The Design and Optimization of Natural Gas Liquefaction Processes: A Review
Abstract
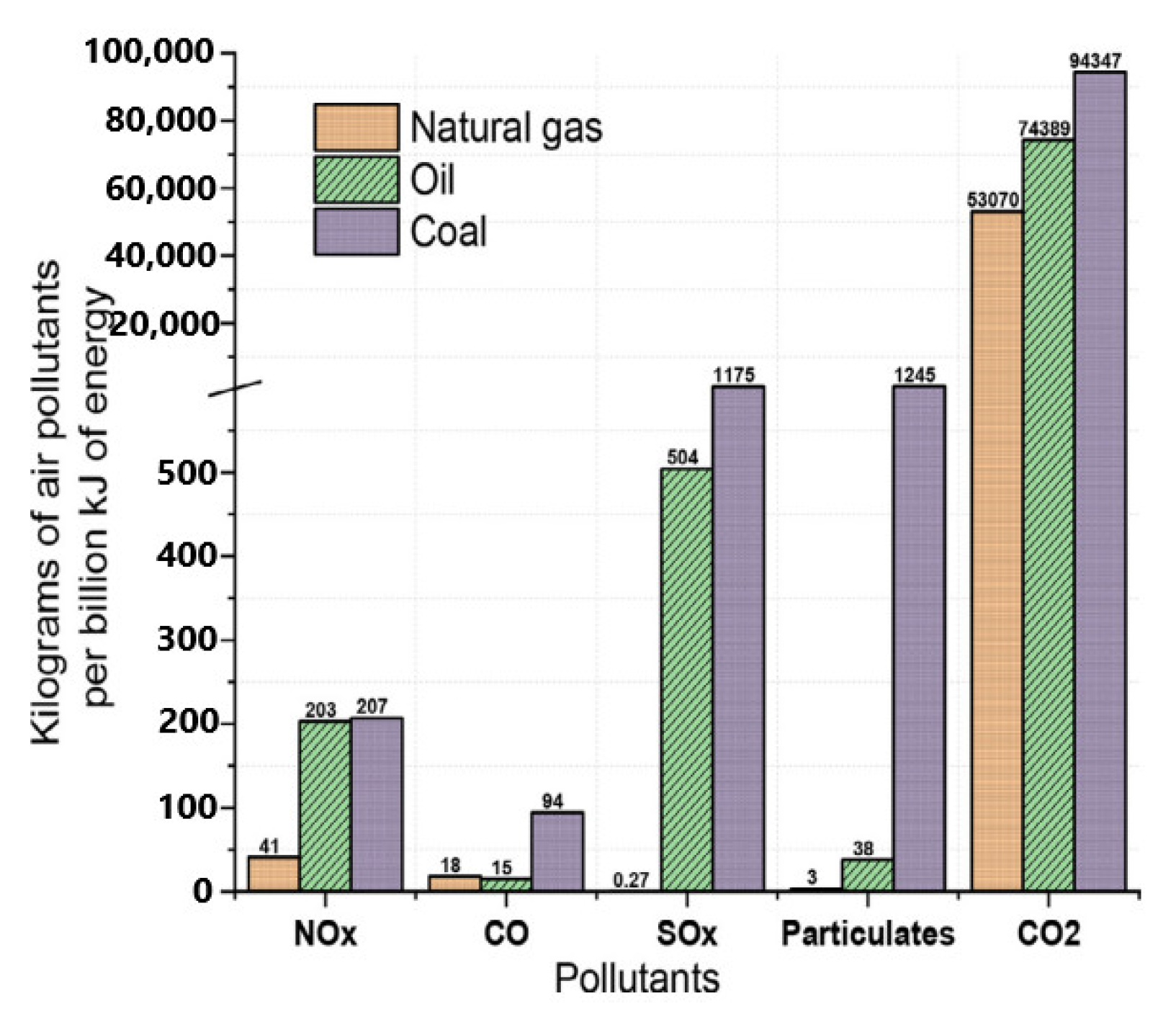
1. Introduction
2. Conventional Liquefied Natural Gas Process
2.1. Cascade Liquefaction Process
2.1.1. Cascade Liquefaction Process with Hydrocarbon-Based Pure Refrigerants
2.1.2. Cascade Liquefaction Process with Other Pure Refrigerants
2.1.3. Cascade Liquefaction Process with Mixed Refrigerants
2.2. Expansion Liquefaction Process
2.2.1. Single Nitrogen Expansion Liquefaction Process
2.2.2. Dual Nitrogen Expansion Liquefaction Process
2.2.3. Triple Nitrogen Expansion Liquefaction Process
2.2.4. Expansion Liquefaction Process with Natural Gas and Nitrogen
2.2.5. Nitrogen Expansion Liquefaction Process with Pre-Cooling
2.2.6. Expansion Liquefaction Process with Other Refrigerants
2.3. Mixed Refrigerant Liquefaction Process
2.3.1. Single Mixed Refrigerant Liquefaction Process (SMR)
2.3.2. Mixed Refrigerant Liquefaction with Propane Pre-Cooling Process (C3MR)
2.3.3. Dual Mixed Refrigerant Liquefaction Process (DMR)
2.3.4. Integrated Process for Co-Production of LNG and NGL Recovery
2.3.5. Research Progress in Mixed Refrigerants
3. Pressurized Liquefied Natural Gas Process
3.1. Comparison between PLNG Process and Conventional LNG Process
3.2. Research Progress in PLNG Process
3.2.1. PLNG Process without CO2 Pretreatment
3.2.2. PLNG Process with CO2 Cryogenic Remove
4. Current Status and Outlook
4.1. Status and Demand Analysis
4.2. Research Prospects
- LNG production is an industrial process with high energy consumption, especially for base-load type LNG production, and a small increase in the cycle efficiency of liquefaction process will have an extremely significant effect on energy saving. Reducing energy consumption and increasing liquefaction capacity and efficiency are still the goals of liquefaction process in medium and large-scale production. Through a detailed analysis of energy loss within various equipment in the whole liquefaction process, and also by improving the liquefaction process and adjusting operational parameters, there is still room and possibility for progress in optimizing the liquefaction process design.
- The liquefaction process that is scaled-down and designed according to the mature process of large-scale liquefaction plant cannot fully meet all the process requirements of small skid-mounted natural gas liquefaction unit. On the one hand, under the premise of ensuring high cycle efficiency, it is still the focus of future research to simplify the process and reduce the size of equipment so as to achieve the skid-mounted process setup. On the other hand, improving the adaptability of the liquefaction process to different gas sources and the flexibility of start and stop operations are also of great practical significance to the application advantages of small skid-mounted natural gas liquefaction units.
- The exploitation of offshore natural gas resources is bound to put forward a great demand for the research of offshore natural gas liquefaction processes. The particularities of offshore operating environment and space provide some new directions for the research of offshore liquefaction processes. For example, the influence of sloshing produced by wind waves and ocean currents on the liquefaction process of FLNG and the influence of different liquefaction processes and refrigerant compositions on safety still needs further research.
- Most of the studies on offshore applications of pressurized liquefaction process are still focused on the liquefaction process. In this respect, a number of important research directions are still open for future research endeavors, e.g., ① further combining dehydration, CO2 removal, and other pretreatment processes to carry out full process simulation, ② to coordinate and optimize the pressurized liquefaction process with pretreatment, storage, and transportation, ③ to deepen the adaptability, safety, and modularity of pressurized liquefaction process at sea conditions and gas source components,④ to overall consider investment costs and operation costs.
- Although PLNG process is proposed for offshore natural gas liquefaction units, its higher requirements for adaptability to different gas sources and the idea of reducing the footprint by eliminating CO2 pre-treatment units coincide with the requirements of small skid-mounted liquefaction units onshore. So, PLNG process may be a strategic possibility as a potential solution for offshore gas.
- Studying natural gas liquefaction process, the specific power consumption or total energy consumption is always used as the optimization objective function and evaluation index. Moreover, some aspects are chosen, such as the relationship between investment and operating costs, analyzing and evaluating the liquefaction process from an economic aspect. Although both energy consumption and economy are among the most important evaluation indicators for liquefaction processes, as mentioned above, different production methods and applications have different requirements for liquefaction processes. Scientific evaluation should comprehensively consider energy consumption, economic and operational adaptability, operability, and safety. In the same respect, an evaluation system should be established in line with the priority of various performance requirements of LNG units for liquefaction process. Conversely, the optimization and comparison of liquefaction process should be also carried out in this evaluation system. At present, research on this aspect is still relatively rare, and further research is needed.
- Various new equipment, such as supersonic cyclone separator and printed circuit heat exchanger, have emerged. The effects and influences caused by their use in natural gas liquefaction processes have become new issues.
- There are still few studies on dynamic simulation of LNG processes. Studying the stability of liquefaction process in disturbance plays an important role in evaluating its adaptability, especially for dynamic simulation under extreme working conditions, so as to better understand dynamic characteristics of the process and provide guidance for actual operation.
- The LNG process is a multi-component feed gas liquefaction production process. Enhancing the energy efficiency of the liquefaction process requires a focus not only on energy consumption at all stages of the natural gas cooling process, but also on the overall improvement in conjunction with the NGL recovery process and BOG cycle re-liquefaction process.
- The deterministic method, stochastic method, and KBO method are suitable for controlling natural gas liquefaction processes and each has strengths and weaknesses with regard to calculation time, multi-objective processing, and convergence. Exploring customized optimization algorithms suitable for different liquefaction processes will be of great help in the design and optimization of natural gas liquefaction processes.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| LNG | Liquefied natural gas |
| FLNG | Floating LNG |
| GHG | Greenhouse gas |
| CO2 | Carbon dioxide |
| NG | Natural gas |
| FLNG-FPSO | LNG-floating production, storage and offloading |
| APCI | Air Products & Chemical Inc. |
| BOG | Boiled off gas |
| JT | Joule-Thomson |
| COP | Coefficient of performance |
| N2O | Nitrous oxide |
| N2 | Nitrogen |
| MFC | Mixed fluid cascade liquefaction process |
| C2H4/C2 | Ethane |
| C3H8/C3 | Propane |
| T0 | Ambient temperature |
| NGL | Natural gas liquid |
| NRU | Nitrogen rejection unit |
| ARC | Absorption refrigeration cycle |
| NH3 | Ammonia |
| H2O | Water |
| LPG | Liquefied petroleum gas |
| HEX | Heat exchanger |
| PNEC | Parallel nitrogen expansion liquefaction process |
| GA | genetic algorithm |
| NEC | Nitrogen expansion liquefaction process |
| SNA | Single nitrogen expansion process with ammonia absorption pre-cooling |
| CH4/C1 | Methane |
| CP | Constant-pressure specific heat capacity |
| Cv | Constant-volume specific heat capacity |
| C3N | Propane-nitrogen |
| MR | Mixed refrigerant |
| SMR | Single mixed refrigerant liquefaction process |
| DMR | Dual mixed refrigerant liquefaction process |
| C3MR | Mixed refrigerant liquefaction process with propane pre-cooling |
| PRICO | Single mixed refrigerant liquefaction process designed by Black–Veatch |
| MTPA | Metric ton per annum |
| MSMR | Modified single mixed refrigerant |
| HK | Heavy key |
| LK | Light key |
| HT | Hydraulic turbine |
| KSMR | Korea single mixed refrigerant |
| PID | Proportion integration differentiation |
| DMC | Dynamic matrix control |
| AP-X™ | mixed refrigerant plus nitrogen expansion liquefaction process |
| WMR | Warm MR |
| CMR | Cold MR |
| CWHE | Coil-wound heat exchangers |
| DWC | Dividing wall column |
| GARO | Gradient assisted robust optimization |
| KBO | Knowledge-based optimization |
| X | Mass fraction |
| MCR | Multi-component refrigerant |
| i-C5H12 | Isopentane |
| HFO-1234yf | 2, 3, 3, 3-Tetrafluoropropylene |
| PLNG | Pressurized LNG |
References
- BP Energy Outlook 2019|News and Insights|Home. Bp Global. Available online: https://www.bp.com/en/global/corporate/news-and-insights/press-releases/bp-energy-outlook-2019.html (accessed on 26 October 2021).
- Lim, W.; Choi, K.; Moon, I. Current Status and Perspectives of Liquefied Natural Gas (LNG) Plant Design. Ind. Eng. Chem. Res. 2013, 52, 3065–3088. [Google Scholar] [CrossRef]
- Liang, F.-Y.; Ryvak, M.; Sayeed, S.; Zhao, N. The role of natural gas as a primary fuel in the near future, including comparisons of acquisition, transmission and waste handling costs of as with competitive alternatives. Chem. Central J. 2012, 6, S4. [Google Scholar] [CrossRef]
- Nawaz, A.; Qyyum, M.A.; Qadeer, K.; Khan, M.S.; Ahmad, A.; Lee, S.; Lee, M. Optimization of mixed fluid cascade LNG process using a multivariate Coggins step-up approach: Overall compression power reduction and exergy loss analysis. Int. J. Refrig. 2019, 104, 189–200. [Google Scholar] [CrossRef]
- Arrhenius, K.; Karlsson, A.; Hakonen, A.; Ohlson, L.; Yaghooby, H.; Büker, O. Variations of fuel composition during storage at Liquefied Natural Gas refuelling stations. J. Nat. Gas Sci. Eng. 2018, 49, 317–323. [Google Scholar] [CrossRef]
- Najibullah Khan, N.B.; Barifcani, A.; Tade, M.; Pareek, V. A case study: Application of energy and exergy analysis for enhancing the process efficiency of a three stage propane pre-cooling cycle of the cascade LNG process. J. Nat. Gas Sci. Eng. 2016, 29, 125–133. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, J.; Zhao, Z.; Luo, J.; Wang, Q.; Chen, G. Development of natural gas liquefaction processes using mixed refrigerants: A review of featured process configurations and performance. J. Zhejiang Univ. Sci. A 2019, 20, 727–780. [Google Scholar] [CrossRef]
- Won, W.; Lee, S.K.; Choi, K.; Kwon, Y. Current trends for the floating liquefied natural gas (FLNG) technologies. Korean J. Chem. Eng. 2014, 31, 732–743. [Google Scholar] [CrossRef]
- Wang, M.; Khalilpour, R.; Abbas, A. Thermodynamic and economic optimization of LNG mixed refrigerant processes. Energy Convers. Manag. 2014, 88, 947–961. [Google Scholar] [CrossRef]
- Qyyum, M.A.; Qadeer, K.; Lee, M. Comprehensive Review of the Design Optimization of Natural Gas Liquefaction Processes: Current Status and Perspectives. Ind. Eng. Chem. Res. 2017, 57, 5819–5844. [Google Scholar] [CrossRef]
- Shell LNG Outlook 2021. Available online: https://www.shell.com/energy-and-innovation/natural-gas/liquefied-natural-gas-lng/lng-outlook-2021.html (accessed on 21 October 2021).
- Kumar, S.; Kwon, H.-T.; Choi, K.-H.; Lim, W.; Cho, J.H.; Tak, K.; Moon, I. LNG: An eco-friendly cryogenic fuel for sustainable development. Appl. Energy 2011, 88, 4264–4273. [Google Scholar] [CrossRef]
- Mazyan, W.; Ahmadi, A.; Ahmed, H.; Hoorfar, M. Market and technology assessment of natural gas processing: A review. J. Nat. Gas Sci. Eng. 2016, 30, 487–514. [Google Scholar] [CrossRef]
- Lee, I.; Park, J.; Moon, I. Key Issues and Challenges on the Liquefied Natural Gas Value Chain: A Review from the Process Systems Engineering Point of View. Ind. Eng. Chem. Res. 2018, 57, 5805–5818. [Google Scholar] [CrossRef]
- Katebah, M.A.; Hussein, M.M.; Shazed, A.; Bouabidi, Z.; Al-musleh, E.I. Rigorous simulation, energy and environmental analysis of an actual baseload LNG supply chain. Comput. Chem. Eng. 2020, 141, 106993. [Google Scholar] [CrossRef]
- Zhang, J.; Meerman, H.; Benders, R.; Faaij, A. Comprehensive review of current natural gas liquefaction processes on technical and economic performance. Appl. Therm. Eng. 2020, 166, 114736. [Google Scholar] [CrossRef]
- He, T.; Karimi, I.A.; Ju, Y. Review on the design and optimization of natural gas liquefaction processes for onshore and offshore applications. Chem. Eng. Res. Des. 2018, 132, 89–114. [Google Scholar] [CrossRef]
- Eldemerdash, U. Technology review of natural gas liquefaction processes. J. Appl. Sci. 2011, 11, 3541–3546. [Google Scholar] [CrossRef]
- Ríos-Mercado, R.Z.; Borraz-Sánchez, C. Optimization problems in natural gas transportation systems: A state-of-the-art review. Appl. Energy 2015, 147, 536–555. [Google Scholar] [CrossRef]
- Chang, H.-M. A thermodynamic review of cryogenic refrigeration cycles for liquefaction of natural gas. Cryogenics 2015, 72, 127–147. [Google Scholar] [CrossRef]
- Khan, M.S.; Karimi, I.A.; Wood, D.A. Retrospective and future perspective of natural gas liquefaction and optimization technologies contributing to efficient LNG supply: A review. J. Nat. Gas Sci. Eng. 2017, 45, 165–188. [Google Scholar] [CrossRef]
- Lin, W.; Xiong, X.; Spitoni, M.; Gu, A. Design and Optimization of Pressurized Liquefaction Processes for Offshore Natural Gas Using Two-Stage Cascade Refrigeration Cycles. Ind. Eng. Chem. Res. 2018, 57, 5858–5867. [Google Scholar] [CrossRef]
- Wu, D. A Preliminary Study on Liquefaction Technology and Storage and Transportation Safety of Natural Gas. Mod. Chem. Res. 2018, 75–76. [Google Scholar]
- Niu, Y.; Feng, L. Application of Petrochemical Industry Process Simulation Software in LNG Engineering. Shanghai Gas 2007, 5–8. [Google Scholar]
- Andress, D. The Phillips Optimized Cascade LNG Process: A Quarter-of-a-Century of Improvements; American Institute of Chemical Engineers: New York, NY, USA, 1996. [Google Scholar]
- Ransbarger, W. A fresh look at LNG process efficiency. In LNG Industry; Palladian Publications: Farnharm, UK, 2007. [Google Scholar]
- Fahmy, M.F.M.; Nabih, H.I.; El-Nigeily, M. Enhancement of the efficiency of the Open Cycle Phillips Optimized Cascade LNG process. Energy Convers. Manag. 2016, 112, 308–318. [Google Scholar] [CrossRef]
- Lee, H.S.; Oh, S.T.; Kim, H.W.; Choi, W.J.; Yoon, J.I.; Lee, S.G. Comparison of Performance of Cryogenic Liquefaction Process with Expander. In Proceedings of the KSPSE Spring Conference; The Society of Air-Conditioning and Refrigerating Engineers of Korea: Jeju, Korea, 2009; pp. 250–254. [Google Scholar]
- Yoon, J.; Lee, H.; Oh, S.; Lee, S.; Choi, K. Characteristics of Cascade and C3MR Cycle on Natural Gas Liquefaction Process. Int. J. Chem. Mol. Eng. 2009, 59, 185–189. [Google Scholar]
- Lee, H.S.; Oh, S.T.; Yoon, J.I.; Lee, S.G.; Choi, K.H. Analysis of Cryogenic Refrigeration Cycle Using Two Stage Intercooler. In Defect and Diffusion Foru; Trans Tech Publications Ltd.: Wollerau, Switzerland; Volume 297, pp. 1146–1151. [CrossRef]
- Oh, S.; Lee, H.; Yi, G.-B.; Yoon, J.; Lee, S. Characteristics of cryogenic liquefaction cycle using two stage compression type. In Proceedings of the SAREK Conference; The Society of Air-Conditioning and Refrigerating Engineers of Korea: Jeju, Korea, 2009; pp. 556–560. [Google Scholar]
- Gang, B.Y. Analysis of the Research on the Application of hydrocarbon in Refrigeration and air conditioning. Refrig. J. 2003, 41–46. [Google Scholar]
- The Economic Impact of Small Scale LNG. PwC. May 2013. Available online: https://xueshu.baidu.com/usercenter/paper/show?paperid=133625d91249ac344bb778d7a54818dd&site=xueshu_se (accessed on 30 August 2022).
- Chang, H.-M.; Chung, M.J.; Lee, S.; Choe, K.H. An efficient multi-stage Brayton–JT cycle for liquefaction of natural gas. Cryogenics 2011, 51, 278–286. [Google Scholar] [CrossRef]
- Yoon, J.-I.; Choi, W.-J.; Lee, S.; Choe, K.; Shim, G.-J. Efficiency of Cascade Refrigeration Cycle Using C3H8, N2O, and N2. Heat Transf. Eng. 2013, 34, 959–965. [Google Scholar] [CrossRef]
- Yoon, J.-I.; Choi, K.-H.; Lee, H.-S.; Kim, H.-J.; Son, C.-H. Assessment of the performance of a natural gas liquefaction cycle using natural refrigerants. Heat Mass Transf. 2015, 51, 95–105. [Google Scholar] [CrossRef]
- Lee, C.-J.; Song, K.; Shin, S.; Lim, Y.; Han, C. Process design for the offshore production of liquefied natural gas with nonflammable refrigerants. Ind. Eng. Chem. Res. 2015, 54, 11106–11112. [Google Scholar] [CrossRef]
- Qingping, L.I.; Meng, W.; Zhang, J.; Han, L.U. Comparison and Selection of Natural Gas Liquefaction Refrigeration Processes. Gas Heat 2012, 32, 4–10. [Google Scholar]
- Vatani, A.; Mehrpooya, M.; Palizdar, A. Energy and exergy analyses of five conventional liquefied natural gas processes: Liquefied natural gas. Int. J. Energy Res. 2014, 38, 1843–1863. [Google Scholar] [CrossRef]
- Ramezani, T.; Nargessi, Z.; Palizdar, A.; Vatani, A. Control Structure Design and Dynamic Simulation of Mixed Fluid Cascade Natural Gas Liquefaction Process. J. Gas Technol. 2019, 5, 19. [Google Scholar]
- Majeed, K.; Qyyum, M.A.; Nawaz, A.; Ahmad, A.; Naqvi, M.; He, T.; Lee, M. Shuffled Complex Evolution-Based Performance Enhancement and Analysis of Cascade Liquefaction Process for Large-Scale LNG Production. Energies 2020, 13, 2511. [Google Scholar] [CrossRef]
- Ding, H.; Sun, H.; Sun, S.; Chen, C. Analysis and optimisation of a mixed fluid cascade (MFC) process. Cryogenics 2017, 83, 35–49. [Google Scholar] [CrossRef]
- Castillo, L.; Majzoub Dahouk, M.; Di Scipio, S.; Dorao, C.A. Conceptual analysis of the precooling stage for LNG processes. Energy Convers. Manag. 2013, 66, 41–47. [Google Scholar] [CrossRef]
- Brodal, E.; Jackson, S.; Eiksund, O. Performance and design study of optimized LNG Mixed Fluid Cascade processes. Energy 2019, 189, 116207. [Google Scholar] [CrossRef]
- Bauer, H.C. Mixed fluid cascade, experience and outlook. In Proceedings of the 12th Topical Conference on Gas Utilization, AIChE 2012 Spring Meeting, Houston, TX, USA, 2–5 April 2012; p. 25a. [Google Scholar]
- Pettersen, J.; Nilsen, Ø.; Vist, S.; Noreng Giljarhus, L.; Fredheim, A.; Aasekjaer, K.; Neeraas, B. Technical and operational innovation for onshore and floating LNG. In Proceedings of the 17th International Conference & Exhibition on Liquefied Natural Gas (LNG 17), Houston, TX, USA, 16–19 April 2013; pp. 1–12. [Google Scholar]
- Eiksund, O.; Brodal, E.; Jackson, S. Optimization of Pure-Component LNG Cascade Processes with Heat Integration. Energies 2018, 11, 202. [Google Scholar] [CrossRef]
- Mehrpooya, M.; Hossieni, M.; Vatani, A. Novel LNG-Based Integrated Process Configuration Alternatives for Coproduction of LNG and NGL. Ind. Eng. Chem. Res. 2014, 53, 17705–17721. [Google Scholar] [CrossRef]
- Ghorbani, B.; Hamedi, M.-H.; Amidpour, M.; Mehrpooya, M. Cascade refrigeration systems in integrated cryogenic natural gas process (natural gas liquids (NGL), liquefied natural gas (LNG) and nitrogen rejection unit (NRU)). Energy 2016, 115, 88–106. [Google Scholar] [CrossRef]
- Mehrpooya, M.; Omidi, M.; Vatani, A. Novel mixed fluid cascade natural gas liquefaction process configuration using absorption refrigeration system. Appl. Therm. Eng. 2016, 98, 591–604. [Google Scholar] [CrossRef]
- Ghorbani, B.; Shirmohammadi, R.; Mehrpooya, M. A novel energy efficient LNG/NGL recovery process using absorption and mixed refrigerant refrigeration cycles—Economic and exergy analyses. Appl. Therm. Eng. 2018, 132, 283–295. [Google Scholar] [CrossRef]
- Ghorbani, B.; Hamedi, M.-H.; Amidpour, M. Exergoeconomic evaluation of an integrated nitrogen rejection unit with LNG and NGL Co-Production processes based on the MFC and absorbtion refrigeration systems. Gas Process. J. 2016, 4, 1–28. [Google Scholar] [CrossRef]
- Austbø, B.; Gundersen, T. Optimization of a Single Expander LNG Process. Energy Procedia 2015, 64, 63–72. [Google Scholar] [CrossRef]
- Chang, H.-M.; Chung, M.J.; Kim, M.J.; Park, S.B. Thermodynamic design of methane liquefaction system based on reversed-Brayton cycle. Cryogenics 2009, 49, 226–234. [Google Scholar] [CrossRef]
- Son, H.; Kim, J.-K. Energy-efficient process design and optimization of dual-expansion systems for BOG (Boil-off gas) Re-liquefaction process in LNG-fueled ship. Energy 2020, 203, 117823. [Google Scholar] [CrossRef]
- Chang, X.; Zhang, Y.; Zhu, J.; Yuxing, L.I.; Zhang, M.; Yang, X. Optimization of the process of nitrogen expansion refrigeration of BOG and the analysis of the adaptability of the sea. Chem. Ind. Eng. Prog. 2017, 36, 1619–1627. [Google Scholar] [CrossRef]
- Khan, M.S.; Lee, S.; Getu, M.; Lee, M. Knowledge inspired investigation of selected parameters on energy consumption in nitrogen single and dual expander processes of natural gas liquefaction. J. Nat. Gas Sci. Eng. 2015, 23, 324–337. [Google Scholar] [CrossRef]
- Finn, A. Are floating LNG facilities viable options? Hydrocarb. Process. 2009, 88, 31. [Google Scholar]
- Yumrutaş, R.; Kunduz, M.; Kanoğlu, M. Exergy analysis of vapor compression refrigeration systems. Exergy Int. J. 2002, 2, 266–272. [Google Scholar] [CrossRef]
- Pang, W.; Qingping, L.I.; Xie, B.; Xichong, Y.U.; Wang, Q. Parameter determination of dual nitrogen expansion technology for gas liquefaction in FLNG unit. Oil Gas Storage Transp. 2017, 36, 108–114. [Google Scholar]
- He, T.; Ju, Y. A novel conceptual design of parallel nitrogen expansion liquefaction process for small-scale LNG (liquefied natural gas) plant in skid-mount packages. Energy 2014, 75, 349–359. [Google Scholar] [CrossRef]
- Kim, M.; Mihaeye, K.; Min, J.; Lee, D.; Park, H.; Nguyen, X.C.; de Regt, C.; Kim, J.; Jang, J. Optimization of Nitrogen Liquefaction Cycle for Small/Medium Scale FLNG. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 1–4 May 2017. [Google Scholar] [CrossRef]
- Qyyum, M.A.; Qadeer, K.; Minh, L.Q.; Haider, J.; Lee, M. Nitrogen self-recuperation expansion-based process for offshore coproduction of liquefied natural gas, liquefied petroleum gas, and pentane plus. Appl. Energy 2019, 235, 247–257. [Google Scholar] [CrossRef]
- Gao, T.; Lin, W.; Gu, A.; Gu, M. Coalbed methane liquefaction adopting a nitrogen expansion process with propane pre-cooling. Appl. Energy 2010, 87, 2142–2147. [Google Scholar] [CrossRef]
- Shen, Y.Y.; Li, J.; Lin, R.Y.; Xi-Chong, Y.U.; Yu, X.C. Simulation of Liquefaction Process and Exergy Efficiency Analysis for FLNG System. J. Qufu Norm. Univ. (Nat. Sci.) 2016, 42, 84–91. [Google Scholar]
- Wang, W.; Wei, D.; Yuxing, L.I.; Zhu, J.; Gas, T.N. Scaling effect analysis on FLNG laboratory and pilot liquefaction tests. Oil Gas Storage Transp. 2018, 37, 462–468. [Google Scholar]
- Wang, W.C.; Wei, D.; Xing, L.Y.; Zhu, J.L. Scaling effect analysis and application study of FLNG liquefaction process system. Nat. Gas Chem. Ind. 2017, 42, 93–99+110. [Google Scholar]
- He, T.B.; Ju, Y.L. Performance improvement of nitrogen expansion liquefaction process for small-scale LNG plant. Cryogenics 2014, 61, 111–119. [Google Scholar] [CrossRef]
- Zhang, J.; Meerman, H.; Benders, R.; Faaij, A. Technical and economic optimization of expander-based small-scale natural gas liquefaction processes with absorption precooling cycle. Energy 2020, 191, 116592. [Google Scholar] [CrossRef]
- Mahabadipour, H.; Ghaebi, H. Development and comparison of two expander cycles used in refrigeration system of olefin plant based on exergy analysis. Appl. Therm. Eng. 2013, 50, 771–780. [Google Scholar] [CrossRef]
- Balmer, R.T. Chapter 14—Vapor and Gas Refrigeration Cycles. In Modern Engineering Thermodynamics; Balmer, R.T., Ed.; Academic Press: Boston, MA, USA, 2011; pp. 535–590. [Google Scholar] [CrossRef]
- Tugcu, A.; Arslan, O. Optimization of geothermal energy aided absorption refrigeration system—GAARS: A novel ANN-based approach. Geothermics 2017, 65, 210–221. [Google Scholar] [CrossRef]
- Mortazavi, A.; Somers, C.; Alabdulkarem, A.; Hwang, Y.; Radermacher, R. Enhancement of APCI cycle efficiency with absorption chillers. Energy 2010, 35, 3877–3882. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, W.; Li, Y.; Wang, W.; Liu, Y. Offshore adaptability of the nitrogen expander liquefaction process with pre-cooling. Appl. Therm. Eng. 2019, 155, 373–385. [Google Scholar] [CrossRef]
- He, T.; Ju, Y. Optimal synthesis of expansion liquefaction cycle for distributed-scale LNG (liquefied natural gas) plant. Energy 2015, 88, 268–280. [Google Scholar] [CrossRef]
- Khan, M.S.; Lee, S.; Hasan, M.; Lee, M. Process knowledge based opportunistic optimization of the N2–CO2 expander cycle for the economic development of stranded offshore fields. J. Nat. Gas Sci. Eng. 2014, 18, 263–273. [Google Scholar] [CrossRef]
- Yuan, Z.; Cui, M.; Xie, Y.; Li, C. Design and analysis of a small-scale natural gas liquefaction process adopting single nitrogen expansion with carbon dioxide pre-cooling. Appl. Therm. Eng. 2014, 64, 139–146. [Google Scholar] [CrossRef]
- Okafor, E.; Ojo, S. Comparative Analyses of an Optimized Dual Expansion, Natural Gas Liquefaction Process. In Proceedings of the SPE Nigeria Annual International Conference and Exhibition, Society of Petroleum Engineers, Lagos, Nigeria, 2–4 August 2016. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Y.; Wang, W.; Liu, Y.; Yu, X. Offshore adaptability of the dual nitrogen expander process with CO2 pre-cooling. Nat. Gas Ind. 2012, 32, 89–95+127–128. [Google Scholar] [CrossRef]
- Xichong, Y.U.; Xie, B.; Yaling, W.U.; Yuxing, L.I.; Wang, W.; Zhu, J. Liquefaction technology of CO2-precooled dinitrogen expansion for large FLNG/FLPG device top module. Oil Gas Storage Transp. 2014, 33, 89–94. [Google Scholar]
- Chen, Z.; Ju, Y. Design and analysis of FLNG liquefaction and NGL recovery process adopting nitrogen expansion cycle with CO2 trans-critical pre-cooling. J. Chem. Eng. Jpn. 2016, 44, 5–11+69. [Google Scholar] [CrossRef]
- Kim, M.; Kim, Y.; Kim, M.; Kim, M.; Min, J. Advanced Liquefaction Cycle for Natural Gas. In Proceedings of the ASME 2018 37th International Conference on Ocean, Offshore and Arctic Engineering, Madrid, Spain, 17–22 June 2018. [Google Scholar]
- Kim, M.; Kim, M.; Lee, K.; Kim, H.; Lee, D.; Min, J.; Kim, J. Advanced Dual Refrigerant Expansion Cycle for LNG Liquefaction. Plant J. 2019, 15, 46–55. [Google Scholar]
- Park, H.; Kim, M.; Kim, C.; Kim, H.; Lee, K.; Kim, M.; Lee, D. Dual Refrigerant LNG Liquefaction Cycle for Offshore FLNG and Its Pilot Plant. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 4–7 May 2020. [Google Scholar] [CrossRef]
- Ding, H.; Sun, H.; He, M. Optimisation of expansion liquefaction processes using mixed refrigerant N2–CH4. Appl. Therm. Eng. 2016, 93, 1053–1060. [Google Scholar] [CrossRef]
- Zheng, Y.P.; Zhang, W.C.; Hao, Z.P.; Jin, J.Q.; Li, S.S. Influence factors analysis of N2-CH4 expansion and refrigeration process. Mod. Chem. Ind. 2015, 35, 165–168. [Google Scholar] [CrossRef]
- Moein, P.; Sarmad, M.; Khakpour, M.; Delaram, H. Methane addition effect on a dual nitrogen expander refrigeration cycle for LNG production. J. Nat. Gas Sci. Eng. 2016, 33, 1–7. [Google Scholar] [CrossRef]
- Liang, P.; Sun, S.; Li, Y.; Hanfei, T.; Zhao, M. Calculation and Thermodynamic Analysis on Liquefaction Processes of Natural Gas with Expanders. J. Xi’an Jiaotong Univ. 2007, 41, 1115–1118+1126. [Google Scholar]
- Cao, W.; Lu, X.; Lin, W.; Gu, A. Parameter comparison of two small-scale natural gas liquefaction processes in skid-mounted packages. Appl. Therm. Eng. 2006, 26, 898–904. [Google Scholar] [CrossRef]
- Ding, H.; Sun, H.; Ming, Z.; Li, Y.; Lian, Y. Seletction of refrigerant composition for energy efficient expansion liquefaction process. Cryog. Supercond. 2014, 42, 81–85. [Google Scholar] [CrossRef]
- He, T.; Lin, W. Design and optimization of nitrogen expansion liquefaction processes integrated with ethane separation for high ethane-content natural gas. Appl. Therm. Eng. 2020, 173, 115272. [Google Scholar] [CrossRef]
- Qyyum, M.A.; Qadeer, K.; Lee, S.; Lee, M. Innovative propane-nitrogen two-phase expander refrigeration cycle for energy-efficient and low-global warming potential LNG production. Appl. Therm. Eng. 2018, 139, 157–165. [Google Scholar] [CrossRef]
- Qyyum, M.A.; Qadeer, K.; Ahmad, A.; Lee, M. Gas–liquid dual-expander natural gas liquefaction process with confirmation of biogeography-based energy and cost savings. Appl. Therm. Eng. 2020, 166, 114643. [Google Scholar] [CrossRef]
- Gao, Y.M.; Wang, X. Simulation and Analysis of N2-CH4 Expansion Liquefaction Process. Build. Energy Environ. 2017, 36, 73–75+83. [Google Scholar]
- Geist, J.M. The role of LNG in energy supply. Int. J. Refrig. 1983, 6, 283–297. [Google Scholar] [CrossRef]
- Khan, M.S.; Lee, S.; Rangaiah, G.P.; Lee, M. Knowledge based decision making method for the selection of mixed refrigerant systems for energy efficient LNG processes. Appl. Energy 2013, 111, 1018–1031. [Google Scholar] [CrossRef]
- Xu, X.; Liu, J.; Jiang, C.; Cao, L. The correlation between mixed refrigerant composition and ambient conditions in the PRICO LNG process. Appl. Energy 2013, 102, 1127–1136. [Google Scholar] [CrossRef]
- Price, B.C.; Hoffart, S.D. LNG Supply Developments Using Distributed LNG Concepts. In Proceedings of the Sixteenth International Conference and Exhibition on Liquefied Natural Gas: LNG 16–GNL 16, Oran, Algeria, 18–21 April 2010. [Google Scholar]
- Barclay, M.; Denton, N. Selecting offshore LNG processes. LNG J. 2005, 10, 34–36. [Google Scholar]
- Meek, H.J.; Cariou, H.; Schier, M. SS: Offshore Floating LNG-LNG FPSO Development-bringing two industries together. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 4–7 May 2009. [Google Scholar]
- Terry, L. Comparison of liquefaction process. LNG J. 1998, 21, 28–33. [Google Scholar]
- Zhang, Y.; Gai, J.; Jiang, H.; Liu, X.; Amp, B. Application of PRICO® liquefaction technology in offshore floating devices. Nat. Gas Ind. 2018, 38, 115–120. [Google Scholar]
- Bukowski, J.; Liu, Y.N.; Boccella, S.; Kowalski, L. Innovations in natural gas liquefaction technology for future LNG plants and floating LNG facilities. In Proceedings of the International Gas Union Research Conference, Seoul, Korea, 19 October 2011. [Google Scholar]
- Talib, J.H.; Price, B. Development of Floating LNG Production Units with Modular/Scalable SMR Processes. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 2–5 May 2011. [Google Scholar]
- Talib, J.H.; Germinder, B. Game-Changing Floating LNG Solutions. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 2–5 May 2016. [Google Scholar]
- Kim, I.H.; Dan, S.; Cho, S.; Lee, G.; Yoon, E.S. Optimization of Single-stage Mixed Refrigerant LNG Process Considering Inherent Explosion Risks. Korean Chem. Eng. Res. 2014, 52, 467–474. [Google Scholar] [CrossRef][Green Version]
- Corneliussen, M.; Samnøy, E. Near Shore FLNG Concept Evaluations (Master thesis, NTNU). Available online: http://hdl.handle.net/11250/2350048 (accessed on 6 April 2018).
- You, W.; Chae, M.; Park, J.; Lim, Y. Potential Explosion Risk Comparison between SMR and DMR Liquefaction Processes at Conceptual Design Stage of FLNG. J. Ocean. Eng. 2018, 32, 95–105. [Google Scholar] [CrossRef]
- Yin, Q. Adaptability and regulating performance of the single mixed refrigerant process. Cryogenics 2010, 173, 1–4. [Google Scholar] [CrossRef]
- Jensen, J.B.; Skogestad, S. Optimal Operation of a Simple LNG Process. IFAC Proc. 2006, 39, 241–246. [Google Scholar] [CrossRef]
- Husnil, Y.A.; Yeo, G.; Lee, M. Plant-wide control for the economic operation of modified single mixed refrigerant process for an offshore natural gas liquefaction plant. Chem. Eng. Res. Des. 2014, 92, 679–691. [Google Scholar] [CrossRef]
- Lee, S.; Long, N.V.D.; Lee, M. Design and Optimization of Natural Gas Liquefaction and Recovery Processes for Offshore Floating Liquefied Natural Gas Plants. Ind. Eng. Chem. Res. 2012, 51, 10021–10030. [Google Scholar] [CrossRef]
- Lee, W.; An, J.; Lee, J.M.; Lim, Y. Design of single mixed refrigerant natural gas liquefaction process considering load variation. Chem. Eng. Res. Des. 2018, 139, 89–103. [Google Scholar] [CrossRef]
- Finn, A.; Johnson, G.; Tomlinson, T. Developments in natural gas liquefaction. Hydrocarb. Process. 1999, 78, 47–59. [Google Scholar]
- Sieminski, A.; Energy Information Administration. International Energy Outlook; U.S. Energy Information Administration: Washington, DC, USA, 2016. [Google Scholar]
- Vatani, A.; Mehrpooya, M.; Palizdar, A. Advanced exergetic analysis of five natural gas liquefaction processes. Energy Convers. Manag. 2014, 78, 720–737. [Google Scholar] [CrossRef]
- Mehrpooya, M.; Ansarinasab, H. Exergoeconomic evaluation of single mixed refrigerant natural gas liquefaction processes. Energy Convers. Manag. 2015, 99, 400–413. [Google Scholar] [CrossRef]
- Niu, G.; Huang, Y.H.; Wang, J. Thermodynamic analysis of liquefied natural gas process. Chem. Eng. 2005, 33, 71–74. [Google Scholar] [CrossRef]
- He, T.; Ju, Y. Design and Optimization of a Novel Mixed Refrigerant Cycle Integrated with NGL Recovery Process for Small-Scale LNG Plant. Ind. Eng. Chem. Res. 2014, 53, 5545–5553. [Google Scholar] [CrossRef]
- Liang, Z.; Li, S.; Tian, J.; Zhu, X.; Mei, Q.; Zhang, L. An experimental study of technical measures for energy saving of CNG compressors. Nat. Gas Ind. 2013, 33, 95–98. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, F.L.; Si, Y.H.; Li, D.; Fan, Z. Exergy analysis of liquefied natural gas process with mixed refrigerant cycle. Mod. Chem. Ind. 2015, 35, 172–175. [Google Scholar] [CrossRef]
- Chen, Z. Study on Mixed Refrigerant Cycle Liquefied Natural Gas Technology. Low Temp. Spec. Gases 2019, 37, 17–23. [Google Scholar]
- Tak, K.; Lee, I.; Kwon, H.; Kim, J.; Ko, D.; Moon, I. Comparison of Multistage Compression Configurations for Single Mixed Refrigerant Processes. Ind. Eng. Chem. Res. 2015, 54, 9992–10000. [Google Scholar] [CrossRef]
- Pham, T.N.; Long, N.V.D.; Lee, S.; Lee, M. Enhancement of single mixed refrigerant natural gas liquefaction process through process knowledge inspired optimization and modification. Appl. Therm. Eng. 2017, 110, 1230–1239. [Google Scholar] [CrossRef]
- Qyyum, M.A.; Long, N.V.D.; Minh, L.Q.; Lee, M. Design optimization of single mixed refrigerant LNG process using a hybrid modified coordinate descent algorithm. Cryogenics 2018, 89, 131–140. [Google Scholar] [CrossRef]
- Qyyum, M.A.; Ali, W.; Long, N.V.D.; Khan, M.S.; Lee, M. Energy efficiency enhancement of a single mixed refrigerant LNG process using a novel hydraulic turbine. Energy 2018, 144, 968–976. [Google Scholar] [CrossRef]
- Qyyum, M.A.; Haider, J.; Qadeer, K.; Lee, M. Performance enhancement of offshore LNG processes by introducing optimal mixed refrigerant self-cooling recuperator. In Proceedings of the 11th International Conference on Applied Energy 2019, Västerås, Sweden, 12–15 August 2019. [Google Scholar]
- Khan, M.S.; Chaniago, Y.D.; Getu, M.; Lee, M. Energy saving opportunities in integrated NGL/LNG schemes exploiting: Thermal-coupling common-utilities and process knowledge. Chem. Eng. Process. Process Intensif. 2014, 82, 54–64. [Google Scholar] [CrossRef]
- Paradowski, H.; Bamba, M.; Bladanet, C. Propane precooling cycles for increased LNG train capacity. In Proceedings of the 14th International Conference and Exhibition on Liquefied Natural Gas, 92973 Paris La Défense, Cedex, France, 21–24 March 2004; pp. 107–124. [Google Scholar]
- Mortazavi, A.; Somers, C.; Hwang, Y.; Radermacher, R.; Rodgers, P.; Al-Hashimi, S. Performance enhancement of propane pre-cooled mixed refrigerant LNG plant. Appl. Energy 2012, 93, 125–131. [Google Scholar] [CrossRef]
- Pan, H.; Li, S.; Li, Y.; Zhu, J. Optimization in the Control Modes of Propane Pre-cooling and Mixed-refrigerant Process. J. Refrig. 2016, 37, 53–58. [Google Scholar]
- Wang, H.; Long, Z.; Liu, J.; Li, L.; Zhang, Y. Research on the Process of Natural Gas Liquefaction C3/MRC. Nat. Gas Oil 2016, 34, 32–35+7–8. [Google Scholar]
- Wang, H. The Industrialized Application of the Three-cascade Mixed-refrigeration Liquefaction Technology about Natural Gas. Refrig. Air Cond. 2014, 28, 191–198. [Google Scholar]
- Wang, B.Q. LNG processing technologies and comparison analysis. Nat. Gas Ind. 2009, 29, 111–113. [Google Scholar] [CrossRef]
- Yiming, M.; Dongjun, W.; Bo, C. Study on mixed refrigerant formulation optimization for natural gas liquefaction. Chem. Eng. Oil Gas 2015, 44, 65–69. [Google Scholar] [CrossRef]
- Chen, G. Development of Natural Gas Liquefaction Process and its Available Energy Analysis. Nat. Gas Oil 2013, 31, 27–32+5. [Google Scholar]
- Biyanto, T.R.; Cordova, H.; Matradji; Priambodo, K.; Sarah, A.Z.; Hermantara, R.C.; Sitorus, C.D. Optimization of energy efficiency in natural gas liquefaction process using plantwide control method. IOP Conf. Ser. Earth Environ. Sci. 2021, 672, 012105. [Google Scholar] [CrossRef]
- Faber, F.; Bliault, A.; Resweber, L.; Jones, P. Floating LNG solutions from the drawing board to reality. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 6–9 May 2002. [Google Scholar]
- Wood, D.; Mokhatab, S.; Economides, M.J. Offshore Natural Gas Liquefaction Process and Development Issues. SPE Proj. Facil. Constr. 2007, 2, 1–7. [Google Scholar] [CrossRef]
- Shin, H.; Lim, Y.K.; Oh, S.-K.; Lee, S.G.; Lee, J.M. Dynamic matrix control applied on propane-mixed refrigerant liquefaction process. Korean J. Chem. Eng. 2017, 34, 287–297. [Google Scholar] [CrossRef]
- Zheng, Y.; Dan, X.; Liu, X.; Li, J. Influence factors analysis of propane-mixed refrigerant natural gas liquefaction process based on HYSYS software model. Chem. Eng. Oil Gas 2013, 42, 473–477. [Google Scholar]
- Akinsipe, O.; Anozie, A.; Babatunde, D. A study of LNG processes to determine the effect of end flash systems on efficiency. Arch. Thermodyn. 2020, 41, 35–63. [Google Scholar] [CrossRef]
- Mortazavi, A.; Alabdulkarem, A.; Hwang, Y.; Radermacher, R. Novel combined cycle configurations for propane pre-cooled mixed refrigerant (APCI) natural gas liquefaction cycle. Appl. Energy 2014, 117, 76–86. [Google Scholar] [CrossRef]
- Rodgers, P.; Mortazavi, A.; Eveloy, V.; Al-Hashimi, S.; Hwang, Y.; Radermacher, R. Enhancement of LNG plant propane cycle through waste heat powered absorption cooling. Appl. Therm. Eng. 2012, 48, 41–53. [Google Scholar] [CrossRef]
- Sun, H.; He Ding, D.; He, M.; Shoujun Sun, S. Simulation and optimization of AP-X process in a large-scale LNG plant. J. Nat. Gas. Sci. Eng. 2016, 32, 380–389. [Google Scholar] [CrossRef]
- Liu, J.; Bai, G.L.; Ming-Lei, J.I. Method for advancement evaluation of natural gas liquefaction process. Chem. Eng. 2016, 44, 69–73+78. [Google Scholar] [CrossRef]
- Šesták, J.; Berggren, G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim. Acta 1971, 3, 1–12. [Google Scholar] [CrossRef]
- Forg, W. Liquefaction of Natural Gas. US4112700 A, 12 September 1978. [Google Scholar]
- Castillo, L.; Nadales, R.; González, C.; Dorao, C.; Viloria, A. Technology selection for liquefied natural gas (LNG) on baseload plants. In Proceedings of the XIX International Gas Convention, Caracas, Venezuela, 2010. [Google Scholar]
- Khan, M.S.; Karimi, I.A.; Lee, M. Evolution and optimization of the dual mixed refrigerant process of natural gas liquefaction. Appl. Therm. Eng. 2016, 96, 320–329. [Google Scholar] [CrossRef]
- Xing, D.X.; Wei, L.; Hua, T.J.; Zhang, Y.T. LNG Liquefaction Process and Field Application Overview. Guangzhou Chem. Ind. 2015, 43, 35–38. [Google Scholar]
- Jin, C.; Son, H.; Lim, Y. Optimization and economic analysis of liquefaction processes for offshore units. Appl. Therm. Eng. 2019, 163, 114334. [Google Scholar] [CrossRef]
- Hwang, J.; Lee, K.-Y. Optimal liquefaction process cycle considering simplicity and efficiency for LNG FPSO at FEED stage. Comput. Chem. Eng. 2014, 63, 1–33. [Google Scholar] [CrossRef]
- Qyyum, M.A.; Duong, P.L.T.; Minh, L.Q.; Lee, S.; Lee, M. Dual mixed refrigerant LNG process: Uncertainty quantification and dimensional reduction sensitivity analysis. Appl. Energy 2019, 250, 1446–1456. [Google Scholar] [CrossRef]
- Husnil, Y.A.; Lee, M. Synthesis of an Optimizing Control Structure for Dual Mixed Refrigerant Process. J. Chem. Eng. Jpn. 2014, 47, 678–686. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, X.; Xiao, K.; Li, Y.; Cheng, B. The Dynamic Performance Analysis of Double Mixed Refrigerant Liquefaction Process for Floating Liquid Natural Gas (FLNG). In Proceedings of the 27th International Ocean and Polar Engineering Conference, San Francisco, CA, USA, 25–30 June 2017; p. ISOPE-I-17-322. [Google Scholar]
- Chang, X.; Zhu, J.; Li, Y.; Jie, C.; Zeng, W. Floating DMR LNG-FPSO process with high efficiency and good offshore adaptability. Nat. Gas Ind. 2017, 37, 88–96. [Google Scholar] [CrossRef]
- Newton, C.L. Dual Mixed Refrigerant Natural Gas Liquefaction with Staged Compression. US. Patent 4525185 A, 24 October 1984. [Google Scholar]
- Liu, Y.N.; Pervier, J.W. Dual Mixed Refrigerant Process. CA1230047 A1, 1984. [Google Scholar]
- Roberts, M.J.; Agrawal, R. Dual Mixed Refrigerant Cycle for Gas Liquefaction. US. Patent 6,119,479, 7 December 1999. [Google Scholar]
- Bukowski, J.D.; Liu, Y.N.; Pillarella, M.R.; Boccella, S.J.; Kennington, W.A. Natural Gas Liquefaction Technology for Floating LNG Facilities; IGRC: Seoul, Korea, 2013. [Google Scholar]
- Lee, I.; Moon, I. Economic Optimization of Dual Mixed Refrigerant Liquefied Natural Gas Plant Considering Natural Gas Extraction Rate. Ind. Eng. Chem. Res. 2017, 56, 2804–2814. [Google Scholar] [CrossRef]
- Ait-Ali, M.A. Optimal Mixed Refrigerant Liquefaction of Natural Gas; Stanford University: Stanford, CA, USA, 1979. [Google Scholar]
- Wang, S.; Liu, L.; Zhang, L.; Du, J.; Wu, K. Conceptual design, simulation and analysis of novel AP-X™ system integrated with NGL recovery process for large-scale LNG plant. J. Chem. Ind. Eng. Soc. China 2019, 70, 508–515. [Google Scholar] [CrossRef]
- Qualls, W.R.; Huang, S.; Elliot, D.; Chen, J.J.; Yao, J.; Zhang, Y. Benefits of integrating NGL extraction and LNG liquefaction technology. In Proceedings of the AIChE Spring National Meeting, 5th Topical Conference on Natural Gas, Atlanta, Georgia, 10–14 April 2005. [Google Scholar]
- Khan, M.S.; Park, J.H.; Chaniago, Y.D.; Lee, M. Energy Efficient Process Structure Design of LNG/NGL Recovery for Offshore FLNG Plant. Energy Procedia 2014, 61, 599–602. [Google Scholar] [CrossRef]
- Cuellar, K.T.; Wilkinson, J.D.; Hudson, P.H.M.; Pierce, P.M.C. CO-producing LNG from cryogenic NGL recovery plants. In Proceedings of the 81th Annual Convention of the Gas Processors Association, Dallas, TX, USA, 12 March 2002. [Google Scholar]
- Hudson, H.M.; Wilkinson, J.D.; Cuellar, K.T.; Pierce, M.C. Integrated liquids recovery technology improves LNG production efficiency. In Proceedings of the 82nd Annual Convention of the Gas Processors Association, San Antonio, TX, USA, 13 March 2003. [Google Scholar]
- Brostow, A.A.; Roberts, M.J. Integrated NGL Recovery in the Production of Liquefied Natural Gas. U.S. Patent Application 13/669,539, 14 March 2013. [Google Scholar]
- Uwitonze, H.; Lee, I.; Hwang, K.S. Alternatives of integrated processes for coproduction of LNG and NGLs recovery. Chem. Eng. Process. Process Intensif. 2016, 107, 157–167. [Google Scholar] [CrossRef]
- Getu, M.; Mahadzir, S.; Long, N.V.D.; Lee, M. Techno-economic analysis of potential natural gas liquid (NGL) recovery processes under variations of feed compositions. Chem. Eng. Res. Des. 2013, 91, 1272–1283. [Google Scholar] [CrossRef]
- Ghorbani, B.; Hamedi, M.-H.; Amidpour, M.; Shirmohammadi, R. Implementing absorption refrigeration cycle in lieu of DMR and C3MR cycles in the integrated NGL, LNG and NRU unit. Int. J. Refrig. 2017, 77, 20–38. [Google Scholar] [CrossRef]
- Wang, M.; Xu, Q. Optimal design and operation for simultaneous shale gas NGL recovery and LNG re-gasification under uncertainties. Chem. Eng. Sci. 2014, 112, 130–142. [Google Scholar] [CrossRef]
- He, T.; Lin, W. Design and optimization of integrated single mixed refrigerant processes for coproduction of LNG and high-purity ethane. Int. J. Refrig. 2020, 119, 216–226. [Google Scholar] [CrossRef]
- Alabdulkarem, A.; Mortazavi, A.; Hwang, Y.; Radermacher, R.; Rogers, P. Optimization of propane pre-cooled mixed refrigerant LNG plant. Appl. Therm. Eng. 2011, 31, 1091–1098. [Google Scholar] [CrossRef]
- Townsend, D.W.; Linnhoff, B. Heat and power networks in process design. Part I: Criteria for placement of heat engines and heat pumps in process networks. AICHE J. 1983, 29, 742–748. [Google Scholar] [CrossRef]
- Song, Y.X.; Qing-Fang, L.I.; Zi-Li, L.I.; Zhu, H.B. A study on selecting composition of mixed refrigerant based on KBO method. Nat. Gas Chem. Ind. 2014, 39, 56–60. [Google Scholar]
- Zhao, M.; Li, Y. Analysis for selecting mixed refrigerant composition based on raw natural gas in propane pre-cooled mixed refrigerant liquefaction process. J. Xi’an Jiaotong Univ. 2010, 44, 108–112. [Google Scholar]
- Zhao, M.; Li, Y. Analysis for interrelations between mixed refrigerant composition and raw natural gas in C3/MRC liquefaction cycle. J. Chem. Ind. Eng. Soc. China 2009, 60, 50–57. [Google Scholar]
- Mortazavi, A.; Alabdulkarem, A.; Hwang, Y.; Radermacher, R. Development of a robust refrigerant mixture for liquefaction of highly uncertain natural gas compositions. Energy 2016, 113, 1042–1050. [Google Scholar] [CrossRef]
- Venkatarathnam, G.; Timmerhaus, K.D. Cryogenic Mixed Refrigerant Processes; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- Yin, Q.S.; Li, H.Y.; Fan, Q.H.; Jia, L.X. Economic Analysis of Mixed-Refrigerant Cycle and Nitrogen Expander Cycle in Small Scale Natural Gas Liquefier. Am. Inst. Phys. 2008, 985, 1159–1165. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, X.P.; He, B.C.; Dou, L.L.; Gao, M.M. Study on energy consumption optimization of mixed refrigerant circulating liquefaction process. Energy Chem. Ind. 2019, 40, 63–69. [Google Scholar]
- Yang, X.L.; Fan, D.D.; Yuan, R.N.; Zhang, X.; Li, W.H. Optimization of operation parameters and the ratio of mixed refrigerant in process of mixed refrigerant cycle. Chem. Eng. 2018, 46, 1–6+20. [Google Scholar] [CrossRef]
- Fan, M.; Zhe, J.; Ji, J.; Liu, S.; Zhang, S.; Petroleum, S.; Branch, Q.; Fuel, C.A. Study on optimization of mixed refrigerant composition based on SVM. Cryog. Supercond. 2017, 45, 32–36+65. [Google Scholar] [CrossRef]
- Xiao, R.G.; Gao, X.; Jin, W.B.; Yao, P.F.; Chen, Y.C. Optimization simulation of natural gas liquefaction process with double mixed refrigerant cycle. Chem. Eng. 2019, 47, 62–67+73. [Google Scholar]
- Khan, M.S.; Lee, M. Design optimization of single mixed refrigerant natural gas liquefaction process using the particle swarm paradigm with nonlinear constraints. Energy 2013, 49, 146–155. [Google Scholar] [CrossRef]
- Qyyum, M.A.; Lee, M. Hydrofluoroolefin-based novel mixed refrigerant for energy efficient and ecological LNG production. Energy 2018, 157, 483–492. [Google Scholar] [CrossRef]
- Jia, R.; Lin, W. Influence of heavy hydrocarbons in mixed refrigerant on natural gas liquefaction processes. J. Chem. Ind. Eng. Soc. China 2015, 66, 379–386. [Google Scholar] [CrossRef]
- Bowen, R.R.; Gentry, M.C.; Nelson, E.D.; Papka, S.D.; Leger, A.T. Pressurized liquefied natural gas (PLNG): A new gas transportation technology. Proc. GasTech. 2005. Available online: http://www.ivt.ntnu.no/ept/fag/tep4215/innhold/LNG%20Conferences/2005/SDS_TIF/050189PR.pdf (accessed on 30 August 2022).
- Xiong, X.; Lin, W.; Gu, A. Design and optimization of offshore natural gas liquefaction processes adopting PLNG (pressurized liquefied natural gas) technology. J. Nat. Gas Sci. Eng. 2016, 30, 379–387. [Google Scholar] [CrossRef]
- Lee, S.H.; Seo, Y.K.; Chang, D.J. Techno-economic Analysis of Acid Gas Removal and Liquefaction for Pressurized LNG. IOP Conf. Ser. Mater. Sci. Eng. 2018, 358, 012066. [Google Scholar] [CrossRef]
- Shen, T.; Gao, T.; Lin, W.; Gu, A. Determination of CO2 Solubility in Saturated Liquid CH4 + N2 and CH4 + C2H6 Mixtures above Atmospheric Pressure. J. Chem. Eng. Data 2012, 57, 2296–2303. [Google Scholar] [CrossRef]
- Xiaochen, H.; Ting, G.; Wensheng, L. Preliminary research on CO2 freeze-out in PLNG process. Cryog. Supercond. 2009, 37, 15–18. [Google Scholar] [CrossRef]
- Nelson, E.D.; Papka, S.D.; Bowen, R.R.; Gentry, M.C.; Leger, A.T. Pressurized LNG: A Paradigm Shift in Gas Transportation. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, Bahrain, 12–15 March 2005. [Google Scholar]
- Papka, S.D.; Gentry, M.C.; Leger, A.T.; Bowen, R.R.; Nelson, E.D. Pressurized LNG: A new technology for gas commercialization. In Proceedings of the Fifteenth International Offshore and Polar Engineering Conference, Seoul, Korea, 19–24 June 2005. [Google Scholar]
- Fairchild, D.P.; Smith, P.P.; Biery, N.E.; Farah, A.M.; Lillig, D.B.; Jackson, T.S.; Sisak, W.J. Pressurized LNG: Prototype container fabrication. In Proceedings of the Fifteenth International Offshore and Polar Engineering Conference, Seoul, Korea, 19–24 June 2005. [Google Scholar]
- Lee, J.H.; Yeol, L.J.; Yoo, S. Development of Innovative LNG Production, Transportation, and Regasification System. In Proceedings of the Offshore Technology Conference, Offshore Technology Conference, Houston, TX, USA, 2–5 May 2011. [Google Scholar] [CrossRef]
- Xiaojun, X.; Wensheng, L.; Anzhong, G. Simulation and optimal design of a natural gas pressurized liquefaction process with gas expansion refrigeration. Nat. Gas Ind. 2013, 33, 97–101. [Google Scholar] [CrossRef]
- Ryan, J.M.; Schaffert, F.W. CO2 recovery by the Ryan/Holmes process. Chem. Eng. Progress 1984, 80, 53–56. [Google Scholar]
- Berstad, D.; Nekså, P.; Anantharaman, R. Low-temperature CO2 Removal from Natural Gas. Energy Procedia 2012, 26, 41–48. [Google Scholar] [CrossRef]
- Lee, J.; Jung, J.; Kim, K. Development of CO2 tolerant LNG production system. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 30 April–3 May 2012. [Google Scholar]
- Zhang, L.; Burgass, R.; Chapoy, A.; Tohidi, B.; Solbraa, E. Measurement and Modeling of CO2 Frost Points in the CO2–Methane Systems. J. Chem. Eng. Data 2011, 56, 2971–2975. [Google Scholar] [CrossRef]
- Xiaojun, X.; Wensheng, L.; Anzhong, G. Feasibility study on cryogenic separation of CH_4-CO_2 binary system. Cryog. Supercond. 2012, 40, 1–4. [Google Scholar] [CrossRef]
- Hart, A.; Gnanendran, N. Cryogenic CO2 capture in natural gas. Energy Procedia 2009, 1, 697–706. [Google Scholar] [CrossRef]
- Maqsood, K.; Pal, J.; Turunawarasu, D.; Pal, A.J.; Ganguly, S. Performance enhancement and energy reduction using hybrid cryogenic distillation networks for purification of natural gas with high CO2 content. Korean J. Chem. Eng. 2014, 31, 1120–1135. [Google Scholar] [CrossRef]
- Lin, W.; Xiong, X.; Gu, A. Optimization and thermodynamic analysis of a cascade PLNG (pressurized liquefied natural gas) process with CO2 cryogenic removal. Energy 2018, 161, 870–877. [Google Scholar] [CrossRef]
- Xiong, X.; Lin, W.; Gu, A. Integration of CO2 cryogenic removal with a natural gas pressurized liquefaction process using gas expansion refrigeration. Energy 2015, 93, 1–9. [Google Scholar] [CrossRef]
- Anzhong, G.; Yumei, S.; Rongshun, W.; Gang, Z. Natural gas liquefaction processes and systems. Cryog. Technol. 2003, 1, 1–6. [Google Scholar]
- Zhengwei, L.S.M.C.L.; Xiaobo, C. Optimal design and analysis on small-scale natural gas liquefaction in nitrogen expander cycle. Cryogenics 2009, 24, 47–51. [Google Scholar] [CrossRef]
- Lü, P.-F.; Lu, Y.W.; Liu, G.L.; Ma, C.F. Parameters Optimization of Small-Scale Natutal Gas Expander Liquefaction Process. K. Cheng Je Wu Li Hsueh Pao/J. Eng. Thermophys. 2011, 32, 1396–1398. [Google Scholar] [CrossRef]
- Cao, W.; Wu, J.; Lu, X.; Lin, W.; Gu, A. Thermodynamic analysis on mixed refrigerant cycle of small scale natural gas liquefaction process in skid-mounted package. J. Chem. Ind. Eng. 2008, 59, 53–59. [Google Scholar]
- He, T.; Ju, Y. Simulation and analysis of liquefaction process for small-scale liquefied natural gas plants in skid-mounted packages. Cryog. Supercond. 2013, 41, 5–9. [Google Scholar] [CrossRef]
- Yang, W.; Cao, X.W.; Sun, L.; Wang, D. Natural gas liquefaction technology research status and progress. Nat. Gas Chem. Ind. 2015, 40, 88–93. [Google Scholar]
- Shan, T.W. Research Framework on FLNG for Operation in China. China Offshore Platf. 2017, 32, 6–10+19. [Google Scholar]
- Lin, W.S.; An-Zhong, G.U.; Zhu, G. Selection for the process for liquefaction plants of natural gas. Vac. Cryog. 2001, 7, 105–109. [Google Scholar]
- Xichong, Y.U.; Xie, B.; Yuxing, L.I.; Liu, M.; Yan, L.I.; Wang, Q.; Zhu, X.; Zhu, J. Optimization of FLNG liquefaction process used for the development of deepwater gas field in the South China Sea. Subsea Pipeline Shipp. 2018, 37, 228–235. [Google Scholar] [CrossRef]
- Hui, P.U.; Chen, J. Study on Comparative Selection of LNG-FPSO Liquefaction Process Schemes. Refrig. Technol. 2011, 31, 31–34. [Google Scholar]



























































Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Wang, J.; Binama, M.; Li, Q.; Cai, W. The Design and Optimization of Natural Gas Liquefaction Processes: A Review. Energies 2022, 15, 7895. https://doi.org/10.3390/en15217895
Gao L, Wang J, Binama M, Li Q, Cai W. The Design and Optimization of Natural Gas Liquefaction Processes: A Review. Energies. 2022; 15(21):7895. https://doi.org/10.3390/en15217895
Chicago/Turabian StyleGao, Lei, Jiaxin Wang, Maxime Binama, Qian Li, and Weihua Cai. 2022. "The Design and Optimization of Natural Gas Liquefaction Processes: A Review" Energies 15, no. 21: 7895. https://doi.org/10.3390/en15217895
APA StyleGao, L., Wang, J., Binama, M., Li, Q., & Cai, W. (2022). The Design and Optimization of Natural Gas Liquefaction Processes: A Review. Energies, 15(21), 7895. https://doi.org/10.3390/en15217895









