Preparation of N-, O-, and S-Tri-Doped Biochar through One-Pot Pyrolysis of Poplar and Urea Formaldehyde and Its Enhanced Removal of Tetracycline from Wastewater
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Biochar with Hybrid Doping of N, O, and S
2.3. Characterization of Biochar with Hybrid Doping of N, O, and S
2.4. Batch TC Adsorption Experiments
3. Results and Discussion
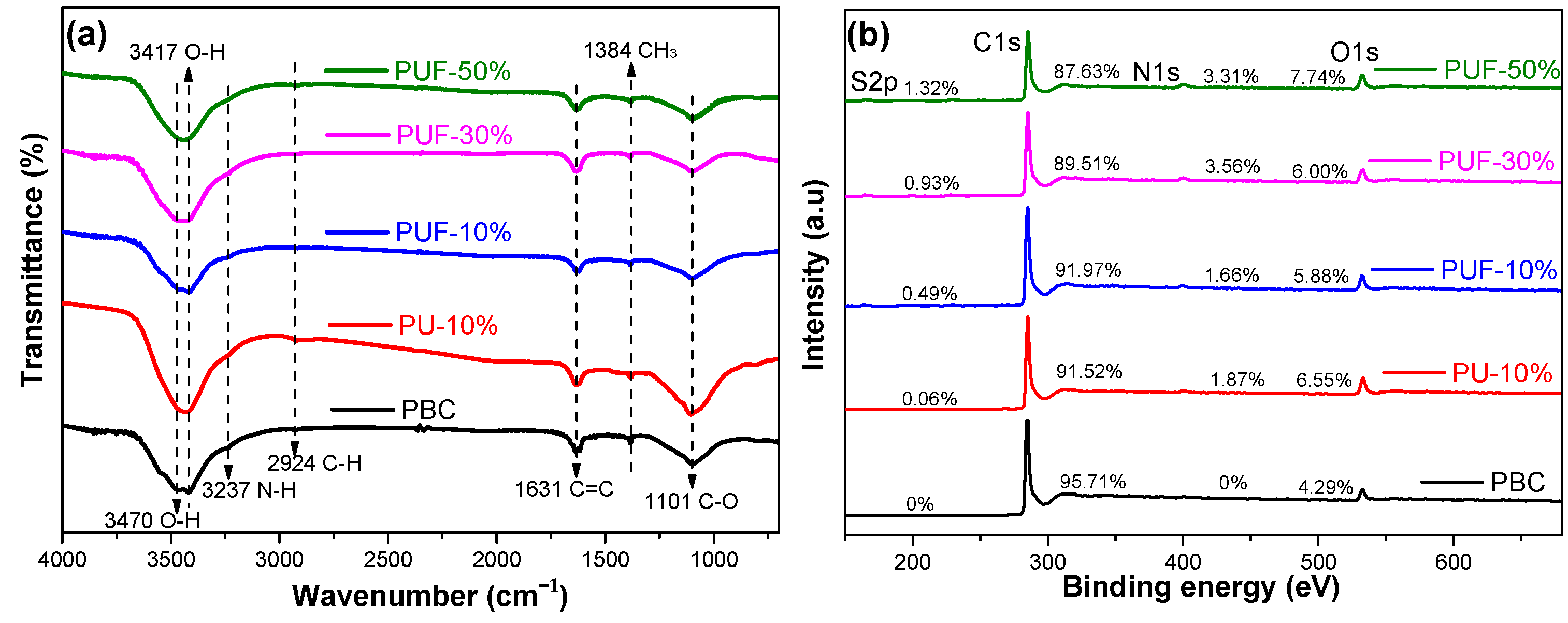
3.1. Characteristics of Biochar with Hybrid Doping of N, O, and S
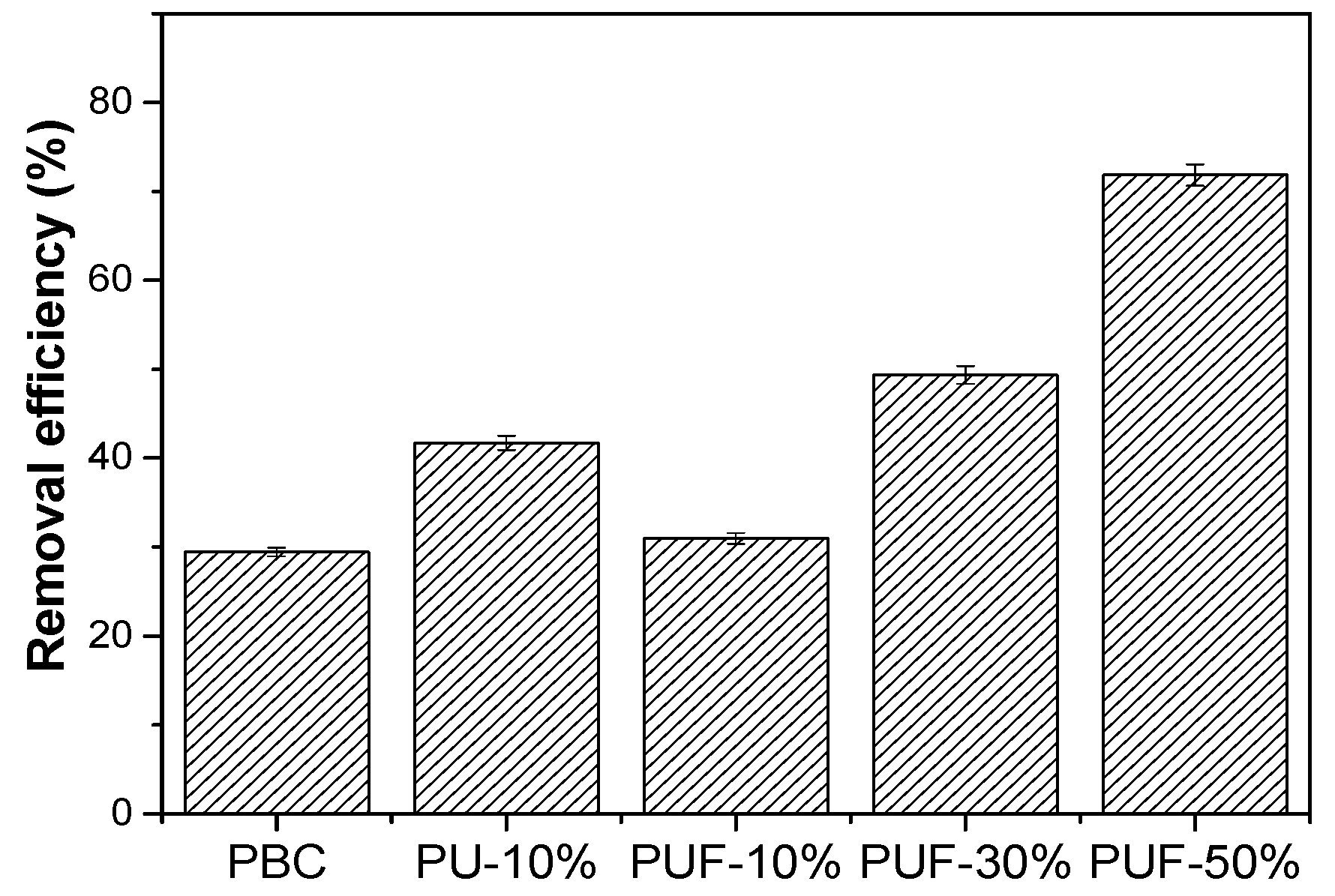
3.2. TC Adsorption Performance of Biochar with Hybrid Doping of N, O, and S
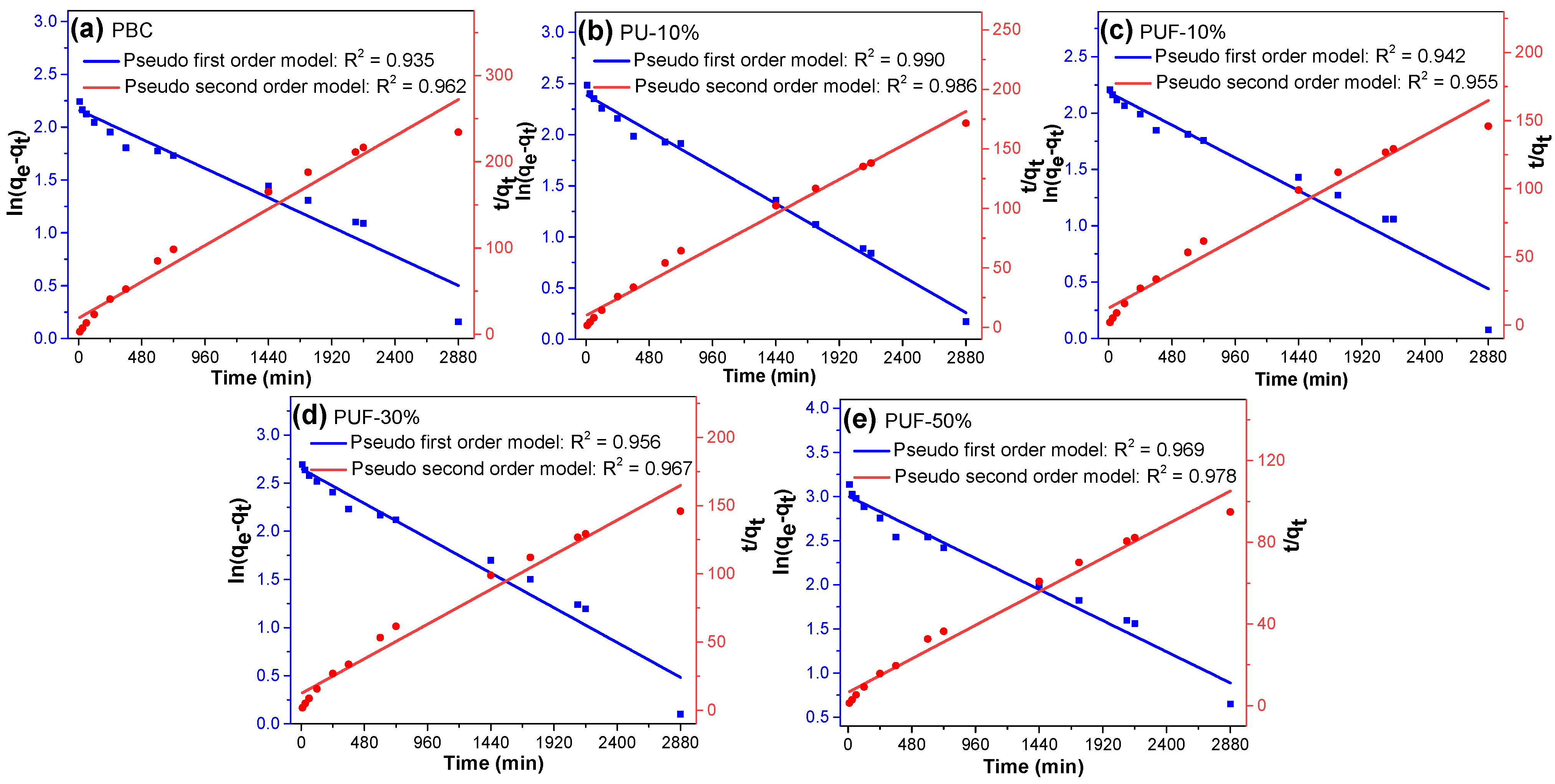
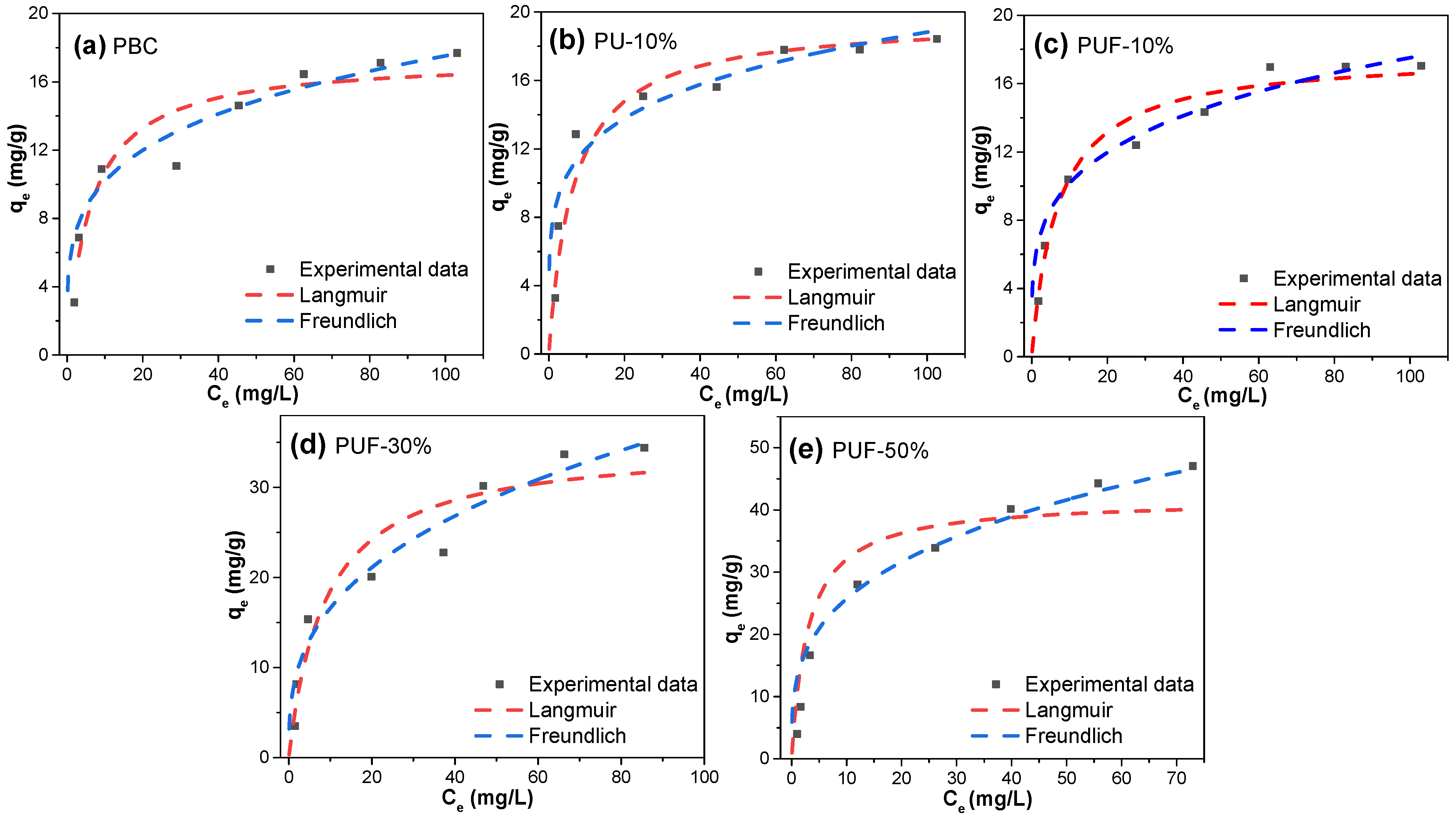
3.3. Adsorption Isotherms and Kinetics
3.4. Further Discussion on the TC Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Xing, B.; Wang, X.; Wang, K.; Zhu, L.; Wang, S. Nitrogen-doped hierarchical porous biochar derived from corn stalks for phenol-enhanced adsorption. Energy Fuels 2019, 33, 12459–12468. [Google Scholar] [CrossRef]
- Cha, J.S.; Park, S.H.; Jung, S.-C.; Ryu, C.; Jeon, J.-K.; Shin, M.-C.; Park, Y.-K. Production and utilization of biochar: A review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Wang, T.; Xue, L.; Liu, Y.; Zhang, L.; Xing, B. N self-doped hierarchically porous carbon derived from biomass as an efficient adsorbent for the removal of tetracycline antibiotics. Sci. Total Environ. 2022, 822, 153567. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Guo, Z.; Zhao, C.; Xu, J. Removal of Cr(VI) from aqueous media by biochar derived from mixture biomass precursors of Acorus calamus Linn. and feather waste. J. Anal. Appl. Pyrolysis 2019, 140, 86–92. [Google Scholar] [CrossRef]
- Hou, Y.; Huang, G.; Li, J.; Yang, Q.; Huang, S.; Cai, J. Hydrothermal conversion of bamboo shoot shell to biochar: Preliminary studies of adsorption equilibrium and kinetics for rhodamine B removal. J. Anal. Appl. Pyrolysis 2019, 143, 104694. [Google Scholar] [CrossRef]
- Song, B.; Cao, X.; Gao, W.; Aziz, S.; Gao, S.; Lam, C.-H.; Lin, R. Preparation of nano-biochar from conventional biorefineries for high-value applications. Renew. Sustain. Energy Rev. 2022, 157, 112057. [Google Scholar] [CrossRef]
- González-Hourcade, M.; Simões dos Reis, G.; Grimm, A.; Dinh, V.M.; Lima, E.C.; Larsson, S.H.; Gentili, F.G. Microalgae biomass as a sustainable precursor to produce nitrogen-doped biochar for efficient removal of emerging pollutants from aqueous media. J. Clean. Prod. 2022, 348, 131280. [Google Scholar] [CrossRef]
- Xu, G.; Han, J.; Ding, B.; Nie, P.; Pan, J.; Dou, H.; Li, H.; Zhang, X. Biomass-derived porous carbon materials with sulfur and nitrogen dual-doping for energy storage. Green Chem. 2015, 17, 1668–1674. [Google Scholar] [CrossRef]
- Yang, G.; Mo, S.; Xing, B.; Dong, J.; Song, X.; Liu, X.; Yuan, J. Effective degradation of phenol via catalytic wet peroxide oxidation over N, S, and Fe-tridoped activated carbon. Environ. Pollut. 2020, 258, 113687. [Google Scholar] [CrossRef]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 56. [Google Scholar] [CrossRef]
- Feng, D.; Guo, D.; Zhang, Y.; Sun, S.; Zhao, Y.; Shang, Q.; Sun, H.; Wu, J.; Tan, H. Functionalized construction of biochar with hierarchical pore structures and surface O-/N-containing groups for phenol adsorption. Chem. Eng. J. 2021, 410, 127707. [Google Scholar] [CrossRef]
- Wang, X.-B.; Yang, S.-Q.; Xu, C.; Ma, H.-D.; Zhang, Z.-H.; Du, Z.-Y.; Li, W.-Y. Effect of boron doping on the performance of Ni/Biochar catalysts for steam reforming of toluene as a tar model compound. J. Anal. Appl. Pyrolysis 2021, 155, 105033. [Google Scholar] [CrossRef]
- Li, Z.; Xing, B.; Ding, Y.; Li, Y.; Wang, S. A high-performance biochar produced from bamboo pyrolysis with in-situ nitrogen doping and activation for adsorption of phenol and methylene blue. Chin. J. Chem. Eng. 2020, 28, 2872–2880. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, B.; Shen, J.; Yan, P.; Kang, J.; Wang, W.; Bi, L.; Zhu, X.; Li, Y.; Wang, S.; et al. Preparation of novel N-doped biochar and its high adsorption capacity for atrazine based on pi-pi electron donor-acceptor interaction. J. Hazard. Mater. 2022, 432, 128757. [Google Scholar] [CrossRef]
- Dinh, V.C.; Hou, C.H.; Dao, T.N. O, N-doped porous biochar by air oxidation for enhancing heavy metal removal: The role of O, N functional groups. Chemosphere 2022, 293, 133622. [Google Scholar] [CrossRef]
- Zhou, Y.; Tan, P.; He, Z.; Zhang, C.; Fang, Q.; Chen, G. CO2 adsorption performance of nitrogen-doped porous carbon derived from licorice residue by hydrothermal treatment. Fuel 2022, 311, 122507. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, M.; Ma, L.; Ren, J.; Ma, P.; Li, B.; Wu, N.; Song, Z.; Huang, L. Nitrogen and sulfur codoped micro-mesoporous carbon sheets derived from natural biomass for synergistic removal of chromium(VI): Adsorption behavior and computing mechanism. Sci. Total Environ. 2020, 730, 138930. [Google Scholar] [CrossRef]
- Guo, R.; Yan, L.; Rao, P.; Wang, R.; Guo, X. Nitrogen and sulfur co-doped biochar derived from peanut shell with enhanced adsorption capacity for diethyl phthalate. Environ. Pollut. 2020, 258, 113674. [Google Scholar] [CrossRef]
- Jang, H.M.; Yoo, S.; Choi, Y.K.; Park, S.; Kan, E. Adsorption isotherm, kinetic modeling and mechanism of tetracycline on Pinus taeda-derived activated biochar. Bioresour. Technol. 2018, 259, 24–31. [Google Scholar] [CrossRef]
- Veiga, P.A.d.S.; Schultz, J.; Matos, T.T.d.S.; Fornari, M.R.; Costa, T.G.; Meurer, L.; Mangrich, A.S. Production of high-performance biochar using a simple and low-cost method: Optimization of pyrolysis parameters and evaluation for water treatment. J. Anal. Appl. Pyrolysis 2020, 148, 104823. [Google Scholar] [CrossRef]
- Kasera, N.; Kolar, P.; Hall, S.G. Nitrogen-doped biochars as adsorbents for mitigation of heavy metals and organics from water: A review. Biochar 2022, 4, 17. [Google Scholar] [CrossRef]
- Mei, Y.; Xu, J.; Zhang, Y.; Li, B.; Fan, S.; Xu, H. Effect of Fe-N modification on the properties of biochars and their adsorption behavior on tetracycline removal from aqueous solution. Bioresour. Technol. 2021, 325, 124732. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, F.; Deng, J.; Wu, Z.; Lei, T.; Tan, M.; Wu, Z.; Qin, X.; Li, H. Mechanism of sulfamic acid modified biochar for highly efficient removal of tetracycline. J. Anal. Appl. Pyrolysis 2021, 158, 105247. [Google Scholar] [CrossRef]
- Dai, Y.; Li, J.; Shan, D. Adsorption of tetracycline in aqueous solution by biochar derived from waste Auricularia auricula dregs. Chemosphere 2020, 238, 124432. [Google Scholar] [CrossRef]
- Jeong, J.; Song, W.; Cooper, W.J.; Jung, J.; Greaves, J. Degradation of tetracycline antibiotics: Mechanisms and kinetic studies for advanced oxidation/reduction processes. Chemosphere 2010, 78, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Pi, X.; Qu, Z.; Sun, F.; Zhang, Z.; Gao, J. Catalytic activation preparation of nitrogen-doped hierarchical porous bio-char for efficient adsorption of dichloromethane and toluene. J. Anal. Appl. Pyrolysis 2021, 156, 105150. [Google Scholar] [CrossRef]
- Gao, W.; Lin, Z.; Chen, H.; Yan, S.; Huang, Y.; Hu, X.; Zhang, S. A review on N-doped biochar for enhanced water treatment and emerging applications. Fuel Process. Technol. 2022, 237, 107468. [Google Scholar] [CrossRef]
- Li, X.; Xu, J.; Shi, J.; Luo, X. Rapid and efficient adsorption of tetracycline from aqueous solution in a wide pH range by using iron and aminoacetic acid sequentially modified hierarchical porous biochar. Bioresour. Technol. 2022, 346, 126672. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Z.; Xing, B.; Li, H.; Ma, Z.; Zhang, W.; Reubroycharoen, P.; Wang, S. Green conversion of bamboo chips into high-performance phenol adsorbent and supercapacitor electrodes by simultaneous activation and nitrogen doping. J. Anal. Appl. Pyrolysis 2021, 155, 105072. [Google Scholar] [CrossRef]
- Qiu, B.; Tao, X.; Wang, H.; Li, W.; Ding, X.; Chu, H. Biochar as a low-cost adsorbent for aqueous heavy metal removal: A review. J. Anal. Appl. Pyrolysis 2021, 155, 105081. [Google Scholar] [CrossRef]
- Gao, W.; Lin, Z.; Chen, H.; Yan, S.; Zhu, H.; Zhang, H.; Sun, H.; Zhang, S.; Zhang, S.; Wu, Y. Roles of graphitization degree and surface functional groups of N-doped activated biochar for phenol adsorption. J. Anal. Appl. Pyrolysis 2022, 167, 105700. [Google Scholar] [CrossRef]
- Xu, D.; Gao, Y.; Lin, Z.; Gao, W.; Zhang, H.; Karnowo, K.; Hu, X.; Sun, H.; Syed-Hassan, S.S.A.; Zhang, S. Application of biochar derived from pyrolysis of waste fiberboard on tetracycline adsorption in aqueous solution. Front. Chem. 2019, 7, 943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hairuddin, M.N.; Mubarak, N.M.; Khalid, M.; Abdullah, E.C.; Walvekar, R.; Karri, R.R. Magnetic palm kernel biochar potential route for phenol removal from wastewater. Environ. Sci. Pollut. Res. Int. 2019, 26, 35183–35197. [Google Scholar] [CrossRef]
- Liu, H.; Dai, P.; Zhang, J.; Zhang, C.; Bao, N.; Cheng, C.; Ren, L. Preparation and evaluation of activated carbons from lotus stalk with trimethyl phosphate and tributyl phosphate activation for lead removal. Chem. Eng. J. 2013, 228, 425–434. [Google Scholar] [CrossRef]
- Wang, L.; Yan, W.; He, C.; Wen, H.; Cai, Z.; Wang, Z.; Chen, Z.; Liu, W. Microwave-assisted preparation of nitrogen-doped biochars by ammonium acetate activation for adsorption of acid red 18. Appl. Surf. Sci. 2018, 433, 222–231. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Chen, M. Progress in the preparation and application of modified biochar for improved contaminant removal from water and wastewater. Bioresour. Technol. 2016, 214, 836–851. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Qi, T.; Zhou, Y.; Liu, C.; Chu, J.; Zhang, Y. Different N-containing functional groups modified mesoporous adsorbents for Cr(VI) sequestration: Synthesis, characterization and comparison. Microporous Mesoporous Mater. 2008, 110, 442–450. [Google Scholar] [CrossRef]
- Hamid, S.B.A.; Chowdhury, Z.Z.; Zain, S.M. Base catalytic approach: A promising technique for the activation of biochar for equilibrium sorption studies of copper, Cu(II) Ions in single solute system. Materials 2014, 7, 2815–2832. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Zhang, R.; Zhang, W.; Yuan, Y.; Lai, B. High-efficiency adsorption of tetracycline by the prepared waste collagen fiber-derived porous biochar. RSC Adv. 2019, 9, 39355–39366. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Yin, Y.X.; Xin, S.; Guo, Y.G.; Wan, L.J. Ionothermal synthesis of sulfur-doped porous carbons hybridized with graphene as superior anode materials for lithium-ion batteries. Chem. Commun. 2012, 48, 10663–10665. [Google Scholar] [CrossRef]
- Liu, P.; Liu, W.-J.; Jiang, H.; Chen, J.-J.; Li, W.-W.; Yu, H.-Q. Modification of bio-char derived from fast pyrolysis of biomass and its application in removal of tetracycline from aqueous solution. Bioresour. Technol. 2012, 121, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Lian, F.; Cui, G.; Liu, Z.; Duo, L.; Zhang, G.; Xing, B. One-step synthesis of a novel N-doped microporous biochar derived from crop straws with high dye adsorption capacity. J. Environ. Manag. 2016, 176, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Kundu, S.; Xia, W.; Busser, W.; Becker, M.; Schmidt, D.A.; Havenith, M.; Muhler, M. The formation of nitrogen-containing functional groups on carbon nanotube surfaces: A quantitative XPS and TPD study. Phys. Chem. Chem. Phys. 2010, 12, 4351–4359. [Google Scholar] [CrossRef]
- Yu, W.; Lian, F.; Cui, G.; Liu, Z. N-doping effectively enhances the adsorption capacity of biochar for heavy metal ions from aqueous solution. Chemosphere 2018, 193, 8–16. [Google Scholar] [CrossRef]
- Zhu, C.; Cao, J.-P.; Yang, Z.; Zhao, X.-Y.; Yi, W.-C.; Feng, X.-B.; Zhao, Y.-P.; Bai, H.-C. Study on hydrodeoxygenation mechanism of anisole over Ni (111) by first-principles calculation. Mol. Catal. 2022, 523, 111402. [Google Scholar] [CrossRef]
- Girods, P.; Dufour, A.; Rogaume, Y.; Rogaume, C.; Zoulalian, A. Thermal removal of nitrogen species from wood waste containing urea formaldehyde and melamine formaldehyde resins. J. Hazard. Mater. 2008, 159, 210–221. [Google Scholar] [CrossRef]
- Chen, W.; Li, K.; Xia, M.; Chen, Y.; Yang, H.; Chen, Z.; Chen, X.; Chen, H. Influence of NH3 concentration on biomass nitrogen-enriched pyrolysis. Bioresour. Technol. 2018, 263, 350–357. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Deditius, A.; Ela, W.P.; Wiśniewski, M.; Gauden, P.A.; Terzyk, A.P.; Furmaniak, S.; Włoch, J.; Kaneko, K.; Neimark, A.V. Super-sieving effect in phenol adsorption from aqueous solutions on nanoporous carbon beads. Carbon 2018, 135, 12–20. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, P.; Faye, M.C.A.S.; Zhang, Y. Characterization of biochar derived from rice husks and its potential in chlorobenzene degradation. Carbon 2018, 130, 730–740. [Google Scholar] [CrossRef]
- Huang, C.W.; Chiu, S.C.; Lin, W.H.; Li, Y.Y. Preparation and characterization of porous carbon nanofibers from thermal decomposition of poly(ethylene glycol). J. Phys. Chem. C. 2008, 112, 926–931. [Google Scholar] [CrossRef]
- Duan, Q.; Li, X.; Wu, Z.; Alsaedi, A.; Hayat, T.; Chen, C.; Li, J. Adsorption of 17beta-estradiol from aqueous solutions by a novel hierarchically nitrogen-doped porous carbon. J. Colloid Interface Sci. 2019, 533, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.; Amano, Y.; Machida, M. Preparation of bean dreg derived N-doped activated carbon with high adsorption for Cr(VI). Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124262. [Google Scholar] [CrossRef]
- Tan, K.L.; Hameed, B.H. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Alizadeh, A.; Abdi, G.; Khodaei, M.M.; Ashokkumar, M.; Amirian, J. Graphene oxide/Fe3O4/SO3H nanohybrid: A new adsorbent for adsorption and reduction of Cr(vi) from aqueous solutions. RSC Adv. 2017, 7, 14876–14887. [Google Scholar] [CrossRef] [Green Version]
- Pouretedal, H.R.; Sadegh, N. Effective removal of Amoxicillin, Cephalexin, Tetracycline and Penicillin G from aqueous solutions using activated carbon nanoparticles prepared from vine wood. J. Water Process Eng. 2014, 1, 64–73. [Google Scholar] [CrossRef]
- Torres-Pérez, J.; Gérente, C.; Andrès, Y. Sustainable Activated Carbons from Agricultural Residues Dedicated to Antibiotic Removal by Adsorption. Chin. J. Chem. Eng. 2012, 20, 524–529. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Qian, F.; Zhou, C.; Zhang, S.; Chen, J. Preparation of magnetic porous carbon from waste hydrochar by simultaneous activation and magnetization for tetracycline removal. Bioresour. Technol. 2014, 154, 209–214. [Google Scholar] [CrossRef]
- Wang, H.; Chu, Y.; Fang, C.; Huang, F.; Song, Y.; Xue, X. Sorption of tetracycline on biochar derived from rice straw under different temperatures. PLoS ONE 2017, 12, e0182776. [Google Scholar] [CrossRef]

| Samples | Yield | Proximate Analysis (wt%, db a) | Elemental Analysis (wt%, daf b) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Volatile | Fixed Carbon | Ash | C | H | O c | N | S | H/C d | O/C d | ||
| PBC e | 20.6 | 5.70 | 90.22 | 4.09 | 97.55 | 1.09 | 1.09 | 0.26 | 0.01 | 0.1341 | 0.0084 |
| PU-10% f | 19.4 | 7.03 | 91.13 | 1.84 | 91.76 | 1.14 | 4.13 | 2.44 | 0.53 | 0.1491 | 0.0338 |
| PUF-10% g | 20.6 | 7.21 | 88.99 | 3.81 | 92.21 | 1.43 | 2.78 | 2.16 | 1.42 | 0.1861 | 0.0226 |
| PUF-30% g | 17.5 | 8.98 | 88.17 | 2.86 | 87.01 | 1.47 | 4.01 | 3.98 | 3.53 | 0.2027 | 0.0346 |
| PUF-50% g | 13.9 | 9.91 | 87.26 | 2.83 | 83.03 | 1.51 | 5.56 | 4.92 | 4.98 | 0.2182 | 0.0502 |
| Samples | Elemental Composition (at.%) | Carbon-Containing Functional Group (at.%) | Nitrogen-Containing Functional Group (at.%) | Sulfur-Containing Functional Group (at.%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | N | O | S | C=C 284.8 eV | C–OH 285.8 eV | C=O 287.2 and 289.2 eV | N-6 398.5 eV | N-5 400.3 eV | N-G 401.2 eV | N-O 403.8 eV | S2P3/2 164.0 eV | S2P1/2 165.2 eV | Sulfate 168.5 eV | |
| PBC | 95.71 | 0 | 4.29 | 0 | 65.5 | 16.4 | 18.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PU-10% | 91.52 | 1.87 | 6.55 | 0.06 | 62.7 | 17.7 | 19.5 | 36.7 | 10.1 | 26.6 | 26.5 | 0 | 0 | 0 |
| PUF-10% | 91.97 | 1.66 | 5.88 | 0.49 | 63.2 | 18.6 | 18.3 | 16.5 | 41.2 | 23.4 | 18.9 | 39.3 | 32.7 | 28.0 |
| PUF-30% | 89.51 | 3.56 | 6.00 | 0.93 | 59.6 | 17.5 | 22.9 | 33.4 | 27.1 | 18.8 | 20.8 | 37.1 | 46.3 | 16.5 |
| PUF-50% | 87.63 | 3.31 | 7.74 | 1.32 | 58.1 | 26.3 | 15.6 | 29.9 | 11.6 | 30.8 | 27.7 | 39.8 | 20.7 | 39.5 |
| Samples | Surface Area (m2/g) | Smicro (m2/g) | Pore Volume (cm3/g) | Pmicro (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|---|---|
| PBC | 477.1 | 440.7 | 0.216 | 0.171 | 1.81 |
| PU-10% | 431.4 | 400.7 | 0.196 | 0.157 | 1.82 |
| PUF-10% | 459.8 | 430.7 | 0.213 | 0.168 | 1.85 |
| PUF-30% | 495.4 | 455.6 | 0.238 | 0.180 | 1.92 |
| PUF-50% | 542.5 | 498.4 | 0.275 | 0.198 | 2.02 |
| Samples | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
| Qe,cal (mg/g) | K1 (min−1) | R2 | Qe,cal (mg/g) | K2 (mg/g/min) | R2 | |
| PBC | 8.70 | 5.77 × 10−4 | 0.935 | 11.35 | 4.21 × 10−4 | 0.962 |
| PU-10% | 10.93 | 7.40 × 10−4 | 0.990 | 16.78 | 3.62 × 10−4 | 0.986 |
| PUF-10% | 8.88 | 6.05 × 10−4 | 0.942 | 11.16 | 3.70 × 10−4 | 0.955 |
| PUF-30% | 14.16 | 7.52 × 10−4 | 0.956 | 18.89 | 0.28 × 10−4 | 0.967 |
| PUF-50% | 20.17 | 7.35 × 10−4 | 0.969 | 29.23 | 1.79 × 10−4 | 0.978 |
| Samples | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| kL (L/mg) | Qmax (mg/g) | R2 | 1/n | kF (mg1−nLng−1) | R2 | |
| PBC | 0.1609 | 17.4 | 0.810 | 0.235 | 5.92 | 0.936 |
| PU-10% | 0.1535 | 19.6 | 0.849 | 0.194 | 7.70 | 0.906 |
| PUF-10% | 0.1439 | 17.7 | 0.805 | 0.236 | 5.90 | 0.943 |
| PUF-30% | 0.1129 | 34.9 | 0.836 | 0.350 | 7.49 | 0.933 |
| PUF-50% | 0.3322 | 41.7 | 0.855 | 0.299 | 12.94 | 0.948 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, W.; Lin, Z.; Yan, S.; Gao, Y.; Zhang, H.; Hu, X.; Sun, H.; Zhang, S. Preparation of N-, O-, and S-Tri-Doped Biochar through One-Pot Pyrolysis of Poplar and Urea Formaldehyde and Its Enhanced Removal of Tetracycline from Wastewater. Energies 2022, 15, 8081. https://doi.org/10.3390/en15218081
Gao W, Lin Z, Yan S, Gao Y, Zhang H, Hu X, Sun H, Zhang S. Preparation of N-, O-, and S-Tri-Doped Biochar through One-Pot Pyrolysis of Poplar and Urea Formaldehyde and Its Enhanced Removal of Tetracycline from Wastewater. Energies. 2022; 15(21):8081. https://doi.org/10.3390/en15218081
Chicago/Turabian StyleGao, Wenran, Zixiang Lin, Shanshan Yan, Yaxuan Gao, Hong Zhang, Xun Hu, Hongqi Sun, and Shu Zhang. 2022. "Preparation of N-, O-, and S-Tri-Doped Biochar through One-Pot Pyrolysis of Poplar and Urea Formaldehyde and Its Enhanced Removal of Tetracycline from Wastewater" Energies 15, no. 21: 8081. https://doi.org/10.3390/en15218081
APA StyleGao, W., Lin, Z., Yan, S., Gao, Y., Zhang, H., Hu, X., Sun, H., & Zhang, S. (2022). Preparation of N-, O-, and S-Tri-Doped Biochar through One-Pot Pyrolysis of Poplar and Urea Formaldehyde and Its Enhanced Removal of Tetracycline from Wastewater. Energies, 15(21), 8081. https://doi.org/10.3390/en15218081







