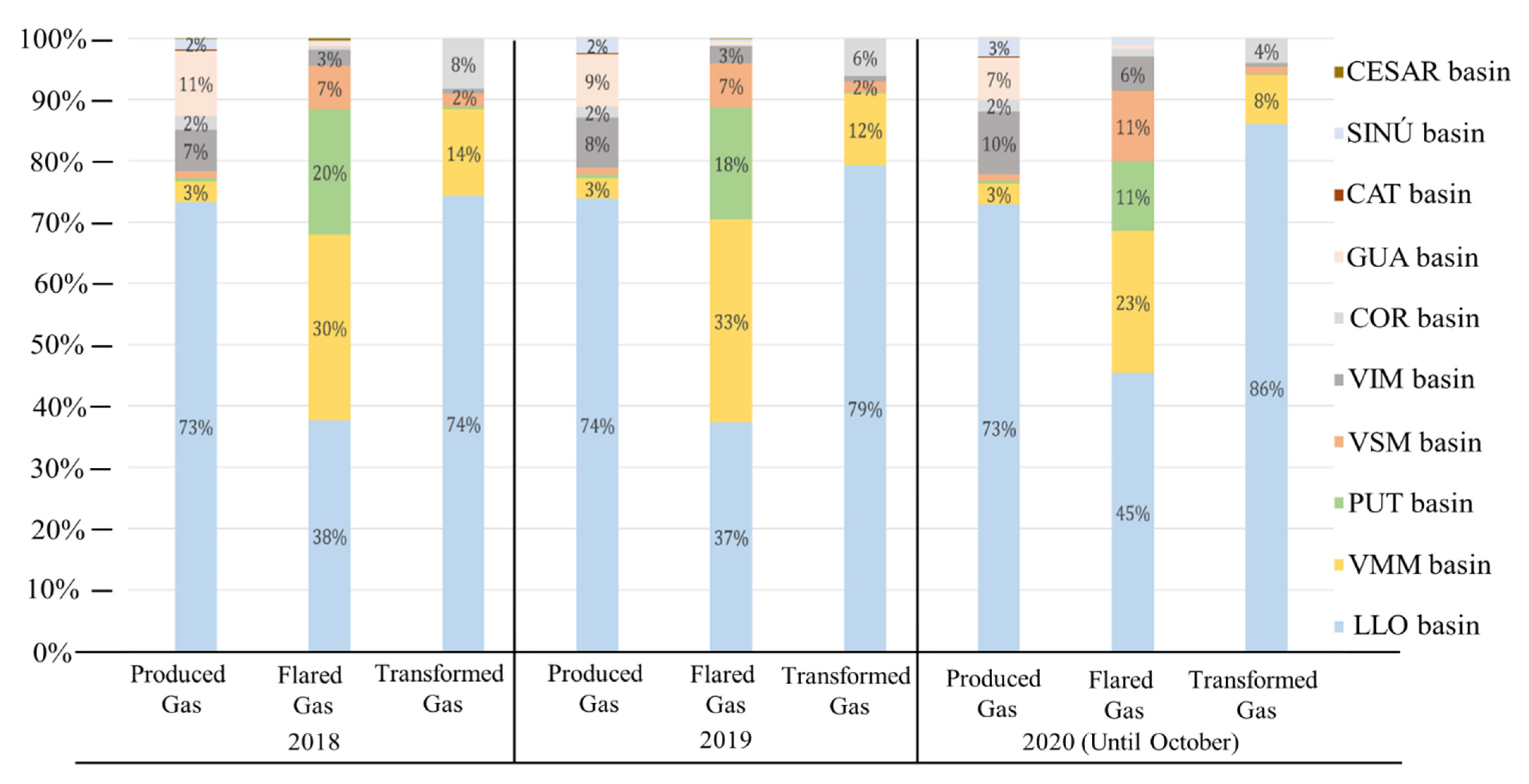
In the following section, the results of the calculations for the gas properties, the selection and simulation in Aspen HYSYS® of the treatment equipment and energy generation processes, and the energetic and technical analysis are presented.
To begin with the properties of the VMM basin natural gas, it must be characterized as a wet gas; its appreciable amounts of liquid hydrocarbons and a methane amount lesser than 85% give this characterization. This gas could be distinguished as an associated gas due to the relation given between rich gas and associated gas, and last, it is characterized as a sour gas for its hydrogen sulfide mol fraction greater than 0.01 (0.021).
3.1. Lower Heating Value VMM Basin Gas
Using Equation (1), the ideal LHV of the raw gas is obtained.
Table 3 shows the ideal LHV of each component of the gas, which must be identified to establish the ideal LHV of the gas mixture.
The ideal LHV of the raw gas equals 929,649.3 kJ/kg mol. This value represents the available energy on the gas as an ideal gas behavior; to obtain the real LHV, the Z factor has to be determined. The pseudocritical pressure and temperature of the gas mixture were estimated with Kay’s method [
40,
41], and due to the presence of H
2S and CO
2, these values were corrected under Wichert and Aziz’s equations [
41]. Then, the pseudoreduced properties were calculated under the initial conditions of pressure and temperature of the raw gas (39.69 psia and 77 °F) and obtained a value of 0.057 and 1.369 as the pseudoreduced pressure and temperature, respectively. Considering the pseudoreduced properties, Beggs and Brill’s correlation [
36,
37,
38,
39,
40,
41] was used to obtain the value of the Z factor for its estimated error of 0.02% in the calculation. Having a compressibility factor of 0.996 for the gas, which means that the real behavior of the gas is closer to the ideal one, and the real LHV obtained equals 933,382.8 kJ/kg mol. This value is compared to make a validation of the results with Aspen HYSYS
®’s properties with a calculation of the raw gas stream, which gives a value of 929,600 kJ/kg mol as the LHV of the raw gas. With a 0.40% error, the available energy calculation is valid, and the one calculated for Aspen HYSYS
® is considered for data analysis.
With the molar density of the raw gas as 0.1115 kg mol/m3, the available energy of the raw gas per volumetric unit is 103,650.4 kJ/m3. This means that 0.95 MMSCFD of the raw gas, which is flared, would produce 12.2 MW. This quantity of energy produced will then be affected by the treatment of the gas and by the efficiency of the power source that uses this flare gas as fuel.
3.2. Gas Treatment
For the selection of the treatment processes that the VMM basin gas needs, different variables were analyzed. Focusing on the components that are necessary to remove in the VMM basin gas is important; hence, the treatment substance and the component that it removes, such as the concentration, plays an important part in the selection. It is crucial to know the advantages that a process could have over the others; Focusing on how the process changes with the influence of different existing components in the VMM basin gas will determine the most suitable treatment to use.
The presence of water in the gas could create hydrates or be corrosive in the presence of CO
2 and H
2S, which is why a dehydration process is necessary. Through a bibliographic review, some dehydrating processes in the petroleum industry are presented in
Table 4.
To select the dehydration process for the VMM basin gas, the dehydration methods are divided into two: the adsorption and the absorption processes.
Table 4 shows that the adsorption processes should not be used for drying gases with concentrations of acid gases, especially CO
2 and H
2S, so, for the effects of this study, the adsorption methods are not going to be considered. The glycol treatment is the most used process in the petroleum industry for water removal, and the selection of the substance (DEG or TEG) to use depends on the process pressure and temperature. Triethylene glycol (TEG) is the most effective one of the glycols and the most used in the petroleum industry and is the best option to deal with the water removal due to the effectiveness and that the conditions are not going to be above 120 °F. After the dehydration process, it will be necessary to remove the contaminant amounts.
Table 5 presents the sulfur removal systems to remove the VMM basin gas contaminants.
It is important to highlight that the sweetening treatment has great importance in the process, because having these contaminants in the gas could be risky for the operation. Sulfhydric acid (H
2S), carbon dioxide (CO
2), carbonyl sulfide (COS), and carbon disulfide (CS
2), and all the sulfur compounds could cause problems in the transport, such as corrosion, pernicious odors, and with the presence of water, it can cause hydrates.
Table 5 shows the advantages of the sulfur removal systems; the DEA process meets the requirements for the VMM basin gas, owing to the good functioning it has when there is the existence of COS and CS
2 in the gas composition and the low presence of CO
2 in the VMM basin gas composition; with the optimum conditions of the temperature and pressure, the COS and CS
2 will help the DEA in the fast removal of CO
2 and H
2S, and then, the diethanolamine (DEA) will remove the COS and CS
2. The two processes used in the gas treatment will help to know the real energy properties that this gas has after its treatment, to improve these properties, and to have a safer operation.
3.3. Power Generation
Once the treatment process was chosen, a technology for power generation had to be selected. The decision context now focused on power sources that can use natural gas as fuel. Two options were preselected as possible alternatives for energy production with the fuel gas obtained: gas turbine cycle (GTC) and gas internal combustion engine cycle.
Figure 2 shows a schematic diagram for the power generation scenarios and the variables that most affected each one, based on the final decision.
One of the most important things that must be considered when selecting the power generation source is the quality of the gas [
28]; in this case, this variable has already been taken into consideration, as the raw gas was previously treated, and the problematic compounds were reduced or eliminated. Therefore, the efficiency of each option is evaluated. Internal combustion engines have, on average, 40% electrical efficiency, while gas turbines electrical efficiency is about 30–35% [
36].
Gas turbines and gas internal combustion engines are one of the most expensive resources, but they can work with any form of natural gas [
28], so, in case compressed natural gas or liquefied natural gas are available, these technologies are still useful. However, previous research has pointed out that gas internal combustion engines are favorable from an economic point of view; by an economic comparison of both gas use technologies, the investment cost, maintenance, and operation cost per year show that internal combustion engines have a better economic performance than gas turbines [
36].
About GHG emissions, gas internal combustion engines have a better performance in terms of pollutant emissions than gas turbines [
27]. While gas turbines generate higher amounts of pollutants, gas internal combustion engines work at low pressures with the least pollution [
25].
Regarding these criteria, a gas internal combustion engine is the most beneficial option as a power source due to the high power output at high efficiency and low emissions, which are also mobile and more flexible in the gas composition.
3.4. Aspen HYSYS Simulation Analysis
Using Aspen HYSYS
® gas treatment and power generation are simulated. Two absorbers, one combustion reactor, compressor K-100, and expansor K-101 are the equipment considered to simulate the process. A volume of 0.95 MMSCFD, taken as the flare gas (raw gas) in one field on VMM basin on December 2020, enters into the dehydration column at 25 °C and 25 psig, necessary conditions of the temperature and pressure for the dehydration process with TEG [
37]. Once dehydrated gas is obtained, the gas passes through the sweetening column where the sour components of the gas are retired. Finishing the gas treatment process, fuel gas is obtained, which reacts with compressed air in the combustion reactor to produce hot gases that finally expand to the atmospheric pressure to generate power. The schematic diagram of the simulation is shown in
Figure 3.
The 0.95 MMSCFD of the VMM basin gas enters the dehydrating process with the composition shown in the “Gas composition analysis and interpretation” section. This important amount of gas, especially the water value, helps to calculate an initial molar flow for the dehydration substance (TEG), knowing that, for 1 lb of H
2O in the gas composition, it is considered appropriate to use 3 gallons of triethylene glycol (TEG) [
37]. The TEG initial inlet molar flow was 0.015 gallons, which is equivalent to 0.0012 kg mol; this substance enters the process with a mol concentration of 99% and with a pressure and temperature of 618.4 kPa and 40 °C respectively. For this process as it was mentioned before, a glycol package is used, because it is the recommended thermodynamic model for a simulation using TEG. To reach the objective of decreasing the largest amount of water in the gas composition, it was necessary to do a sensibility analysis by starting with the initial inlet molar flow, this analysis guides the process to an optimum inlet molar flow value of TEG, as it shows in
Figure 4.
Figure 4 shows the behavior between the TEG inlet molar flow against the mole fraction of H
2O in the outlet gas after the dehydration process, resulting in an optimal inlet amount of TEG of 0.5 kg mol/h. In this case, the independent variable of the process is the TEG Inlet molar flow, showing that the decrease of the mole fraction of H
2O depends on the increase of the amount of TEG that enters the dehydration absorber.
After the gas dehydration, another treatment was simulated in Aspen HYSYS, the sweetening process. For this operation, the treatment substance was diethanolamine (DEA) entering the sweetening absorber with a concentration of 40% in weight. For the calculation of the initial DEA, the inlet flow in Equation (2) was used:
where
Q is the sour gas to be processed in MSm
3/day,
y is the acid gas concentration in sour gas in mol%, and
x is the amine concentration in liquid solution in mass% [
42,
43,
44,
45,
46,
47,
48,
49,
50]. Using the formula, an initial DEA inlet flow was estimated as 106.26 m
3/h, reaching the objective of decreasing the contaminants (H
2S, CO
2, COS, and CS
2) to zero. To optimize the high amount of DEA entering the process, due to this volume removing some of the pentane quantity, a sensibility analysis was made in
Figure 5.
Figure 5 shows a comparison between the DEA volume flow and the pentane mole fraction. Firstly, it is important to clarify that the range of volumes of the DEA flow presented in the chart is from the calculated initial DEA inlet flow to the minimum volume where the number of contaminants started to appear. Then, the comparison between these variables was important to increase the heating value of the resulting gas (fuel gas) due to the great heating value provided by pentane. The independent variable in this analysis was the DEA volume flow, showing that, by decreasing the flow entering the absorber, the mole fraction of pentane will increase and that the gas composition is highly related to the entering flow of DEA. As a result, for the removal of the contaminants and the higher amount of pentane, the minimum volume where the number of contaminants started to appear (approximately 13.93 m
3/h) is the optimized value for the DEA flow [
48,
49,
50,
51,
52]. Another variable to take into consideration for the gas composition was the DEA inlet temperature, as shown in
Figure 6.
To estimate the correct DEA inlet temperature,
Figure 6 shows a sensibility analysis between this temperature and the mole fraction of the component’s H
2O, pentane, and the contaminant CS
2. Increasing the DEA inlet temperature will have an important change in the gas composition; the water value and the pentane amount (in a small amount) increased, and the contaminant CS
2 started to appear in the composition again. Therefore, it is crucial to maintain a low DEA inlet temperature (20 °C) for the removal of the contaminants and to control the amount of water in the fuel gas.
The gas treatment was an important step to find a gas composition for the optimal performance in the power generation process.
Table 6 shows the fuel gas composition, seeing that the amount of the contaminants decreased considerably to zero, and the water mole fraction was increased from 0.008 to 0.0011, representing that the dehydrating and sweetening processes achieved their purposes. However, it is important to establish that one of the goals of the treatment was to increase the heating value of the raw gas and show the real heating value of this gas after the necessary treatment processes. Consequently, a comparison was made between these values, knowing that the raw gas had a heating value of 929,600 kJ/Kmol, and the heating value of the fuel gas was 226,500 kcal/Kmol, which was equivalent to 947,676 kJ/Kmol, it was correct to affirm that the goal was reached; additionally, the real and optimal heating value of the VMM basin gas was revealed.
Table 6 shows the composition of the fuel gas that enters the power generation process with a molar flow of 45.12 Kmol/h, a smaller amount compared with the raw gas inlet (47.32 Kmol/h) due to the gas treatment processes.
The gas internal combustion engine configuration consists of one combustion chamber, one compressor, and an expander. First, air that comes from the atmosphere enters the compressor K-100, where the pressure is increased from the standard conditions to 22.5 bar to enter the combustion reactor with the same pressure conditions as the fuel gas. Then, the compressed air passes to a combustion chamber with the fuel gas, where combustion takes place. The generated hot gases go to a gas engine where they expand to atmospheric pressure, and the gas energy is converted to mechanical energy, which generates electricity. Equations (3)–(7) showed a stoichiometric reaction model that occurs in the combustion chamber; these chemical reactions are assumed to be ideal, and other components such as CO are not produced.
3.5. Energy and Environmental Evaluation
After treating, 0.95 MMSCFD of VMM basin gas has an estimated value of available energy of 11.8 MW; this represents a loss of 0.4 MW due to the volume of contaminants removed during the gas treatment. The total gross power produced by the process is 5133 kW, which represents an electrical efficiency of 43.5% in the internal combustion engine. This result is approximate to the one obtained by Rahimpour et al. [
20,
21,
22], in which, with a gas turbine, 2130 MW was produced from 365.5 MMSCFD. In the same conditions this value equaled 5.53 MW produced by 0.95 MMSCFD. However, it has to be considered that, in this previous research, the raw gas was not previously treated, which could mean the presence of corrosion and hydrate formation; also, the heating value was not improved, and the use of a gas turbine instead of a gas internal combustion engine has a lower long-term performance due to the variables considered in
Section 3.3. In addition, the results show that the performance of the scenarios depends on the gas flow rate, as Mohmmad Hidari et al. [
23,
24,
25,
48,
49,
50,
51,
52] concluded in his research.
Subtracting the energy demand of the compressor, 2842 kW total net power is produced; it is the available energy to supply the energy consumption of the gas treatment and estimates the energy quantity of the consumption model field that can be supplied.
Using the enthalpy of the inlet flow lines and outlet flow lines, the energy consumption by the absorbers is estimated. Approximately, each absorber requires 629.292 kW to work with the flow volume of raw gas, as the treatment gas configuration considers two absorbers (dehydrating process and sweetening process); then, 1258.584 kW is considered as the energy consumption for the treatment processes.
Considering
Table 7, where the values of energy consumption are shown, the energy evaluation is made, exposing that the needed energy requirements for the gas treatments can be supplied with the energy produced, and this amount is sufficient to meet the VMM basin’s field model energy needs four times, similar to the results obtained by E.M Wallace et al., who calculated the requirement that the need to be supplied for powering drilling and hydraulic operations, which consume much more energy, and concluded that the flared gas is more than enough to supply these energy requirements [
28].
Establishing a volume of gas required per day to supply the model field energy requirements is important to recognize by the field operator, the period of project sustainability using the gas reserves in the reservoir, and its flow. In this case, with the properties of the VMM basin gas, the volume of gas required per day to supply the model field energy requirements is approximately 0.7 MMSCFD.
The amount of diesel, the most common fuel used in the petroleum industry, needed to produce the same amount of energy (5133 kW) is 435 kg; this is equivalent to 135.19 gallons, which produces an average of 1392 kg CO
2 [
28,
53,
54,
55], while 1249 kg CO
2 is emitted when this amount of energy is produced with the flare gas. Therefore, using the VMM basin flared gas instead of diesel to produce the same quantity of energy reduces the CO
2 emissions by 11.44%.
Supplying the gross energy consumption of the model field implies using 12.66 gal/day of diesel, which equals
$27.09 USD/day. Assuming this energy consumption is constant in a year, taking advantage of the VMM basin gas implies money savings of USD 9887 per year. Additionally, to estimate the financial output from the power generation, the cost of selling electricity in Colombia in 2021 is USD 0.15 kWh. This means that the VMM basin flared gas could cost approximately USD 770 with the energy generation produced; this equals the net incomes that were lost by flaring the VMM basin gas. On the other hand, the previously stated results are suitable for the natural gas utilization process. Obtaining convincing results for Colombia exaprolols for Latin American countries, it should be noted that the processes mentioned have the potential for application in African countries such as Nigeria [
53,
54,
55,
56].