Experimental and Numerical Study on the Explosion Dynamics of the Non-Uniform Liquefied Petroleum Gas and Air Mixture in a Channel with Mixed Obstacles
Abstract
:1. Introduction
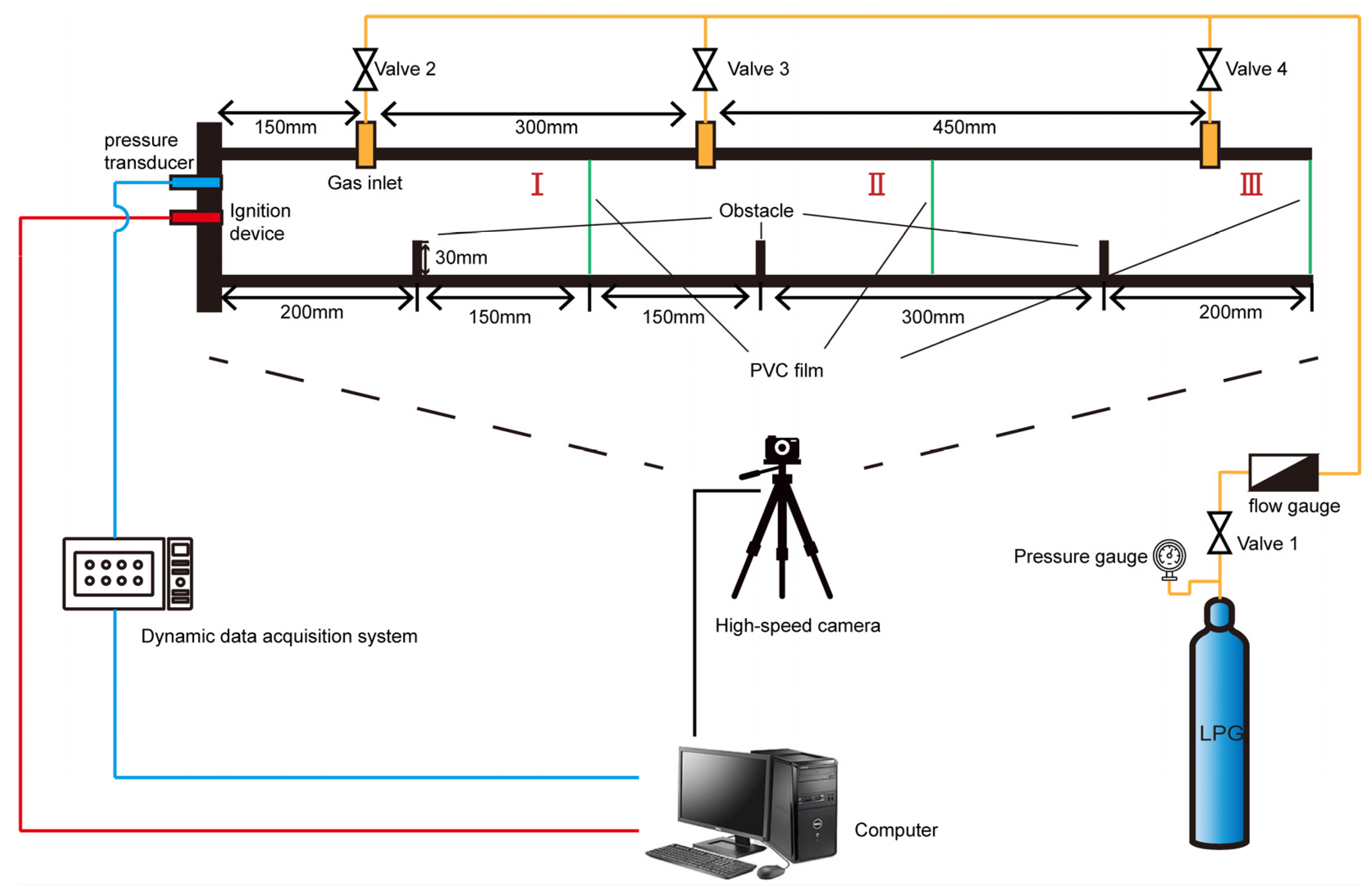
2. Experiment Setup
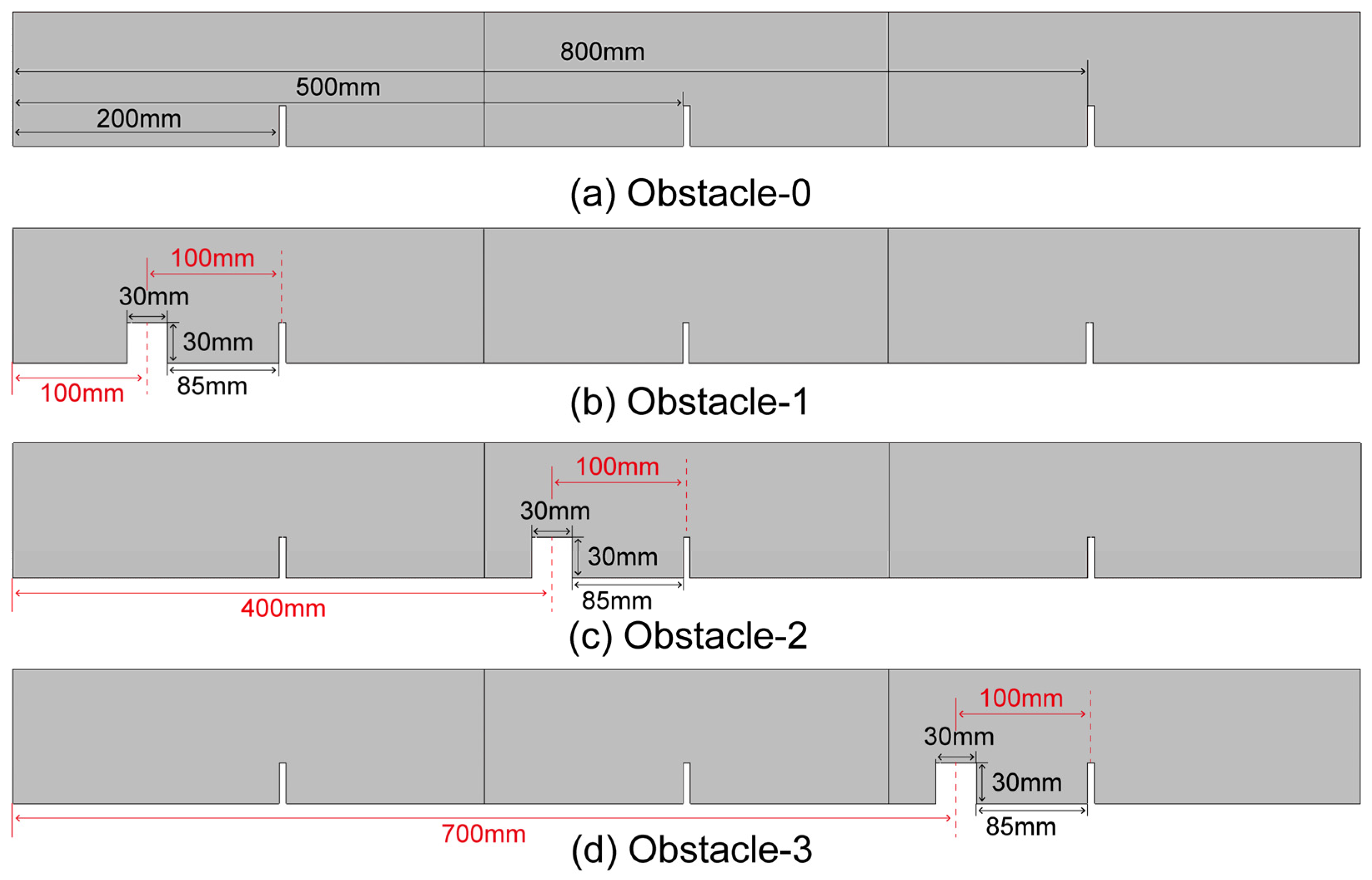
2.1. Geometrical Structure of the Square Duct with Flat Obstacles
2.2. Experimental Methods
3. Numerical Model
3.1. Materials
3.2. Boundary and Initial Conditions
4. Results and Discussion
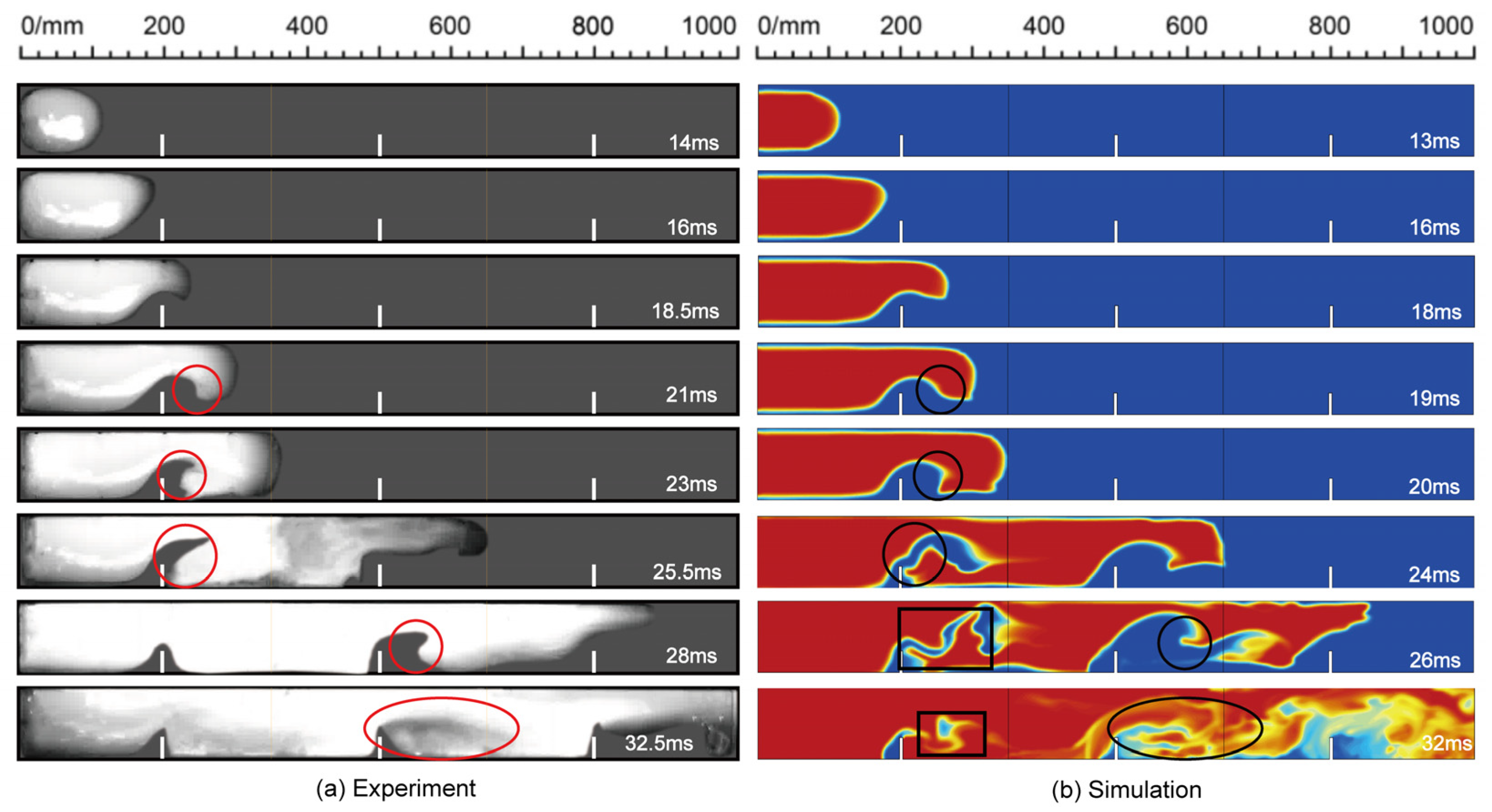
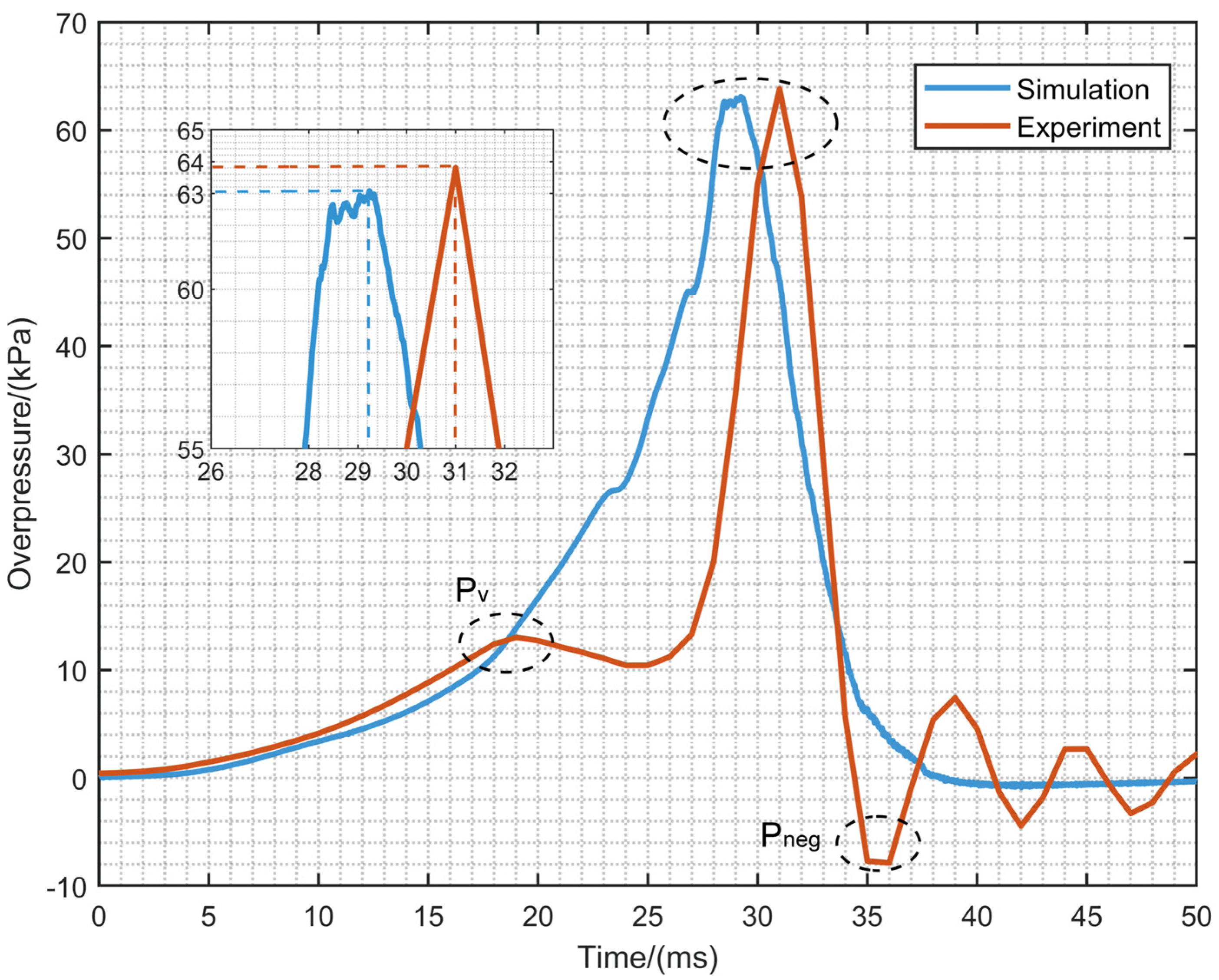
4.1. Numerical Verification
4.2. Model with Rectangular Obstacle
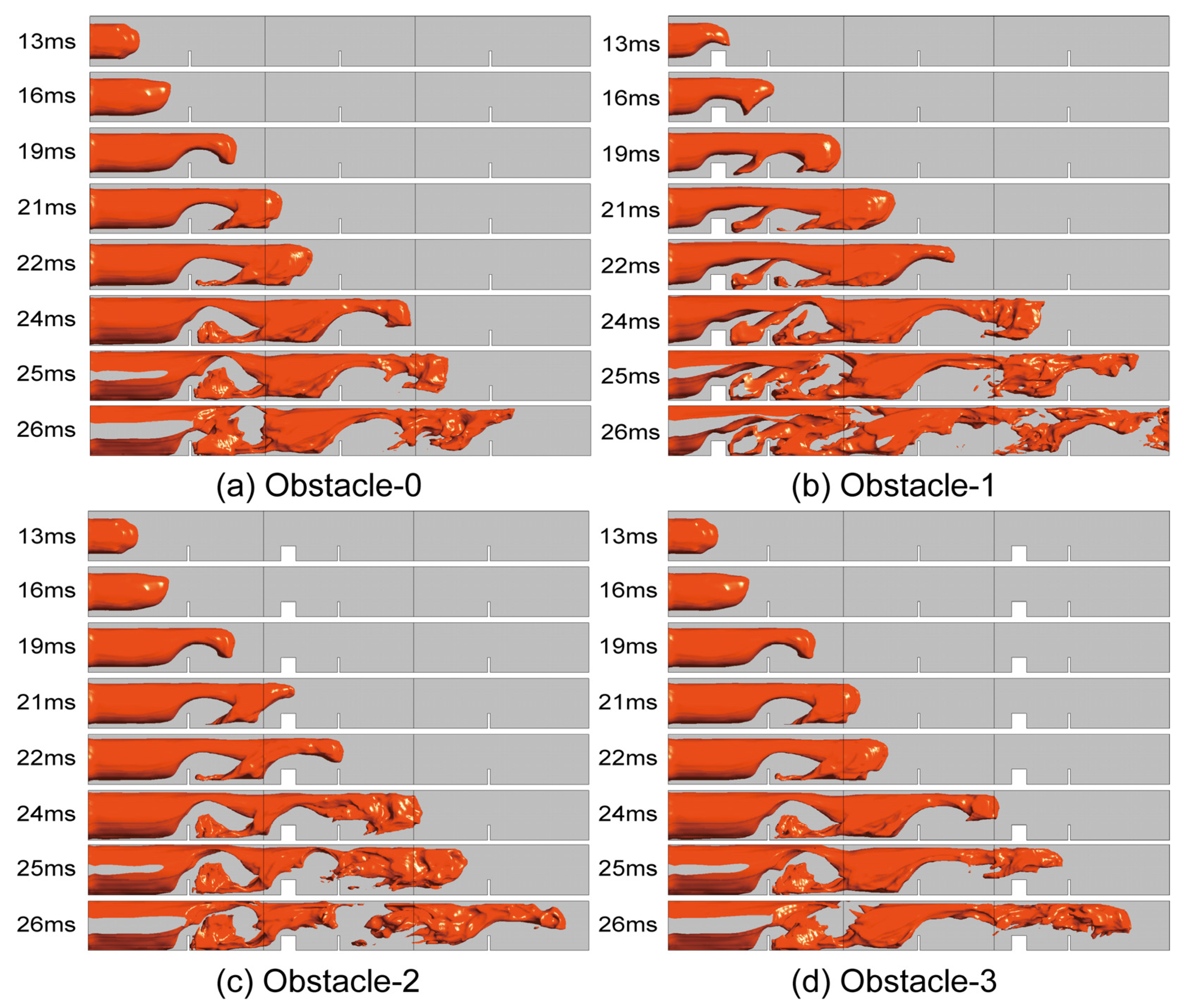
4.3. The Structure of Flame
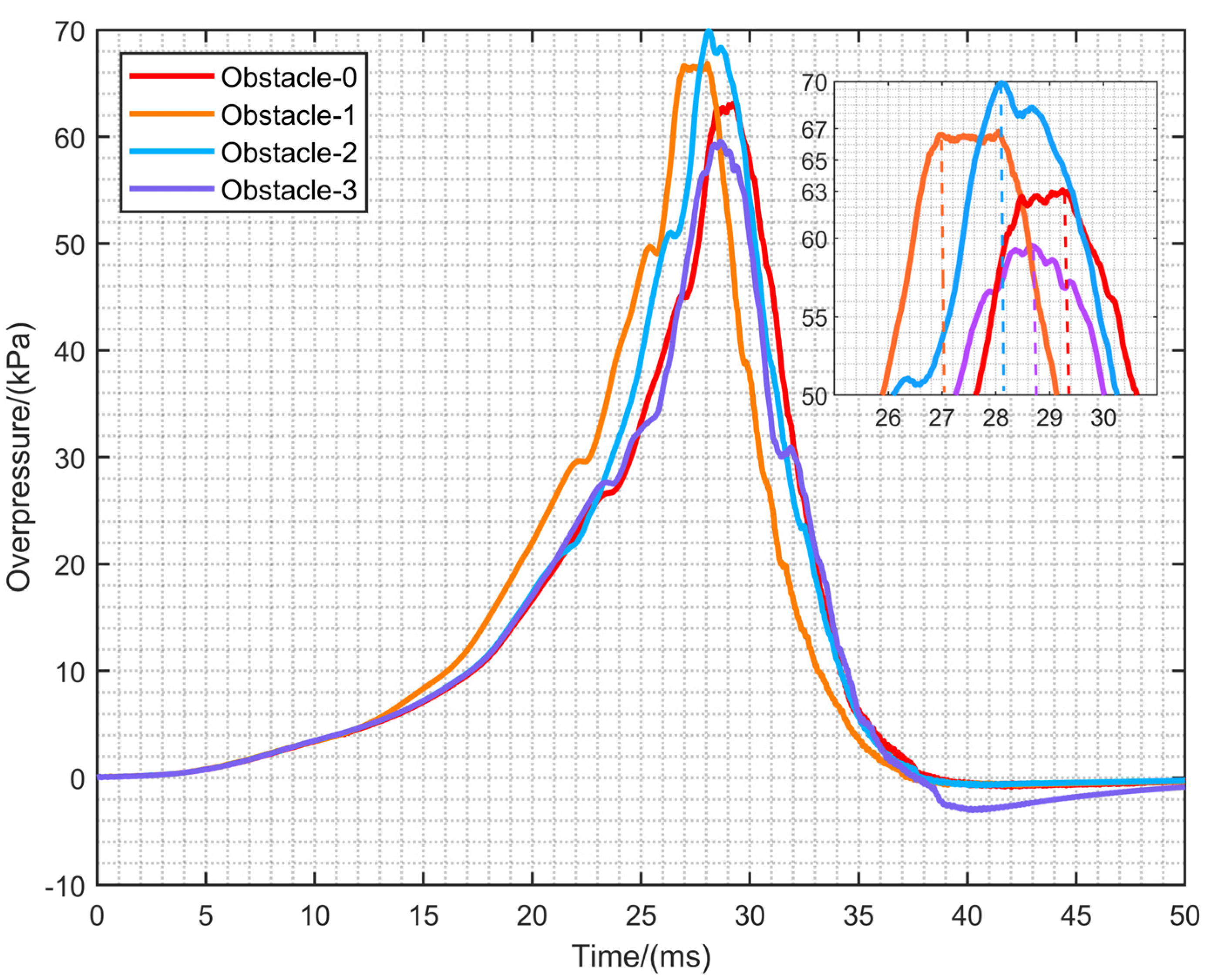
4.4. The Overpressure Dynamics
5. Conclusions
- (1)
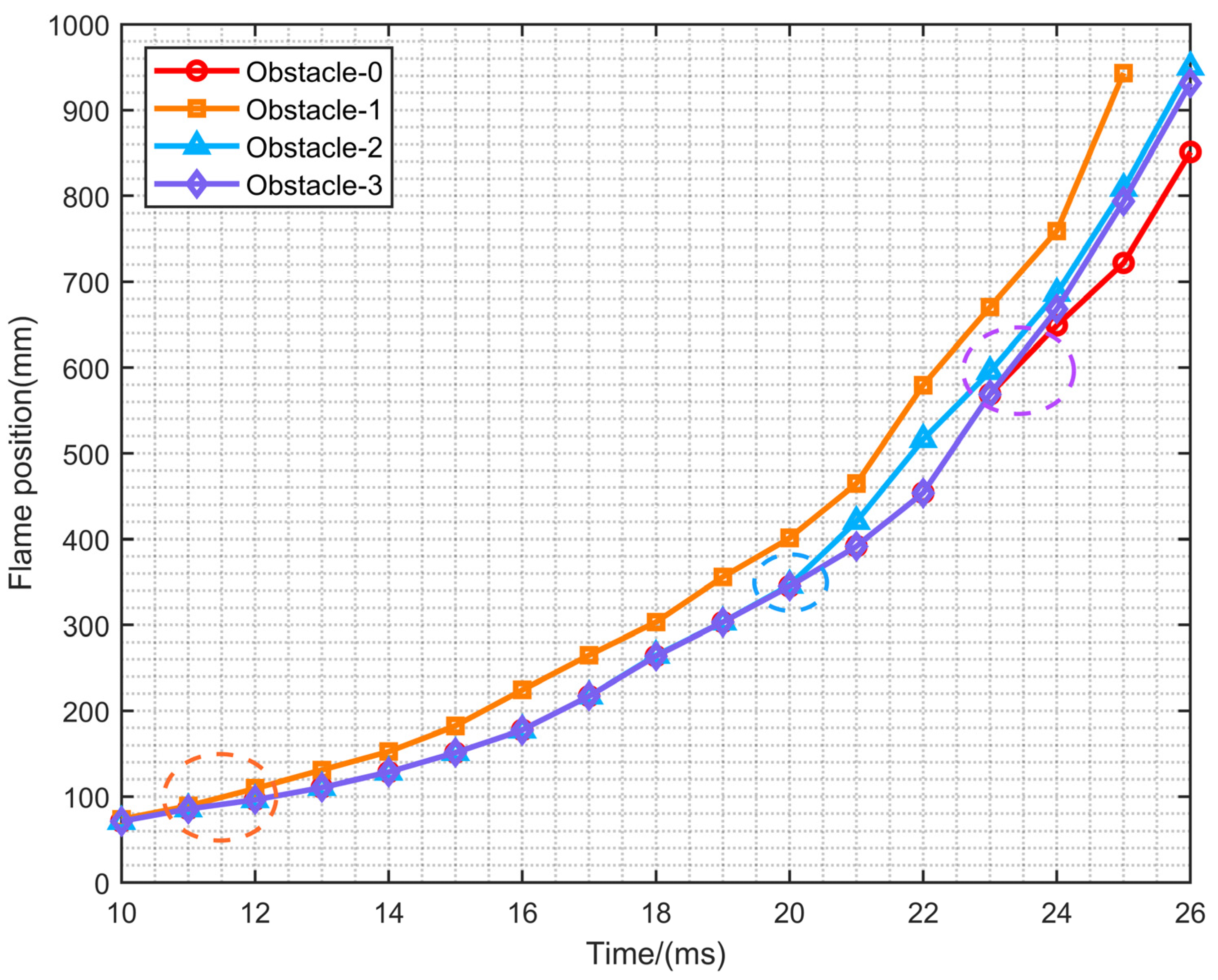
- When the rectangular obstacle is 100 mm away from the ignition source, a slender flame appeared between the rectangular obstacle and the first flat obstacle, which leads to the final formation of a special reflux structure in the duct. The lateral branch of the flame between obstacles is the most unique, and appears in this configuration. The flame is the first to rush out of the duct. After that, Obstacle-2 and Obstacle-3 rush out of the duct successively. The flame of Obstacle-0 without a rectangular obstacle is the last to exit the pipe. This result is consistent with the order of contact with the rectangular obstacle.
- (2)
- The closer the rectangular obstruction is to the ignition source, the shorter the time required to reach the maximum overpressure. However, the maximum overpressure is obtained by rectangular obstacles in a moderate position, namely Obstacle-2. In addition, when the maximum flame area occurs, the overpressure value also reaches the maximum, and there is a close relationship between the two.
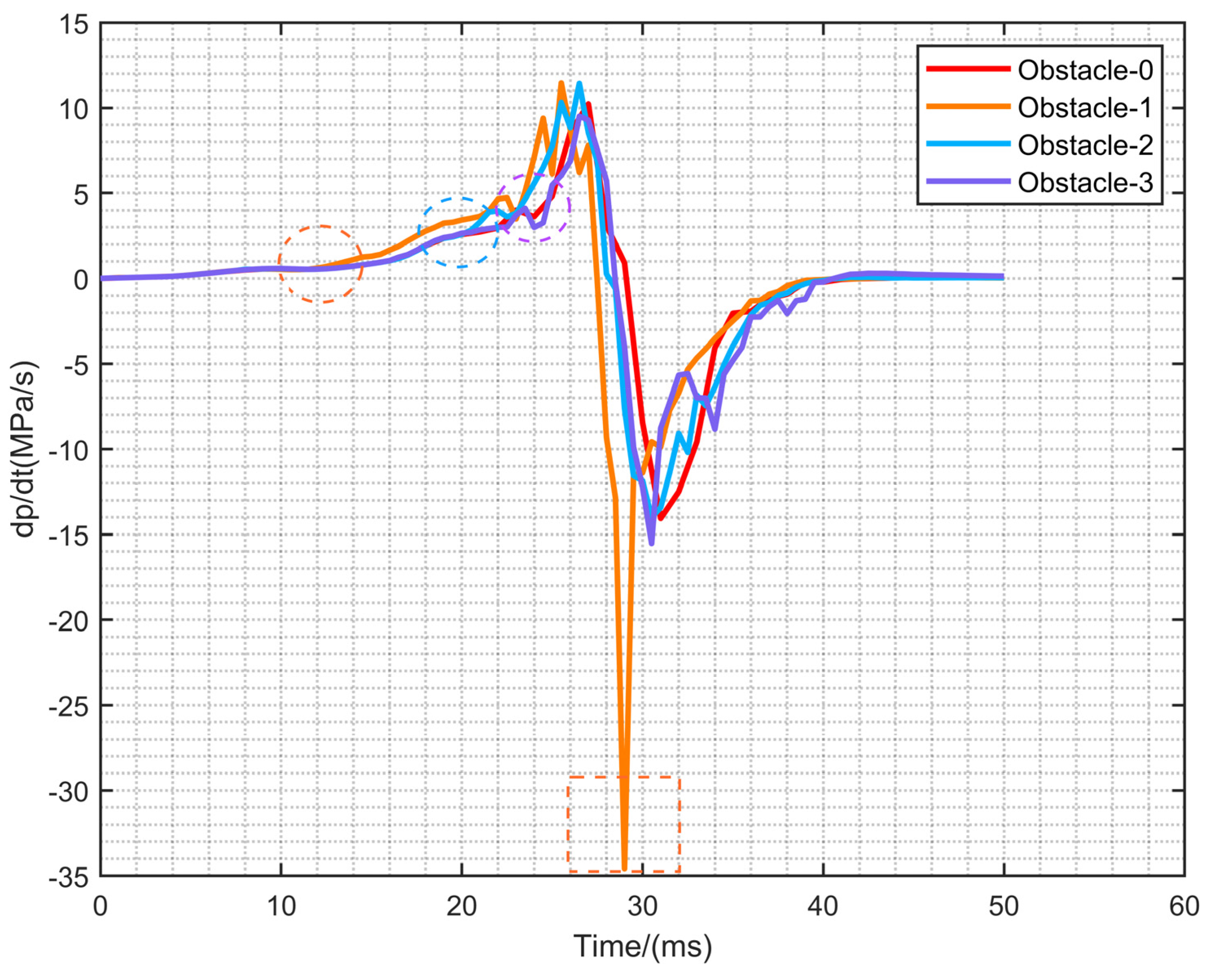
- (3)
- The growth rate of overpressure increases significantly after contact with the rectangular obstacle. The moment coincides with the mutation time of the front tip of the flame, overpressure and area of flame after the flame touches the rectangular obstacle. With Obstacle-1, the rectangular obstacle closest to the ignition source, the overpressure growth rate has the fastest decline, reaching −35 MPa/s, which is much less than the other three configurations.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, G.; Hong, B.; Hu, H.; Shao, B.; Jiang, W.; Li, C.; Guo, J. Risk Management of Island Petrochemical Park: Accident Early Warning Model Based on Artificial Neural Network. Energies 2022, 15, 3278. [Google Scholar] [CrossRef]
- Guo, J.; Wang, J.; Zhu, B.; Hong, B.; Li, C.; He, J.; Chen, M.H. A Risk Evaluation Method of Coastal Oil Depots for Heavy Rainfall Vulnerability Assessment. Sustainability 2022, 14, 6902. [Google Scholar] [CrossRef]
- Biezma, M.; Andrés, M.; Agudo, D.; Briz, E. Most fatal oil & gas pipeline accidents through history: A lessons learned approach. Eng. Fail. Anal. 2020, 110, 104446. [Google Scholar] [CrossRef]
- Hao, B.; Gao, J.; Guo, B.; Ai, B.; Hong, B.; Jiang, X. Numerical Simulation of Premixed Methane–Air Explosion in a Closed Tube with U-Type Obstacles. Energies 2022, 15, 4909. [Google Scholar] [CrossRef]
- Wang, J.; Fan, Z.; Wu, Y.; Zheng, L.; Pan, R.; Wang, Y. Effect of abrupt changes in the cross-sectional area of a pipe on flame propagation characteristics of CH4/air mixtures. ACS Omega 2021, 6, 15126–15135. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Luo, Z.; Cheng, F.; Wang, T.; Lin, H.; Liu, H. A comparative investigation of premixed flame propagating of combustible gases-methane mixtures across an obstructed closed tube. Fuel 2020, 289, 119766. [Google Scholar] [CrossRef]
- Elshimy, M.; Ibrahim, S.; Malalasekera, W. Numerical studies of premixed hydrogen/air flames in a small-scale combustion chamber with varied area blockage ratio. Int. J. Hydrogen Energy 2020, 45, 14979–14990. [Google Scholar] [CrossRef]
- Sheng, Z.; Yang, G.; Li, S.; Shen, Q.; Sun, H.; Jiang, Z.; Liao, J.; Wang, H. Modeling of turbulent deflagration behaviors of premixed hydrogen-air in closed space with obstacles. Process Saf. Environ. Prot. 2022, 161, 506–519. [Google Scholar] [CrossRef]
- Luo, G.; Tu, J.-Q.; Qian, Y.-L.; Jin, K.-K.; Ye, T.-J.; Bai, Y.; Gao, S. Impacts of Rectangular Obstacle Lengths on Premixed Methane–Air Flame Propagation in a Closed Tube. Combust. Explos. Shock Waves 2022, 58, 10–21. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Wang, Z.; Tian, W.; Wang, Z. The effect of orifice plates with different shapes on explosion propagation of premixed methane–air in a semi-confined pipeline. J. Loss Prev. Process Ind. 2021, 71, 104498. [Google Scholar] [CrossRef]
- Huang, C.; Chen, X.; Liu, L.; Zhang, H.; Yuan, B.; Li, Y. The influence of opening shape of obstacles on explosion characteristics of premixed methane-air with concentration gradients. Process Saf. Environ. Prot. 2021, 150, 305–313. [Google Scholar] [CrossRef]
- Fang, H.; Xue, H.; Tang, W. Blast wave propagation characteristics in FPSO: Effect of cubical obstacles. Ocean Eng. 2022, 250, 111022. [Google Scholar] [CrossRef]
- Eduardo, F.-T.; Mario, S.-S.; Antonio, L.S.; Forman, A.W. Minimum ignition energy of methanol-air mixtures. Combust. Flame 2016, 171, 234–236. [Google Scholar]
- Xing, H.; Qiu, Y.; Sun, S.; Wang, M.; Li, B.; Xie, L. Experimental study of overpressure and temperature field behaviors of a methane-air mixture with different ignition positions, solid structure obstacles and initial turbulence levels. Fuel 2020, 287, 119446. [Google Scholar] [CrossRef]
- Zhou, N.; Mei, Y.; Li, X.; Chen, B.; Huang, W.-Q.; Zhao, H.-J.; Yuan, X.-J. Numerical simulation of the influence of vent conditions on the characteristics of hydrogen explosion in confined space. Combust. Theory Model. 2021, 26, 241–259. [Google Scholar] [CrossRef]
- Ahumada, C.B.; Wang, Q.; Petersen, E.L. Effects of unequal blockage ratio and obstacle spacing on wave speed and overpressure during flame propagation in stoichiometric H2/O2. Shock. Waves 2020, 30, 755–767. [Google Scholar] [CrossRef]
- Dai, Q.; Zhang, S.; Zhang, S.; Sun, H.; Huang, M. Large Eddy Simulation of Premixed CH4/Air Deflagration in a Duct with Obstacles at Different Heights. ACS Omega 2021, 6, 27140–27149. [Google Scholar] [CrossRef]
- Jia, H.; Cui, B.; Duan, Y.; Zheng, K. Study on the Influence of Vent Shape and Blockage Ratio on the Premixed Gas Explosion in the Chamber with a Small Aspect Ratio. ACS Omega 2022, 7, 22787–22796. [Google Scholar] [CrossRef]
- Boeck, L.; Lapointe, S.; Melguizo-Gavilanes, J.; Ciccarelli, G. Flame propagation across an obstacle: OH-PLIF and 2-D simulations with detailed chemistry. Proc. Combust. Inst. 2017, 36, 2799–2806. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Du, Y.; Liang, J.; Wang, S.; Wang, B.; Qi, S. Characteristics of gasoline–air mixture explosions with different obstacle configurations. J. Energy Inst. 2018, 91, 194–202. [Google Scholar] [CrossRef]
- Wen, X.; Ding, H.; Su, T.; Wang, F.; Deng, H.; Zheng, K. Effects of obstacle angle on methane–air deflagration characteristics in a semi-confined chamber. J. Loss Prev. Process Ind. 2017, 45, 210–216. [Google Scholar] [CrossRef]
- Yang, X.; Yu, M.; Zheng, K.; Luan, P.; Han, S. An experimental study on premixed syngas/air flame propagating across an obstacle in closed duct. Fuel 2020, 267, 117200. [Google Scholar] [CrossRef]
- Na’Inna, A.M.; Somuano, G.B.; Phylaktou, H.N.; Andrews, G.E. Flame acceleration in tube explosions with up to three flat-bar obstacles with variable obstacle separation distance. J. Loss Prev. Process Ind. 2015, 38, 119–124. [Google Scholar] [CrossRef]
- Palymskiy, I.B.; Fomin, P.A.; Gharehdash, S. On control of convection intensity of the reacting equilibrium gas. Comput. Therm. Sci. Int. J. 2019, 11, 297–314. [Google Scholar] [CrossRef]
- Heilbronn, D.; Barfuss, C.; Sattelmayer, T. Deflagration-to-detonation transition in H2-CO-Air mixtures in a partially obstructed channel. Int. J. Hydrogen Energy 2021, 46, 12372–12383. [Google Scholar] [CrossRef]
- Saeid, M.H.S.; Khadem, J.; Emami, S. Numerical investigation of the mechanism behind the deflagration to detonation transition in homogeneous and inhomogeneous mixtures of H2-air in an obstructed channel. Int. J. Hydrogen Energy 2021, 46, 21657–21671. [Google Scholar] [CrossRef]
- Rudy, W.; Teodorczyk, A. Numerical Simulations of DDT Limits in Hydrogen-Air Mixtures in Obstacle Laden Channel. Energies 2020, 14, 24. [Google Scholar] [CrossRef]
- Liu, D.; Liu, Z.; Xiao, H. Flame acceleration and deflagration-to-detonation transition in narrow channels filled with stoichiometric hydrogen-air mixture. Int. J. Hydrogen Energy 2022, 47, 11052–11067. [Google Scholar] [CrossRef]
- Xiao, H.; Oran, E.S. Flame acceleration and deflagration-to-detonation transition in hydrogen-air mixture in a channel with an array of obstacles of different shapes. Combust. Flame 2020, 220, 378–393. [Google Scholar] [CrossRef]
- Coates, A.M.; Mathias, D.L.; Cantwell, B.J. Numerical investigation of the effect of obstacle shape on deflagration to detonation transition in a hydrogen–air mixture. Combust. Flame 2019, 209, 278–290. [Google Scholar] [CrossRef]
- Leo, Y.; Zhang, B. Explosion behavior of methane-air mixtures and Rayleigh-Taylor instability in the explosion process near the flammability limits. Fuel 2022, 324, 124730. [Google Scholar] [CrossRef]
- Luo, Z.; Kang, X.; Wang, T.; Su, B.; Cheng, F.; Deng, J. Effects of an obstacle on the deflagration behavior of premixed liquefied petroleum gas-air mixtures in a closed duct. Energy 2021, 234, 121291. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, C.; Xiu, G.; Zhu, H. Evolution of premixed stoichiometric hydrogen/air flame in a closed duct. Energy 2019, 176, 265–271. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, X. Study on the dynamic process of in-duct hydrogen-air explosion flame propagation under different blocking rates. Int. J. Hydrogen Energy 2022, 47, 18857–18876. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, X. Flame propagation of premixed hydrogen-air explosion in a closed duct with obstacles. Int. J. Hydrogen Energy 2020, 46, 2684–2701. [Google Scholar] [CrossRef]
- Zheng, K.; Song, C.; Yang, X.; Wu, J.; Jiang, J.; Xing, Z. Effect of obstacle location on explosion dynamics of premixed H2/CO/air mixtures in a closed duct. Fuel 2022, 324, 124703. [Google Scholar] [CrossRef]
- Xiao, G.; Wang, S.; Mi, H.; Khan, F. Analysis of obstacle shape on gas explosion characteristics. Process Saf. Environ. Prot. 2022, 161, 78–87. [Google Scholar] [CrossRef]
- Nguyen, T.; Strebinger, C.; Bogin, G.; Brune, J. A 2D CFD model investigation of the impact of obstacles and turbulence model on methane flame propagation. Process Saf. Environ. Prot. 2020, 146, 95–107. [Google Scholar] [CrossRef]
- Han, S.; Yu, M.; Yang, X.; Li, H.; Ma, Z. Flame propagation mode transition of premixed syngas-air mixtures in a closed duct. Fuel 2022, 318, 123649. [Google Scholar] [CrossRef]
- Li, Y.; Bi, M.; Li, B.; Zhou, Y.; Gao, W. Effects of hydrogen and initial pressure on flame characteristics and explosion pressure of methane/hydrogen fuels. Fuel 2018, 233, 269–282. [Google Scholar] [CrossRef]
- Azadboni, R.K.; Wen, J.X.; Heidari, A.; Wang, C. Numerical modeling of deflagration to detonation transition in inhomogeneous hydrogen/air mixtures. J. Loss Prev. Process Ind. 2017, 49, 722–730. [Google Scholar] [CrossRef]
- Gao, J.; Ai, B.; Hao, B.; Guo, B.; Hong, B.; Jiang, X. Effect of Obstacles Gradient Arrangement on Non-Uniformly Distributed LPG–Air Premixed Gas Deflagration. Energies 2022, 15, 6872. [Google Scholar] [CrossRef]
- Wu, Q.; Yu, M.; Zheng, K. Experimental investigation on the effect of obstacle position on the explosion behaviors of the non-uniform methane/air mixture. Fuel 2022, 320, 123989. [Google Scholar] [CrossRef]
- Zheng, K.; Wu, Q.; Chen, C.; Xing, Z.; Hao, Y.; Yu, M. Explosion behavior of non-uniform methane/air mixture in an obstructed duct with different blockage ratios. Energy 2022, 255, 124603. [Google Scholar] [CrossRef]
- Li, J.; Jiang, X.; Li, J.; YU, B.; Zhang, L.; Zhao, Y. Study on influence of length-diameter ratio on explosion characteristics and flame propagation laws of gasoline-air mixture in pipeline. J. Saf. Sci. Technol. 2020, 16, 88–94. [Google Scholar]
- Chen, P.; Sun, Y.; Li, Y.; Luo, G. Experimental and LES investigation of premixed methane/air flame propagating in an obstructed chamber with two slits. J. Loss Prev. Process Ind. 2017, 49, 711–721. [Google Scholar] [CrossRef]
- Chen, P.; Guo, S.; Li, Y.; Zhang, Y. Experimental and LES investigation of premixed methane/air flame propagating in a tube with a thin obstacle. Combust. Theory Model. 2016, 21, 274–292. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Lian, Z. Explosion hazards of LPG-air mixtures in vented enclosure with obstacles. J. Hazard. Mater. 2017, 334, 59–67. [Google Scholar] [CrossRef]
- Chen, P.; Li, Y.; Huang, F.; Guo, S.; Liu, X. Experimental and LES investigation of premixed methane/air flame propagating in a chamber for three obstacle BR configurations. J. Loss Prev. Process Ind. 2016, 41, 48–54. [Google Scholar] [CrossRef]
- Gharehdash, S.; Sainsbury, B.-A.L.; Barzegar, M.; Palymskiy, I.B.; Fomin, P.A. Field Scale Modelling of Explosion-Generated Crack Densities in Granitic Rocks Using Dual-Support Smoothed Particle Hydrodynamics (DS-SPH). Rock Mech. Rock Eng. 2021, 54, 4419–4454. [Google Scholar] [CrossRef]
- Charlette, F.; Meneveau, C.; Veynante, D. A power-law flame wrinkling model for LES of premixed turbulent combustion Part I: Non-dynamic formulation and initial tests. Combust. Flame 2002, 131, 159–180. [Google Scholar] [CrossRef]
- Pan, C.; Wang, X.; Sun, H.; Zhu, X.; Zhao, J.; Fan, H.; Liu, Y. Large-eddy simulation and experimental study on effects of single-dual sparks positions on vented explosions in a channel. Fuel 2022, 322, 124282. [Google Scholar] [CrossRef]
- Li, G.; Du, Y.; Wang, S.; Qi, S.; Zhang, P.; Chen, W. Large eddy simulation and experimental study on vented gasoline-air mixture explosions in a semi-confined obstructed pipe. J. Hazard. Mater. 2017, 339, 131–142. [Google Scholar] [CrossRef] [PubMed]




| Propane Concentration (%) | Heat of Combustion (J/kg) | Laminar Flame Speed (m/s) | Unburnt Fuel Mass Fraction |
|---|---|---|---|
| 4.80 | 6.0485 × 107 | 0.37 | 0.0845 |
| 4.03 | 5.0375 × 107 | 0.38 | 0.0602 |
| 3.84 | 4.7884 × 107 | 0.36 | 0.0547 |
| Boundary | Acoustic Wave Model | Shear Condition | Thermal | Species |
|---|---|---|---|---|
| Pressure-Outlet | Non-Reflection | / | 300 K | 0 |
| Wall | / | No-Slip | 0 W/m2 | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, B.; Gao, J.; Hao, B.; Ai, B.; Hong, B.; Jiang, X. Experimental and Numerical Study on the Explosion Dynamics of the Non-Uniform Liquefied Petroleum Gas and Air Mixture in a Channel with Mixed Obstacles. Energies 2022, 15, 7999. https://doi.org/10.3390/en15217999
Guo B, Gao J, Hao B, Ai B, Hong B, Jiang X. Experimental and Numerical Study on the Explosion Dynamics of the Non-Uniform Liquefied Petroleum Gas and Air Mixture in a Channel with Mixed Obstacles. Energies. 2022; 15(21):7999. https://doi.org/10.3390/en15217999
Chicago/Turabian StyleGuo, Bingang, Jianfeng Gao, Bin Hao, Bingjian Ai, Bingyuan Hong, and Xinsheng Jiang. 2022. "Experimental and Numerical Study on the Explosion Dynamics of the Non-Uniform Liquefied Petroleum Gas and Air Mixture in a Channel with Mixed Obstacles" Energies 15, no. 21: 7999. https://doi.org/10.3390/en15217999






