Analysis of a Costly Fiberglass-Polyester Air Filter Fire
Abstract
:1. Introduction
2. The Facility and the Fire Incident
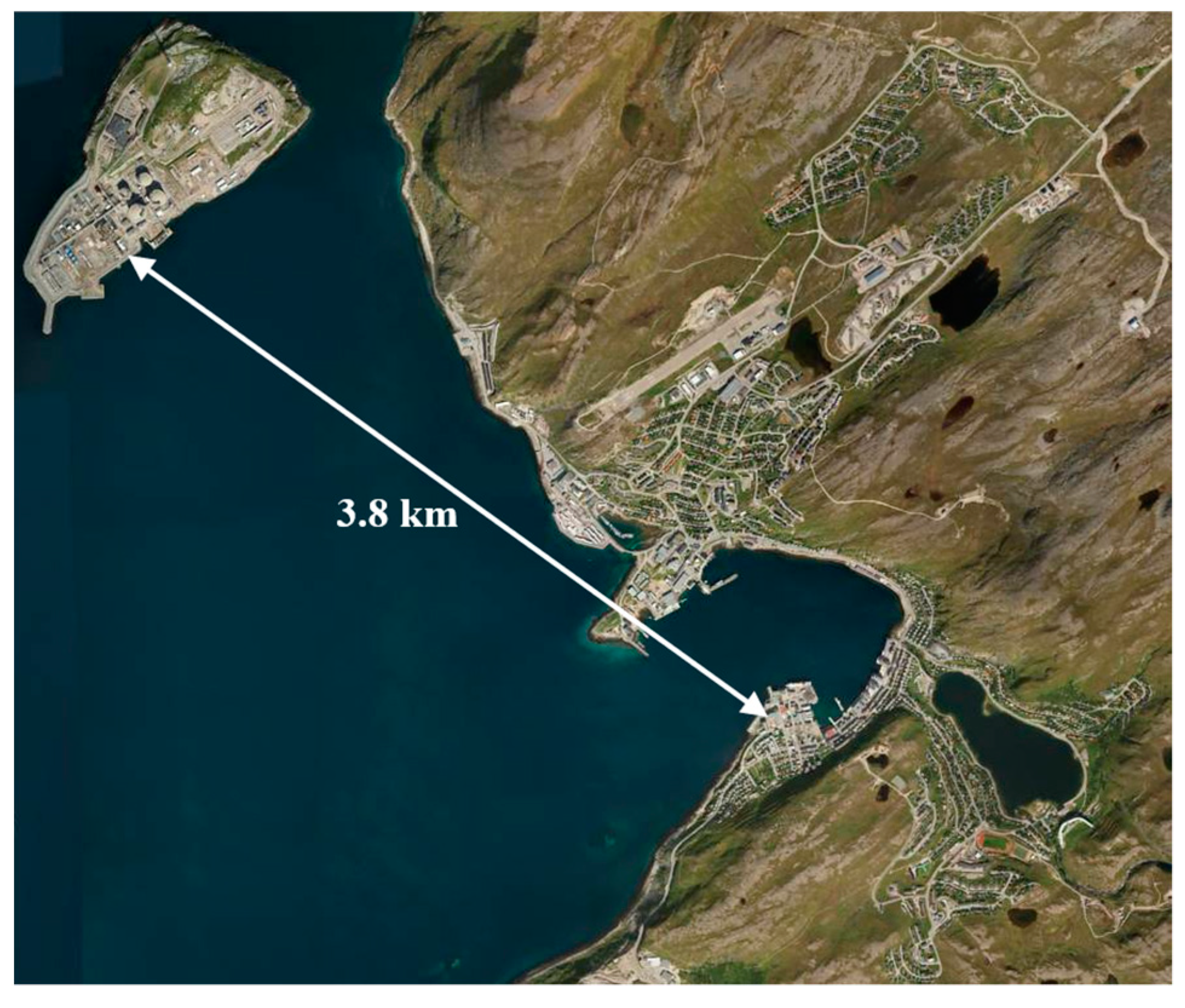
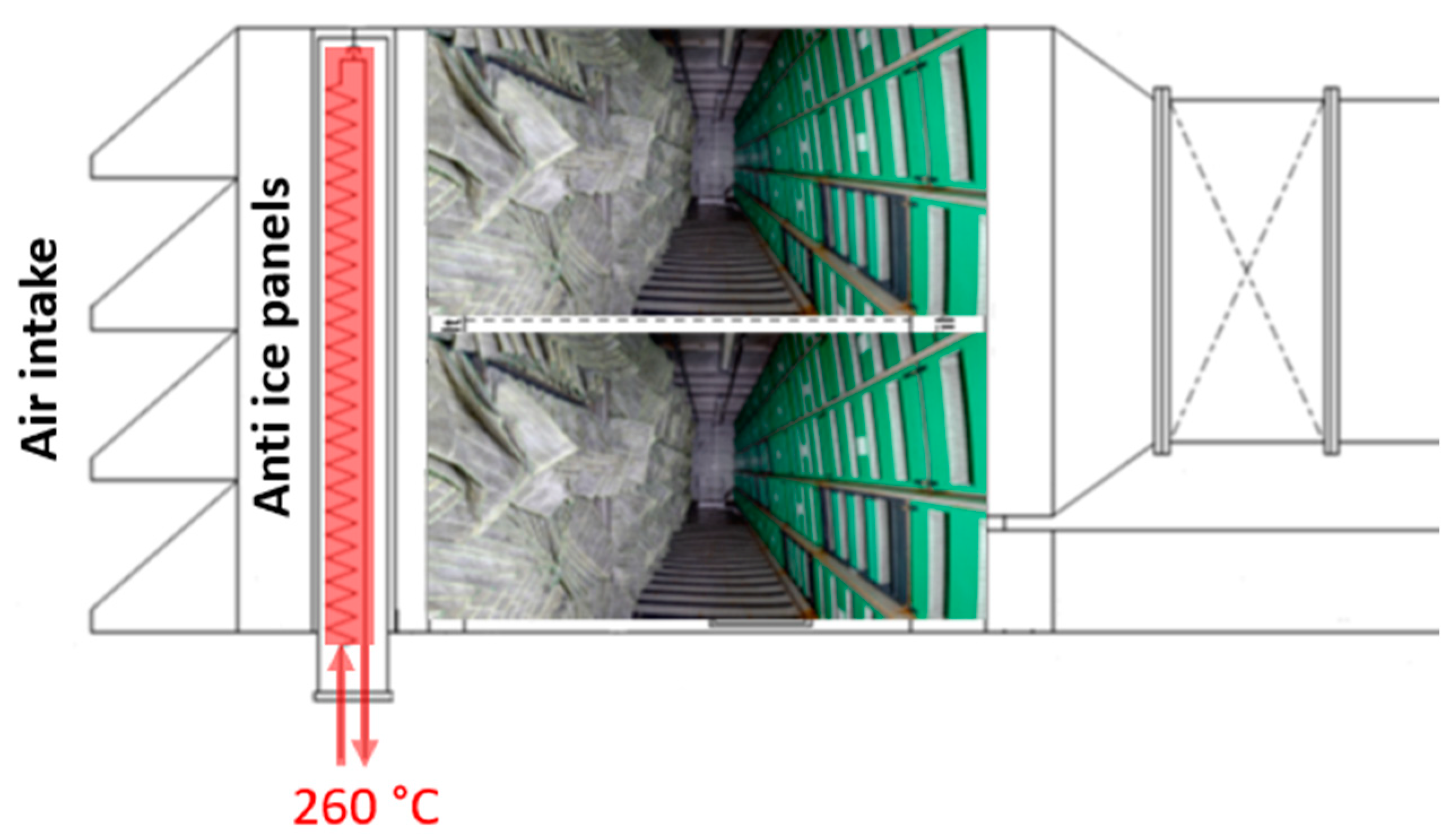
2.1. The Facility
2.2. Sequence of Events
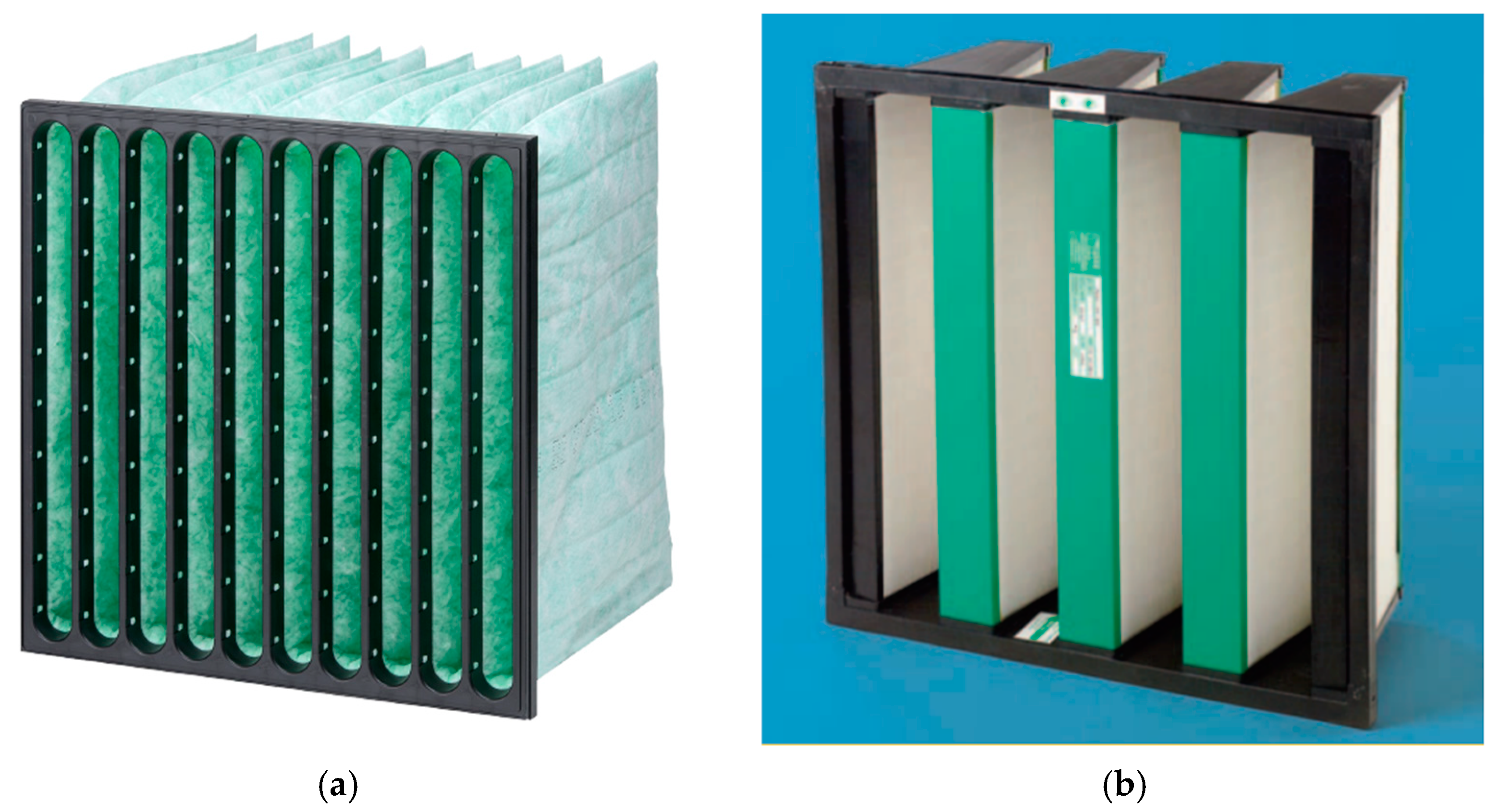
3. Materials and Methods
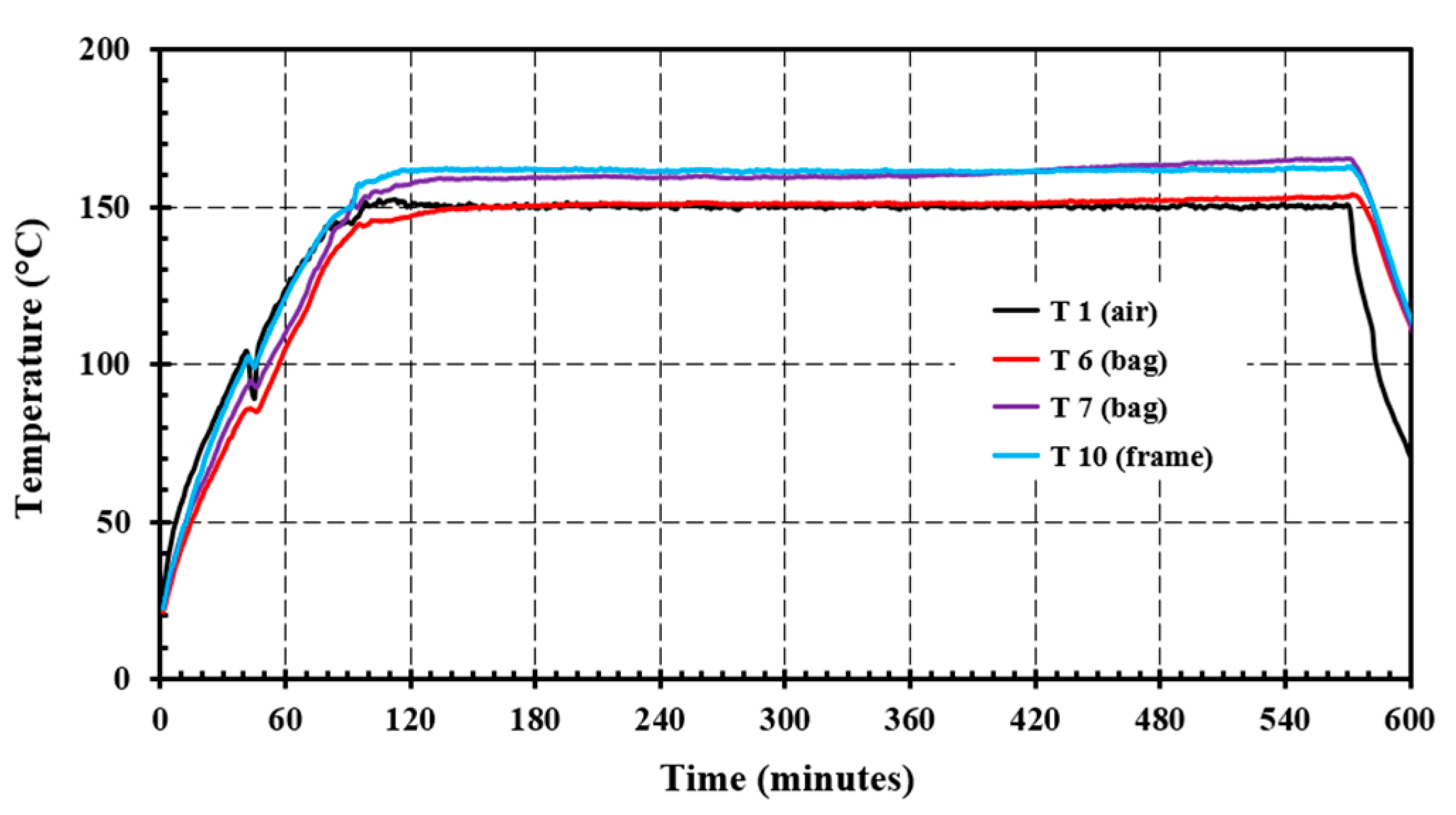
4. Results
4.1. Initial Ignition Test Results
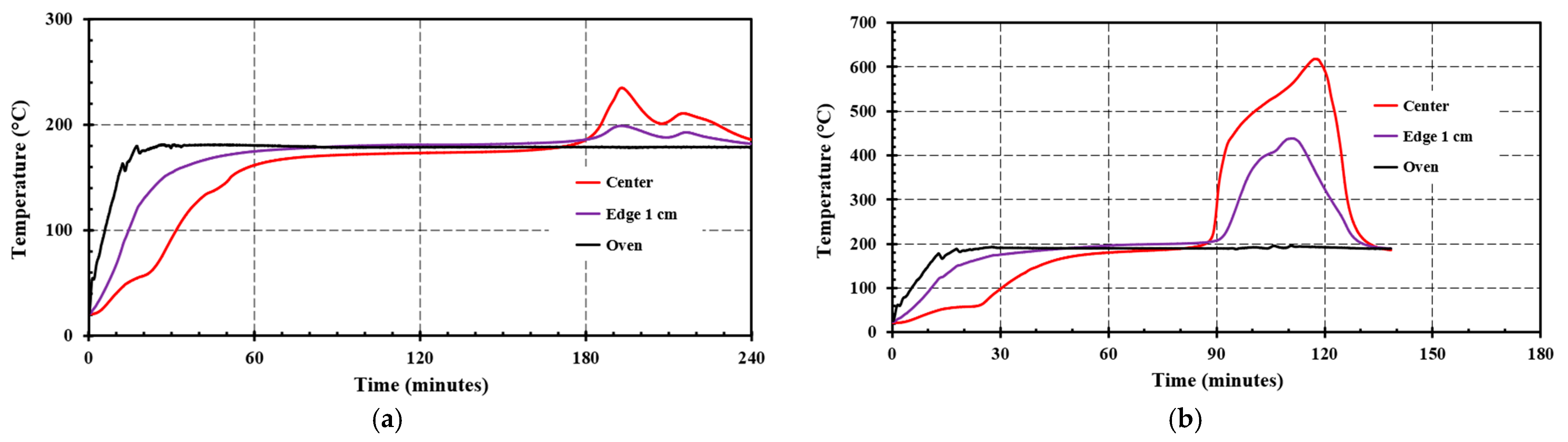
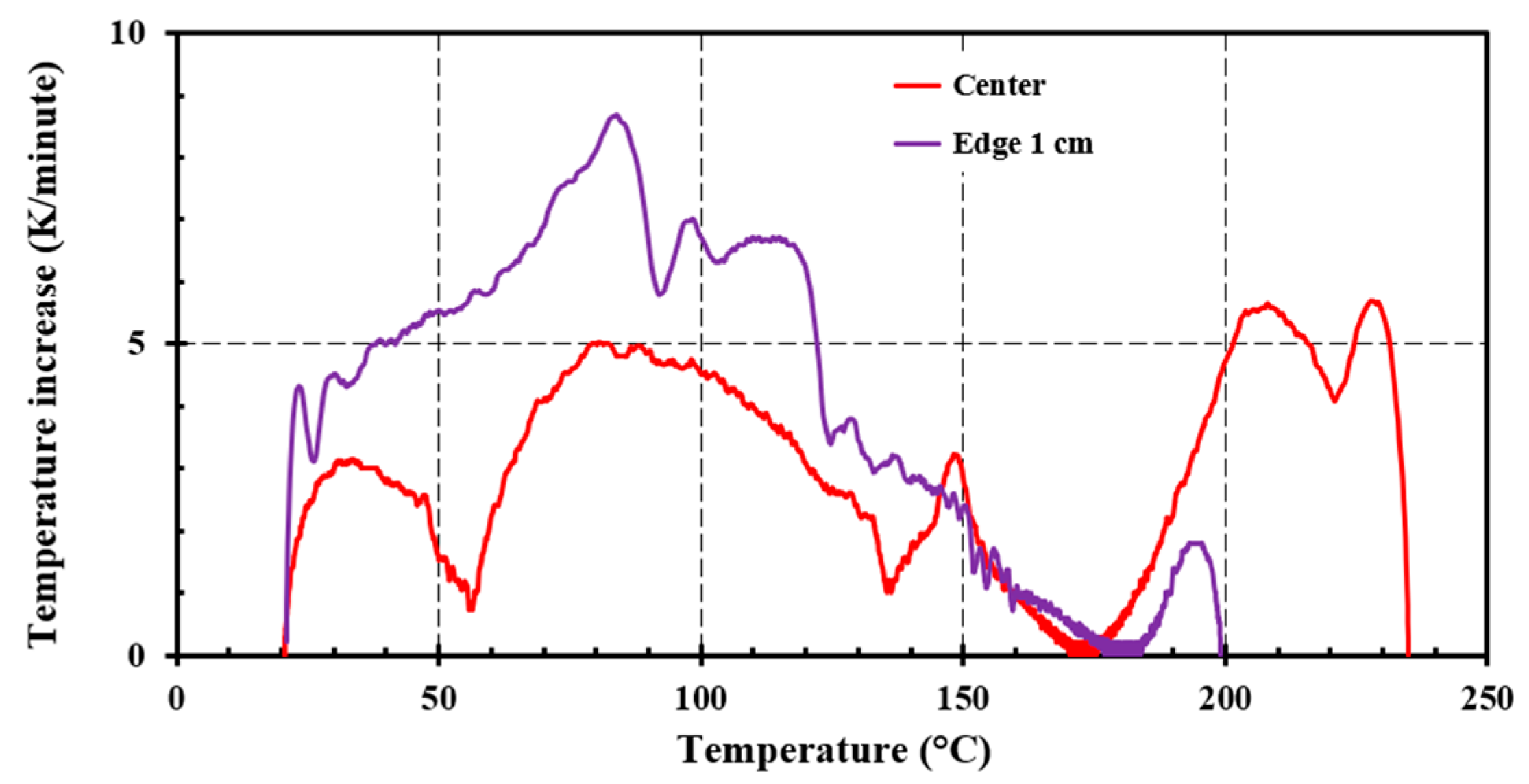
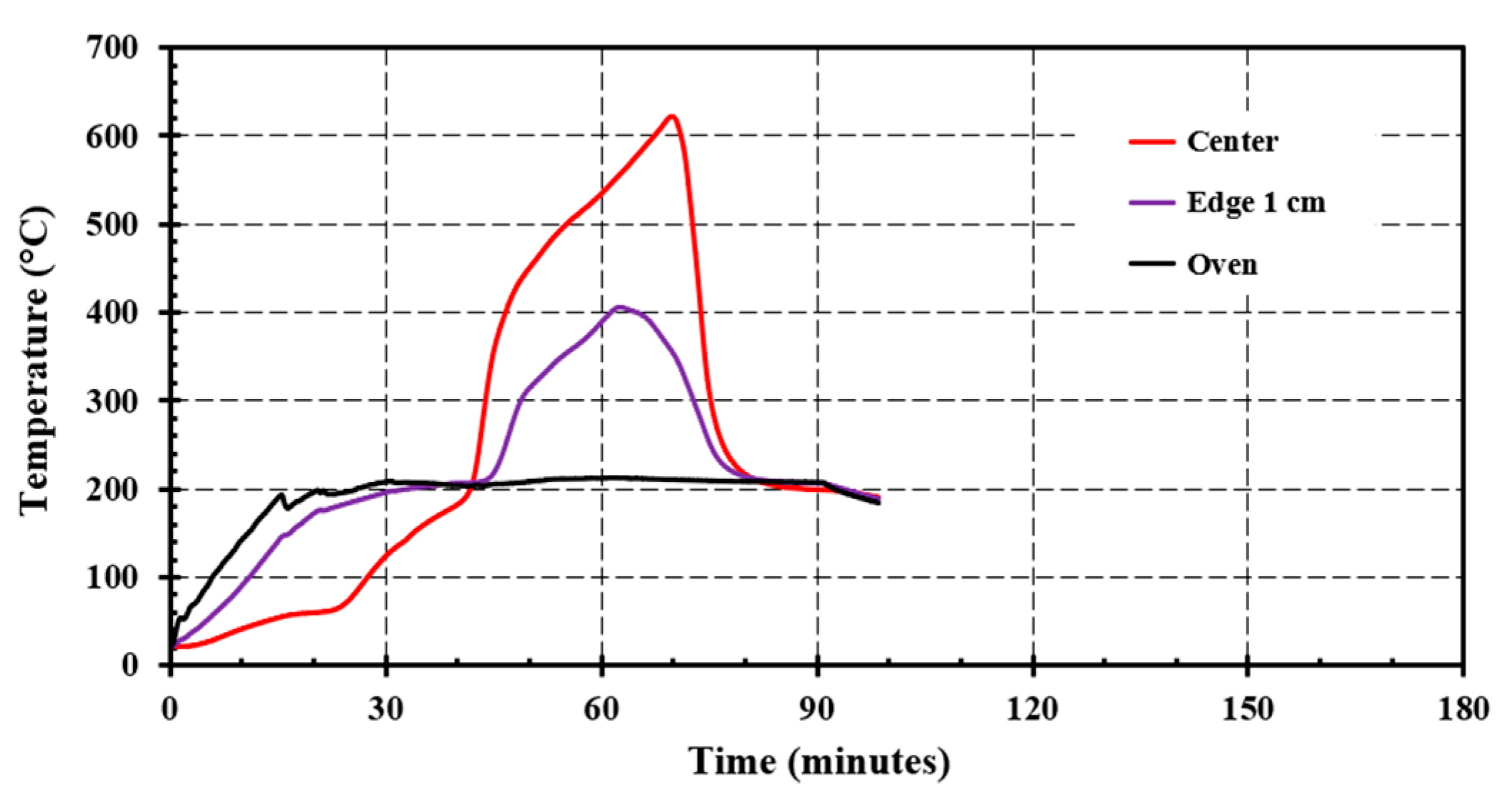
4.2. Small Scale Self-Heating Tests
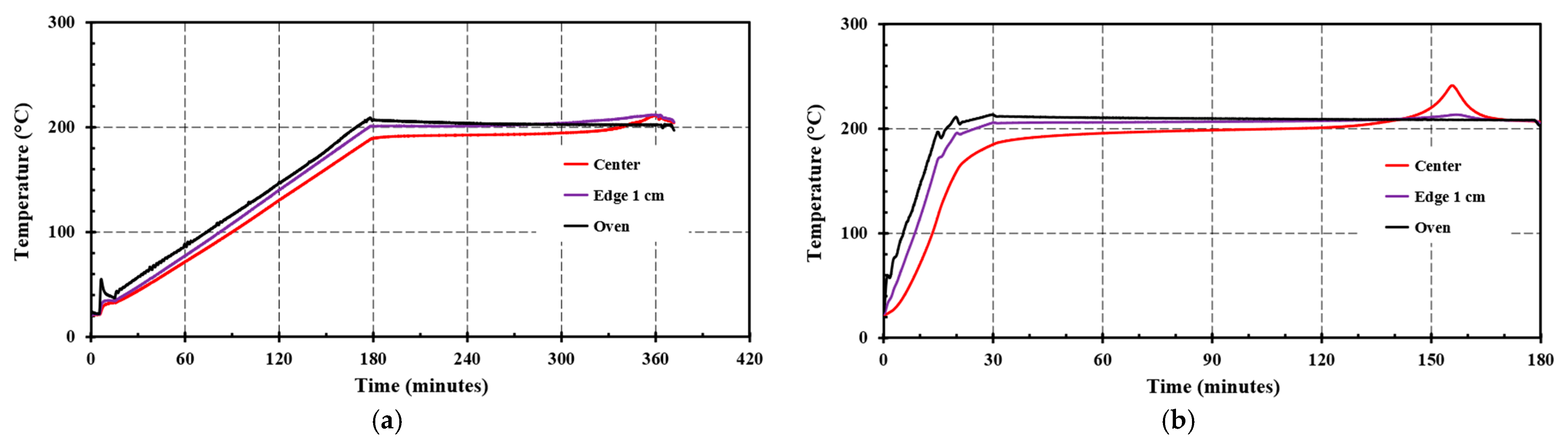
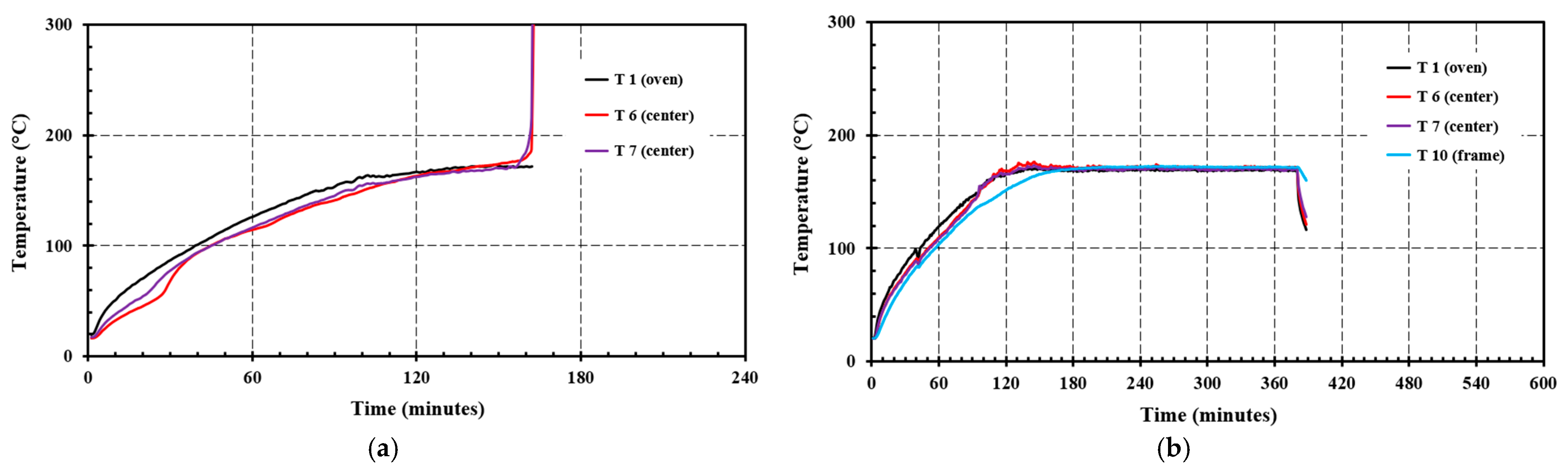
4.3. Filter Cassette Self-Heating Tests
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drysdale, D.D.; Sylvester-Evans, R. The explosion and fire on the Piper Alpha platform, 6 July 1988. A. case study. Phil. Trans. R. Soc. A Math. Phys. Eng. Sci. 1998, 356, 2929–2951. [Google Scholar] [CrossRef]
- Holmstrom, D.; Altamirano, F.; Banks, J.; Joseph, G.; Kaszniak, M.; Mackenzie, C.; Shroff, R.; Cohen, H.; Wallace, S. CSB investigation of the explosions and fire at the BP Texas City refinery on March 23, 2005. Proc. Safety Progr. 2006, 25, 345–349. [Google Scholar] [CrossRef]
- Zhu, Y.; Qian, X.M.; Liu, Z.Y.; Huang, P.; Yuan, M.Q. Analysis and assessment of the Qingdao crude oil vapor explosion accident: Lessons learnt. J. Loss Prev. Proc. Ind. 2015, 33, 289–303. [Google Scholar] [CrossRef]
- Bakka, M.S.; Eriksen, B.; Johansen, O.J.; Oltedal, H.-L.-; Nissestad, O.A.; Log, T. Brann i luftinntak på gassturbin GTG4, Hammerfest LNG 28.09.2020 (En. Fire in air intake at gas turbine GTG4, Hammerfest LNG 28.09.2020); Report no. A MMP L2 2020-18; Equinor: Forus, Norway, 2021. [Google Scholar]
- Hallan, B.; Rundell, L.R.; Thorsen, A.J.; Steinmakk, A.-H.; Sundby, T. Rapport etter gransking av brann i luftinntak til GTG4 på Hammerfest LNG, Melkøya (En. Investigation report after fire in air intake for GTG4 at Hammerfest LNG, Melkøya); Norwegian Petroleum Safety Authority investigation report, activity 001901043; Equinor: Stavanger, Norway, 2021. (In Norwegian) [Google Scholar]
- Agapiou, A. Damage Proxy Map of the Beirut Explosion on 4th of August 2020 as Observed from the Copernicus Sensors. Sensors 2020, 20, 6382. [Google Scholar] [CrossRef] [PubMed]
- Camfil. Hi-Flo XLT filterpåse. Byggvarudeklaration, Camfil Sweden, Stockholm, Sweden. 2015. Available online: www.camfil.com/damdocuments/46633/1217698/ebvd-hi-flo-xlt.pdf (accessed on 1 December 2020).
- Drysdale, D.D. An Introduction to Fire Dynamics, 2nd ed.; John Wiley: New York, NY, USA, 1999; ISBN 0-471-97291-6. [Google Scholar]
- McGrattan, K.; Hostikka, S.; McDermott, R.; Floyd, J.; Weinschenk, C.; Overholt, K. Fire dynamics simulator user’s guide. NIST Special Publ. 2013, 1019, 339. [Google Scholar] [CrossRef]
- McGrattan, K.; Hostikka, S.; McDermott, R.; Floyd, J.; Weinschenk, C.; Overholt, K. Fire dynamics simulator technical reference guide volume 1: Mathematical model. NIST Special Publ. 2013, 1018, 175. [Google Scholar] [CrossRef]
- Skjold, T.; van Wingerden, K. Investigation of an explosion in a gasoline purification plant. Proc. Safety Progr. 2013, 32, 268–276. [Google Scholar] [CrossRef]
- McAllister, P.M.; Dyche, J.L.; Graves, R.C. Investigation and actions after an internal air compressor filter fire. Proc. Safety Progr. 2013, 32, 96–101. [Google Scholar] [CrossRef]
- Larsen, A.; Sande, E.; Glette, S.H.; Jensen, J.E. Report after Fire in Ventilation Hood at Petrojarl Knarr 24.3.2015; Investigation report, 411003011; The Petroleum Safety Authority: Stavanger, Norway, 2015. (In Norwegian) [Google Scholar]
- Government of Norway. Investigation—Fire in HVAC room onboard Petrojarl Knarr, Teekay Petrojarl TKPJ-01-S-97-RA-00001-001, 2015; Government of Norway: Oslo, Norway, 2015.
- Stølen, R. Fire in HVAC Room on Board Petrojarl Knarr; F15 20141-2:1; SP Fire Research: Trondheim, Norway, 2015. [Google Scholar]
- Torero, J.L.; Gerhard, J.I.; Martins, M.F.; Zanoni, M.A.B.; Rashwan, T.L.; Brown, J.K. Processes defining smouldering combustion: Integrated review and synthesis. Progr. Energy Combust. Sci. 2020, 81, 100869. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, N.; Yuan, H.; Chen, H.; Xie, X.; Zhang, L.; Rein, G. Smouldering and its transition to flaming combustion of polyurethane foam: An experimental study. Fuel 2022, 309, 122249. [Google Scholar] [CrossRef]
- Morgan, A.B.; Knapp, G.; Stoliarov, S.I.; Levchik, S.V. Studying smoldering to flaming transition in polyurethane furniture subassemblies: Effects of fabrics, flame retardants, and material type. Fire Mater. 2021, 45, 56–67. [Google Scholar] [CrossRef]
- Hagen, B.C.; Meyer, A.K. From smoldering to flaming fire: Different modes of transition. Fire Safety J. 2021, 121, 103292. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, K.; Wang, S. Effect of porosity on ignition and burning behavior of cellulose materials. Fuel 2022, 322, 124158. [Google Scholar] [CrossRef]
- Graham, L.L.B.; Applegate, G.B.; Thomas, A.; Ryan, K.C.; Saharjo, B.H.; Cochrane, M.A. A Field Study of Tropical Peat Fire Behaviour and Associated Carbon Emissions. Fire 2022, 5, 62. [Google Scholar] [CrossRef]
- Gogola, K.; Rogala, T.; Magdziarczyk, M.; Smoliński, A. The Mechanisms of Endogenous Fires Occurring in Extractive Waste Dumping Facilities. Sustainability 2020, 12, 2856. [Google Scholar] [CrossRef] [Green Version]
- Xu, K.; Tian, X.; Cao, Y.; He, Y.; Xia, Y.; Quan, F. Suppression of Smoldering of Calcium Alginate Flame-Retardant Paper by Flame-Retardant Polyamide-66. Polymers 2021, 13, 430. [Google Scholar] [CrossRef]
- Korobeinichev, O.; Shaklein, A.; Trubachev, S.; Karpov, A.; Paletsky, A.; Chernov, A.; Sosnin, E.; Shmakov, A. The Influence of Flame Retardants on Combustion of Glass Fiber-Reinforced Epoxy Resin. Polymers 2022, 14, 3379. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Luo, S.; Du, X.; Li, Q.; Cheng, S. Improvement the Flame Retardancy and Thermal Conductivity of Epoxy Composites via Melamine Polyphosphate-Modified Carbon Nanotubes. Polymers 2022, 14, 3091. [Google Scholar] [CrossRef]
- Korobeinichev, O.; Karpov, A.; Shaklein, A.; Paletsky, A.; Chernov, A.; Trubachev, S.; Glaznev, R.; Shmakov, A.; Barbot’ko, S. Experimental and Numerical Study of Downward Flame Spread over Glass-Fiber-Reinforced Epoxy Resin. Polymers 2022, 14, 911. [Google Scholar] [CrossRef]
- Juita; Dlugogorski, B.Z.; Kennedy, E.M.; Mackie, J.C. Oxidation reactions and spontaneous ignition of linseed oil. Proc. Comb. Inst. 2011, 33, 2625–2632. [Google Scholar] [CrossRef]
- Bjørge, J.S.; Metallinou, M.-M.; Log, T.; Frette, Ø. Method for Measuring Cooling Efficiency of Water Droplets Impinging onto Hot Metal Discs. Appl. Sci. 2018, 8, 953. [Google Scholar] [CrossRef] [Green Version]
- Bjørge, J.S.; Bjørkheim, S.A.; Metallinou, M.M.; Log, T.; Frette, Ø. Influence of acetone and sodium chloride additives on cooling efficiency of water droplets impinging onto hot metal surfaces. Energies 2019, 12, 2358. [Google Scholar] [CrossRef] [Green Version]
- Bjørge, J.S.; Metallinou, M.M.; Kraaijeveld, A.; Log, T. Small Scale Hydrocarbon Fire Test Concept. Technologies 2017, 5, 72. [Google Scholar] [CrossRef] [Green Version]
- Bjørge, J.S.; Gunnarshaug, A.; Log, T.; Metallinou, M.-M. Study of Industrial Grade Thermal Insulation as Passive Fire Protection up to 1200 °C. Safety 2018, 4, 41. [Google Scholar] [CrossRef] [Green Version]
- Bakka, M.S.; Handal, E.K.; Log, T. Analysis of a High-Voltage Room Quasi-Smoke Gas Explosion. Energies 2020, 13, 601. [Google Scholar] [CrossRef] [Green Version]
- Handal, E.; Sandvik, O.I.; de Jong, H.; Bondevik, T.B.; Ovesen, R.V. Fire in Compressor House; Report no. A MMP L1 2020–2023; Equinor: Forus, Norway, 2021. [Google Scholar]
- Metallinou, M.M. Single-and double-loop organizational learning through a series of pipeline emergency exercises. J. Contingencies Crisis Manag. 2017, 26, 530–543. [Google Scholar] [CrossRef]
- Metallinou, M.M. Liquefied Natural Gas as a New Hazard; Learning Processes in Norwegian Fire Brigades. Safety 2019, 5, 11. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Log, T.; Gunnarshaug, A. Analysis of a Costly Fiberglass-Polyester Air Filter Fire. Energies 2022, 15, 7719. https://doi.org/10.3390/en15207719
Log T, Gunnarshaug A. Analysis of a Costly Fiberglass-Polyester Air Filter Fire. Energies. 2022; 15(20):7719. https://doi.org/10.3390/en15207719
Chicago/Turabian StyleLog, Torgrim, and Amalie Gunnarshaug. 2022. "Analysis of a Costly Fiberglass-Polyester Air Filter Fire" Energies 15, no. 20: 7719. https://doi.org/10.3390/en15207719





