Interaction between Droplets and Particles as Oil–Water Slurry Components
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
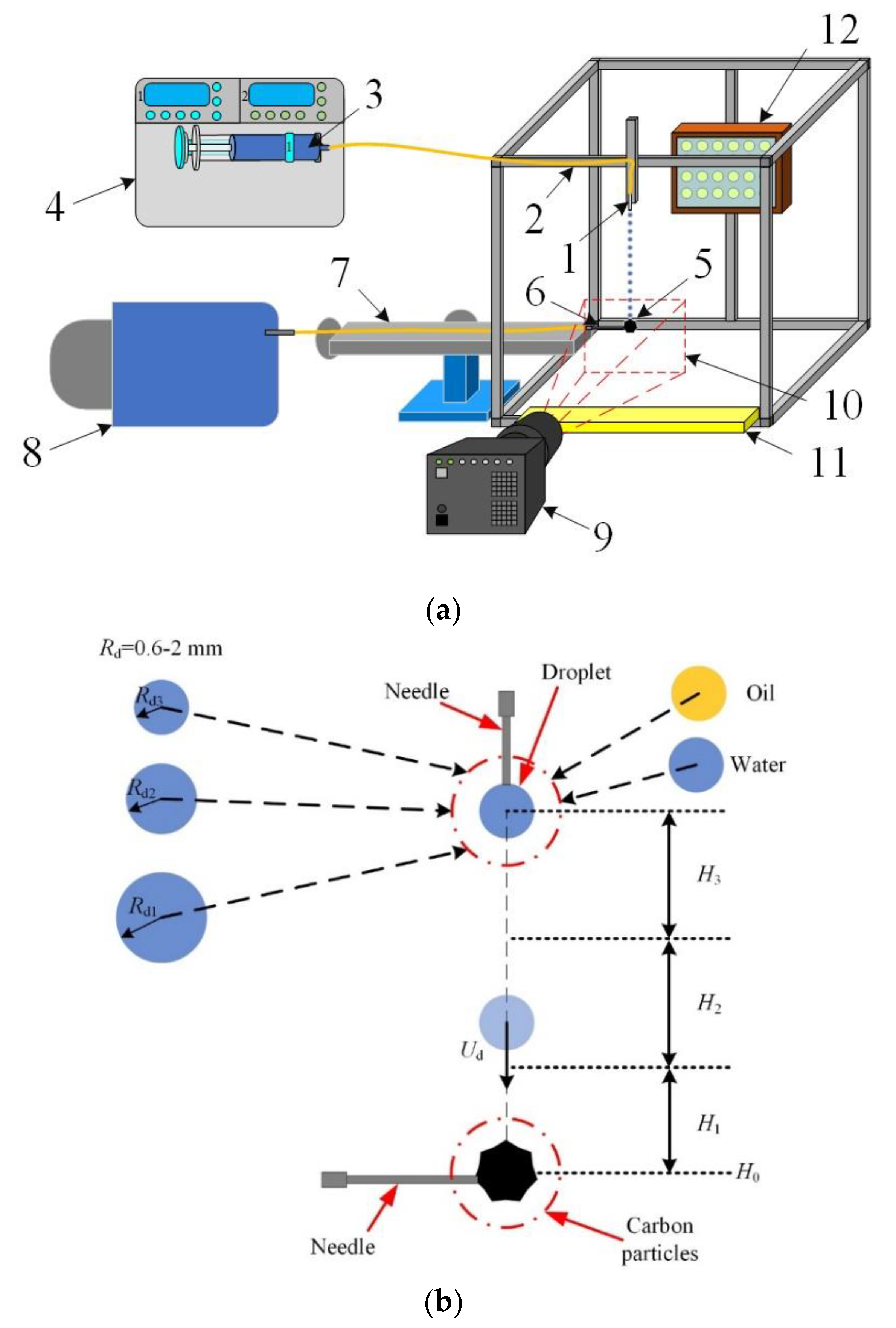
2.2. Setup and Experimental Procedures
3. Results
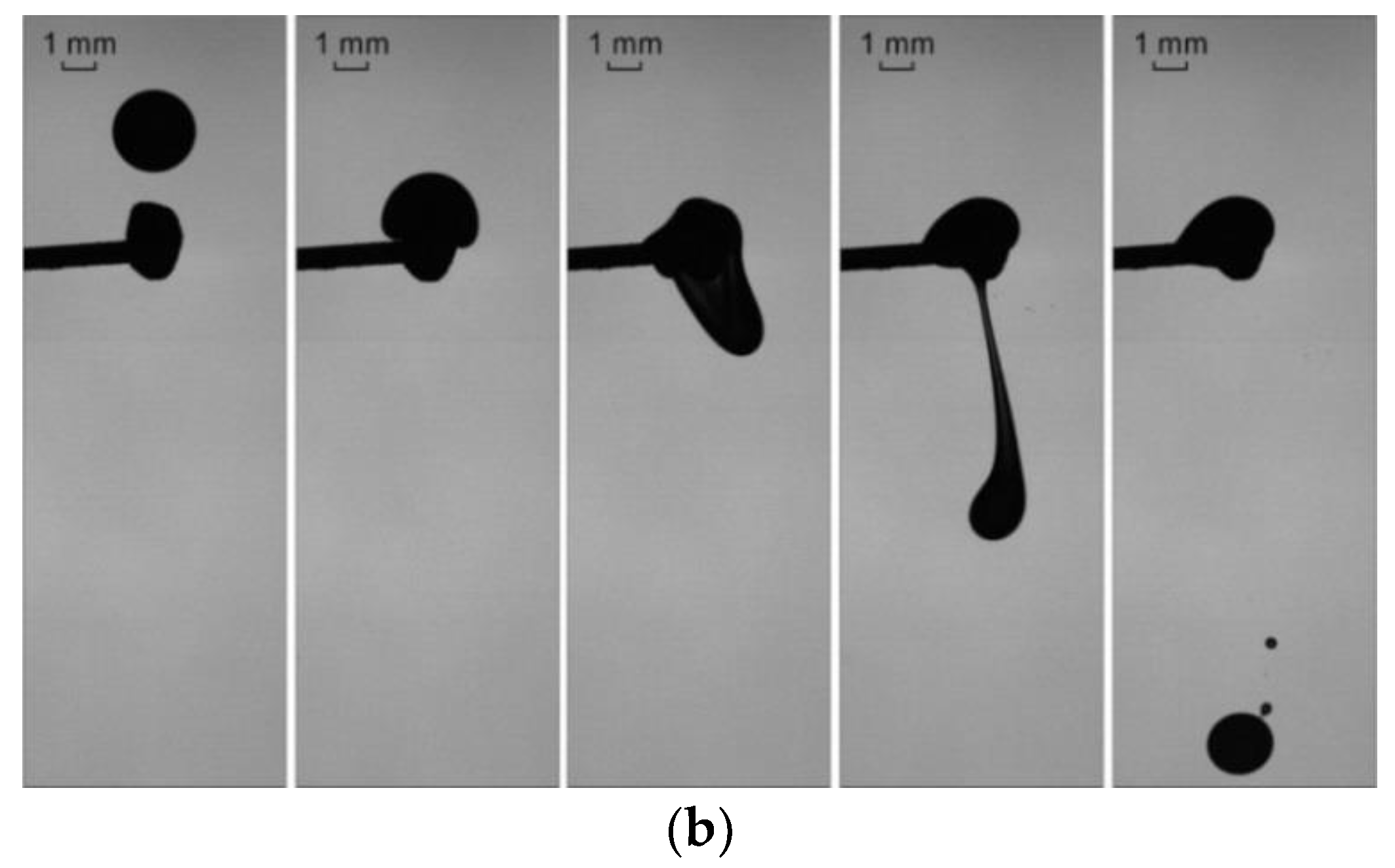
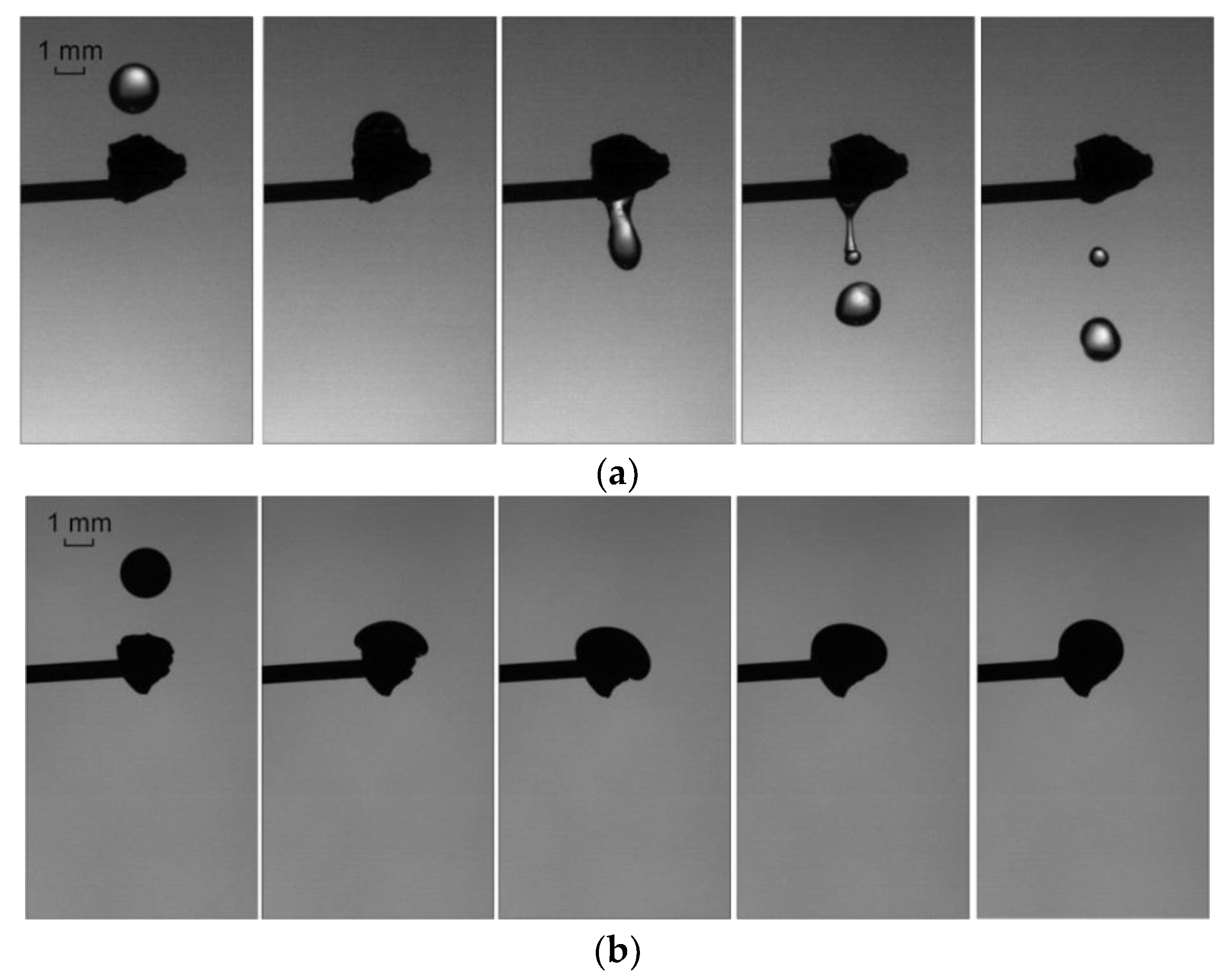
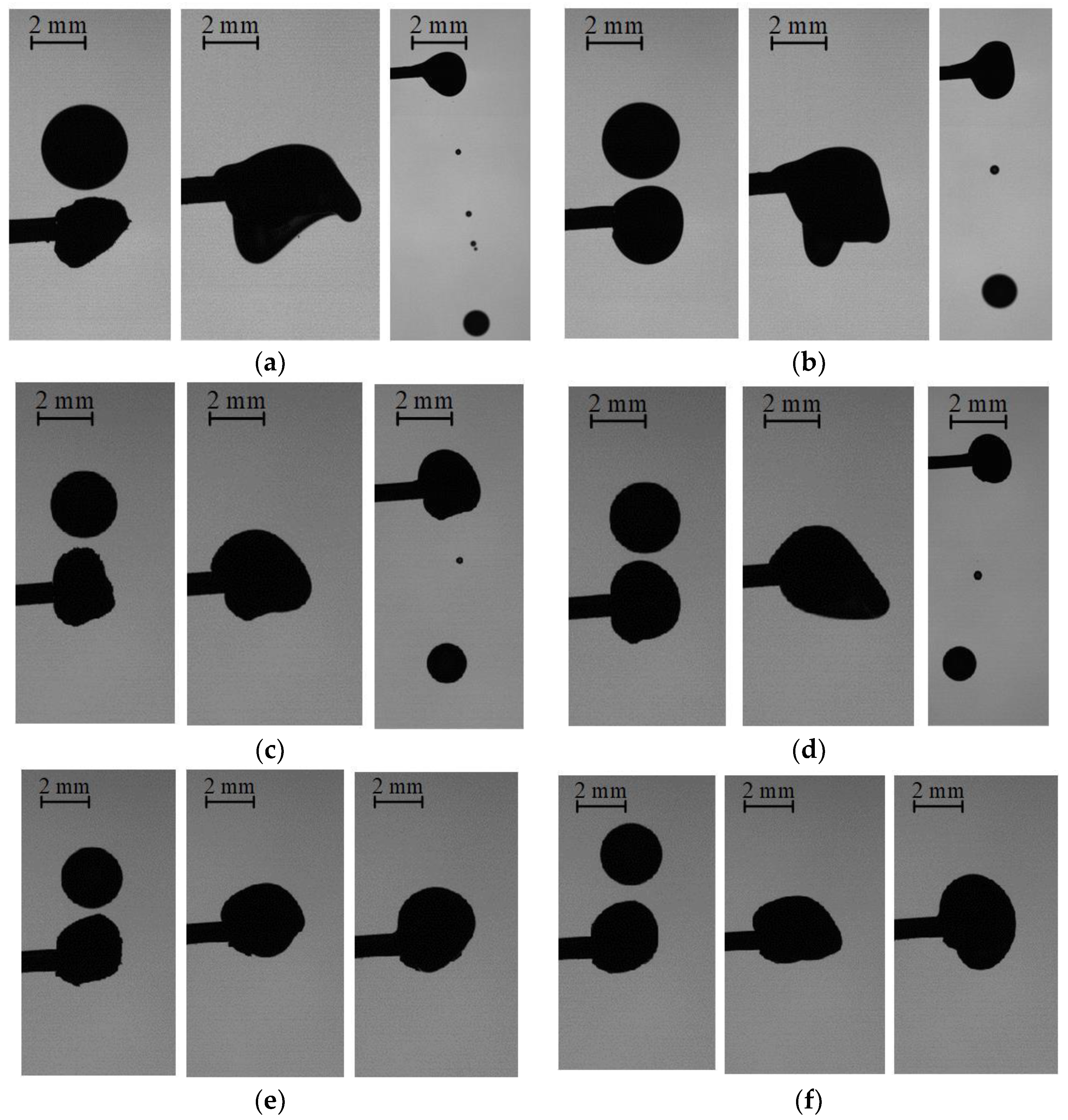
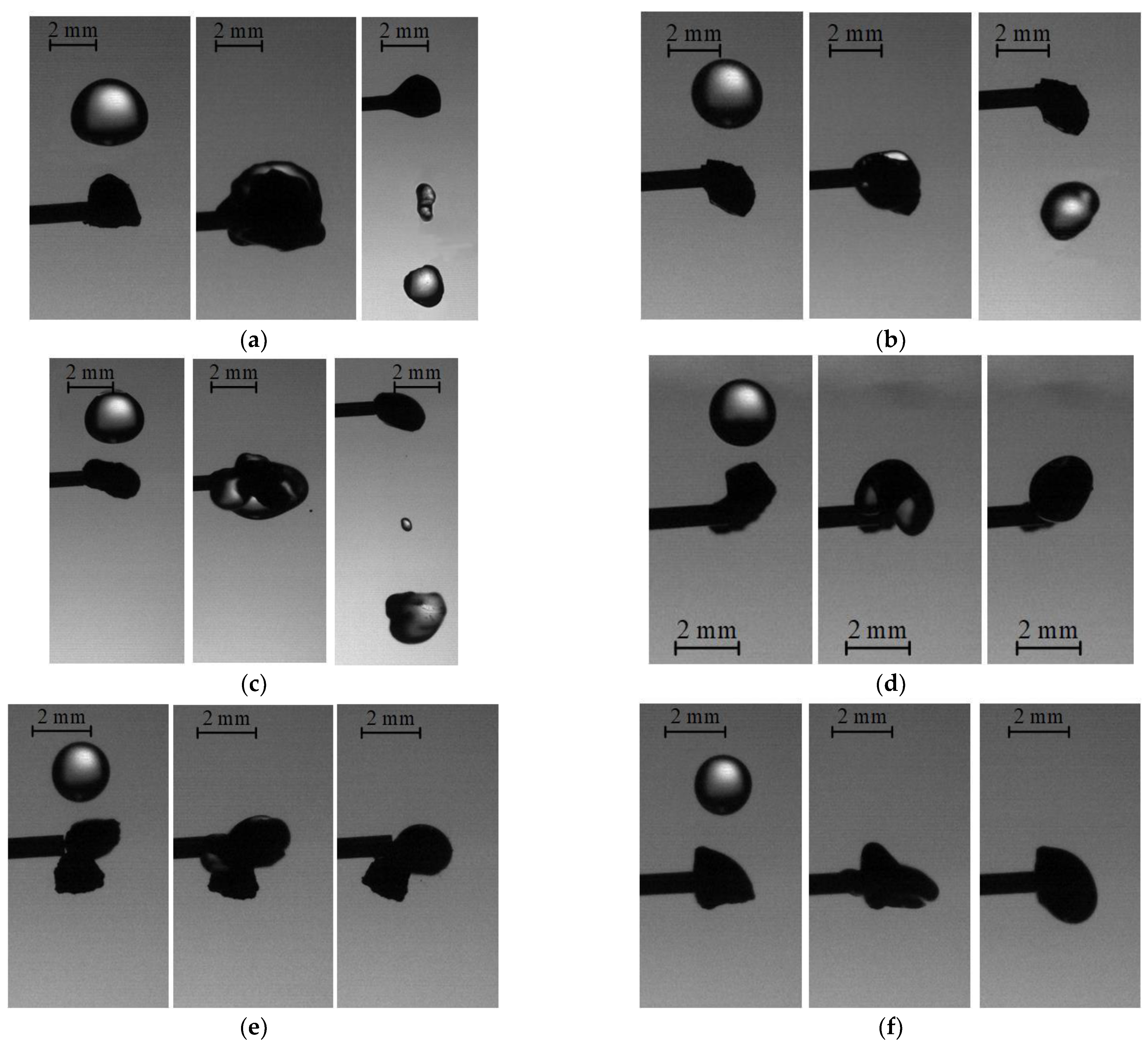
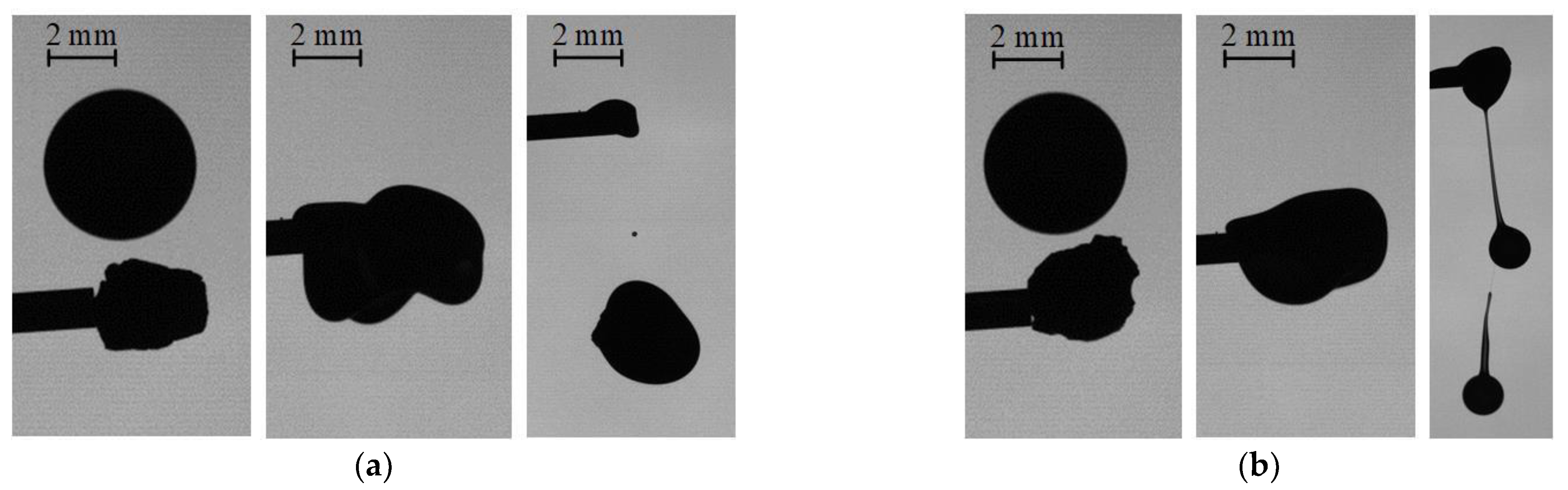
3.1. Collisions of Liquid Droplets with Solid Particles
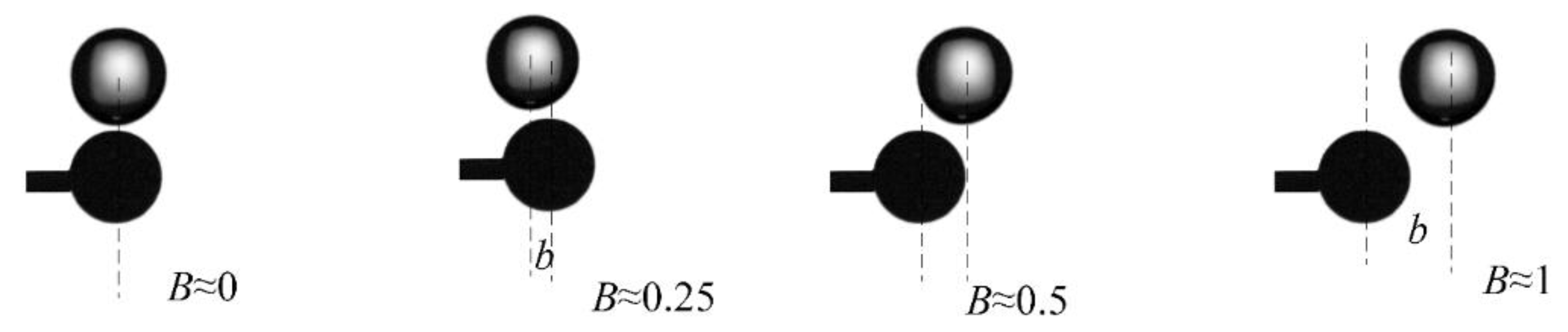
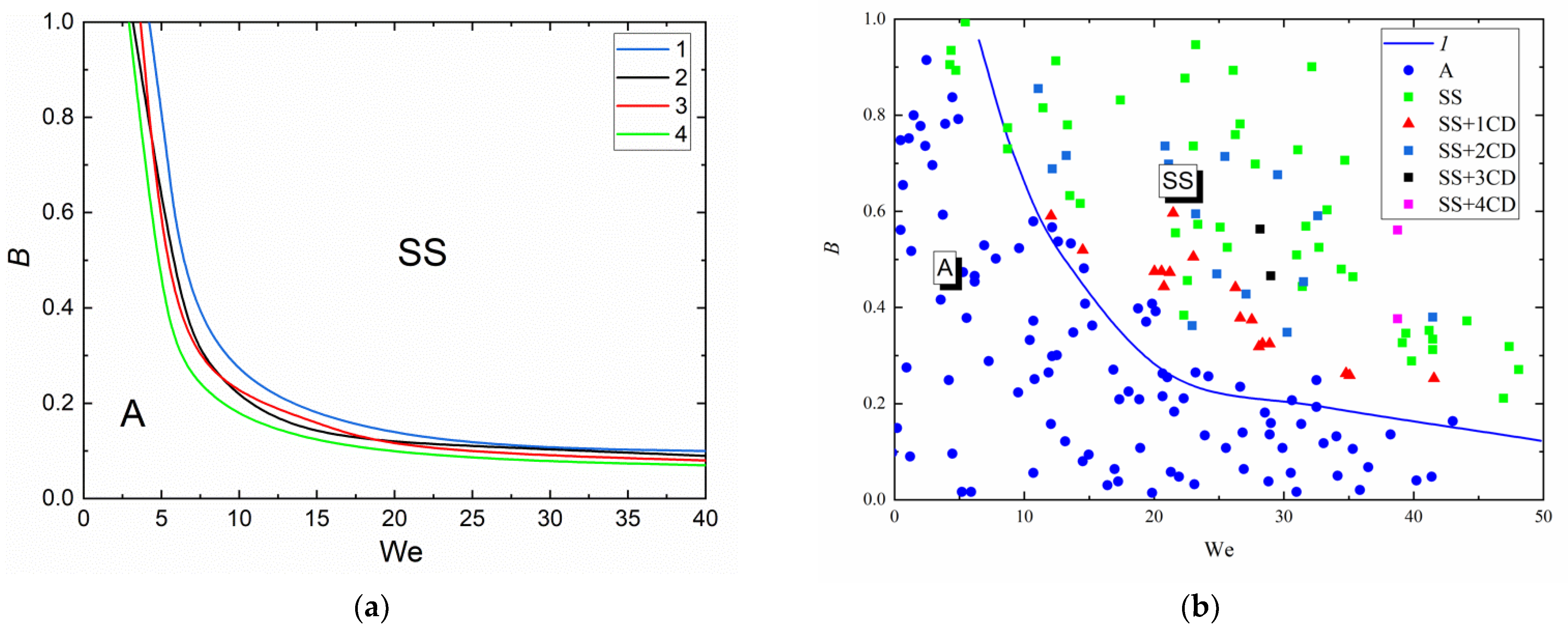
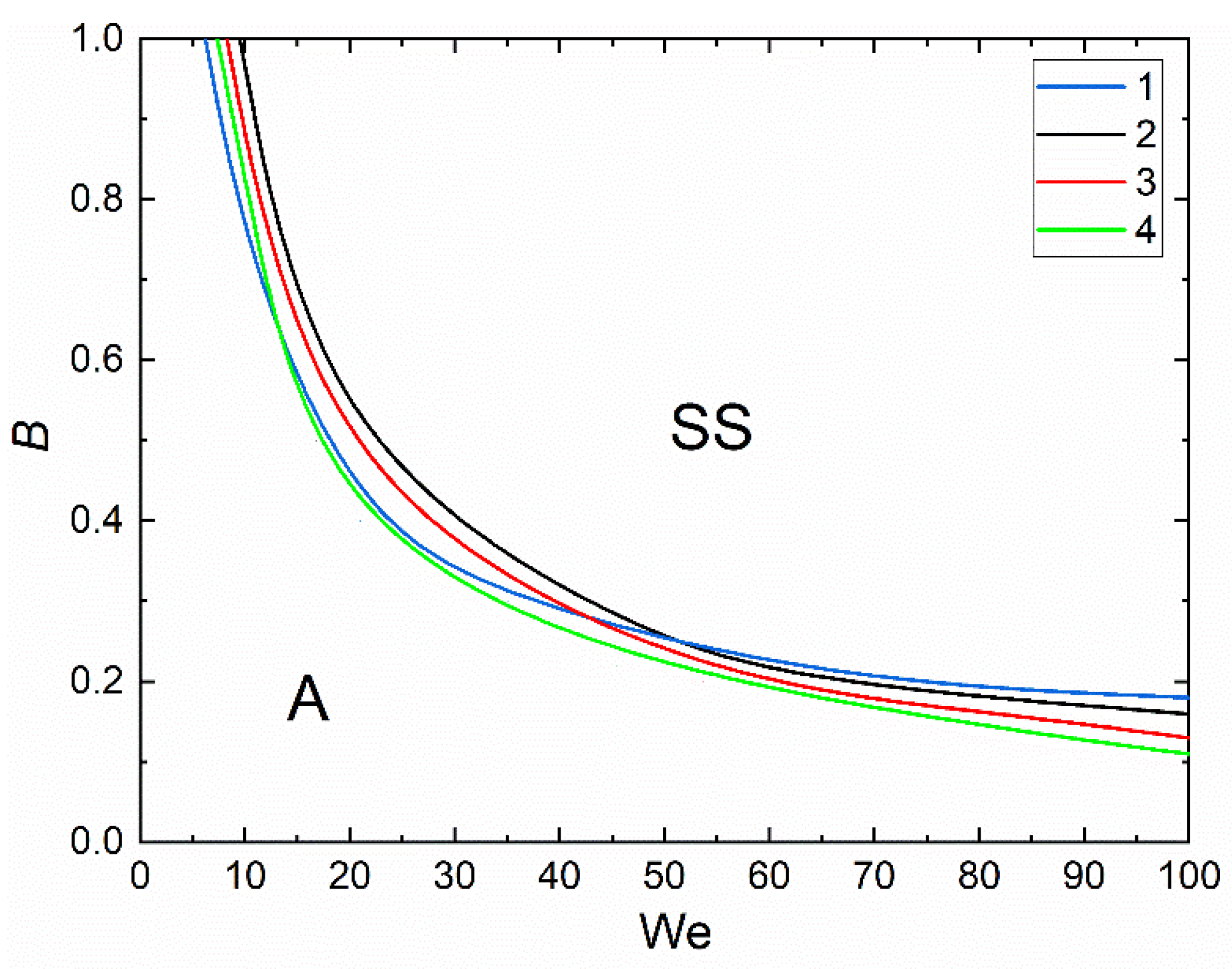
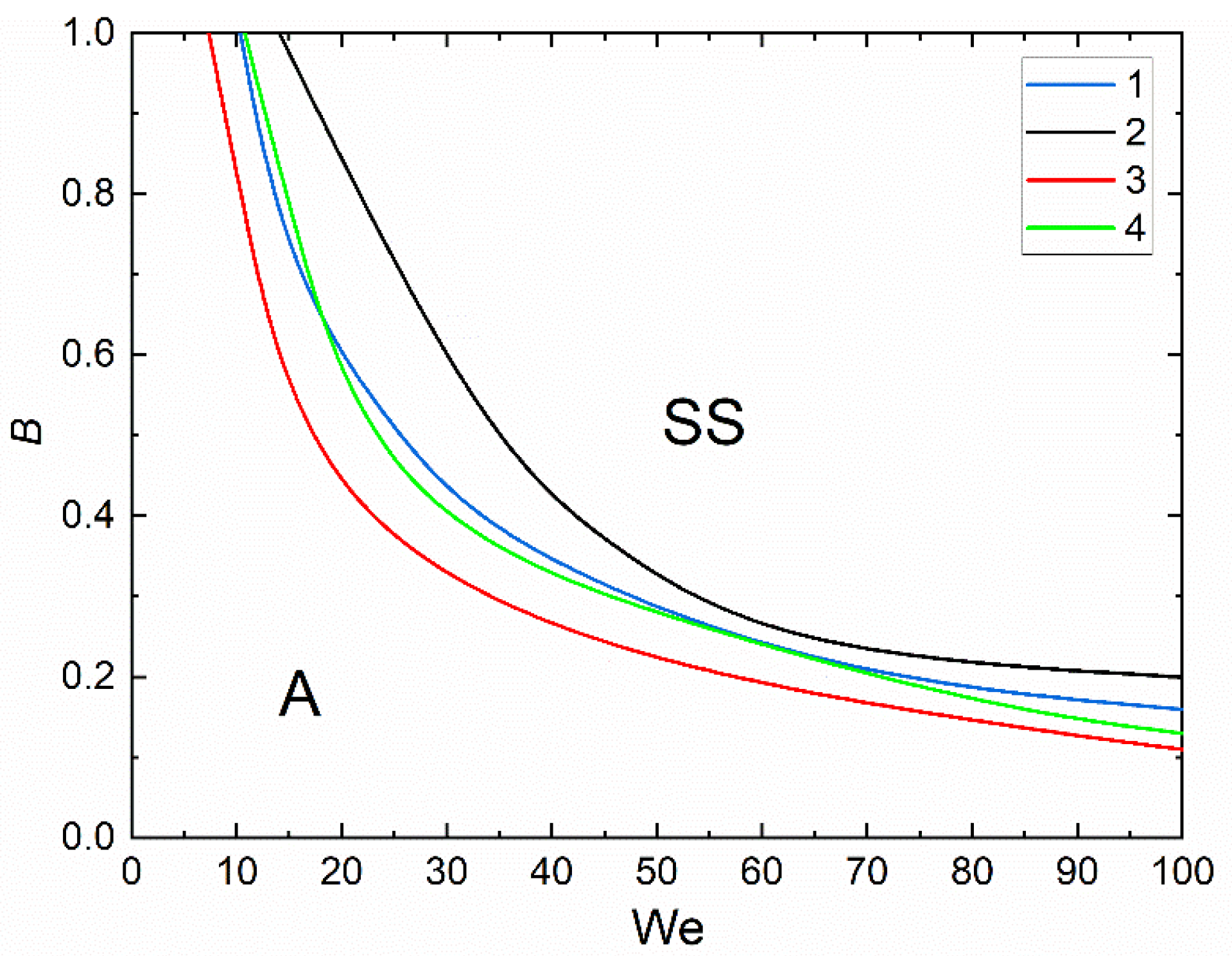
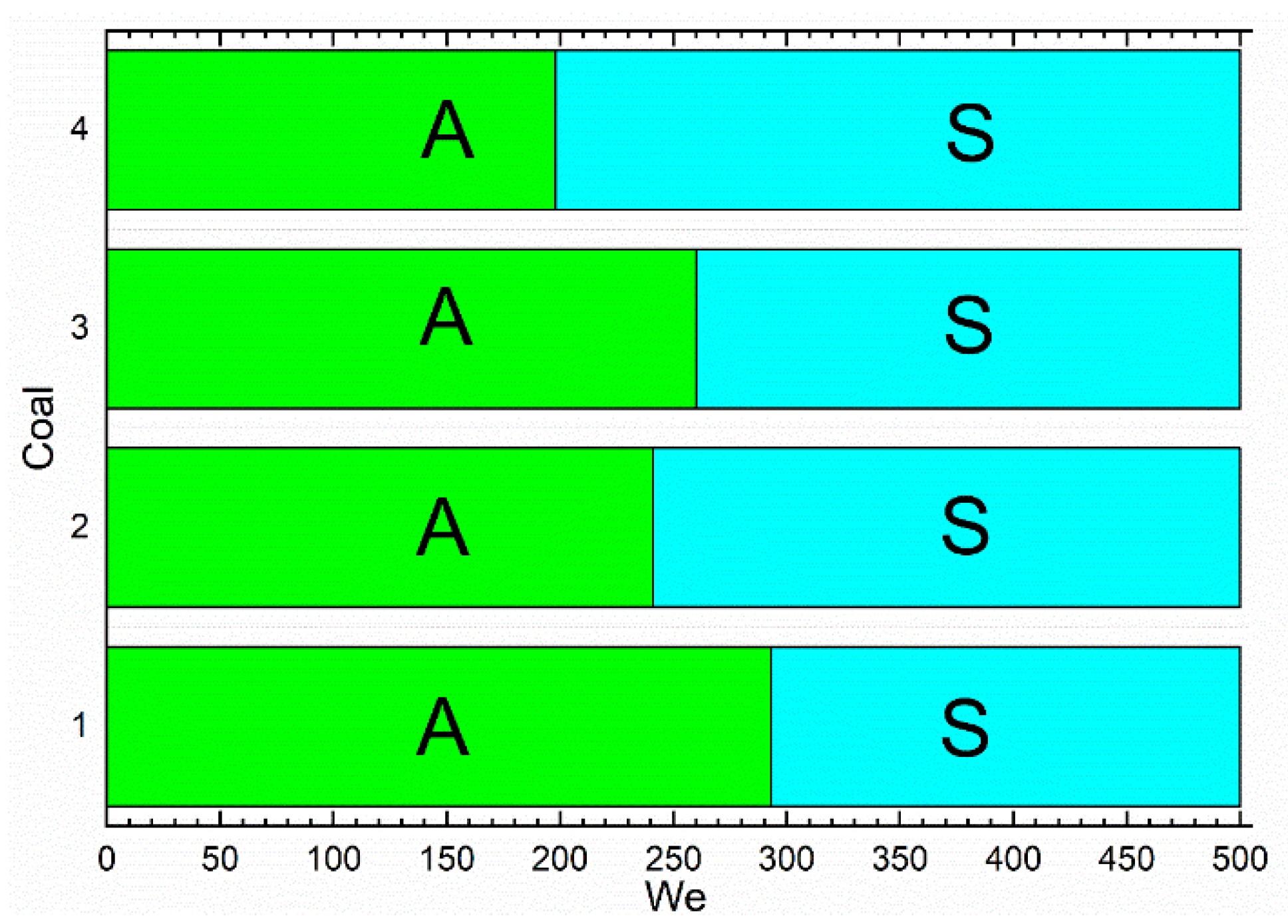
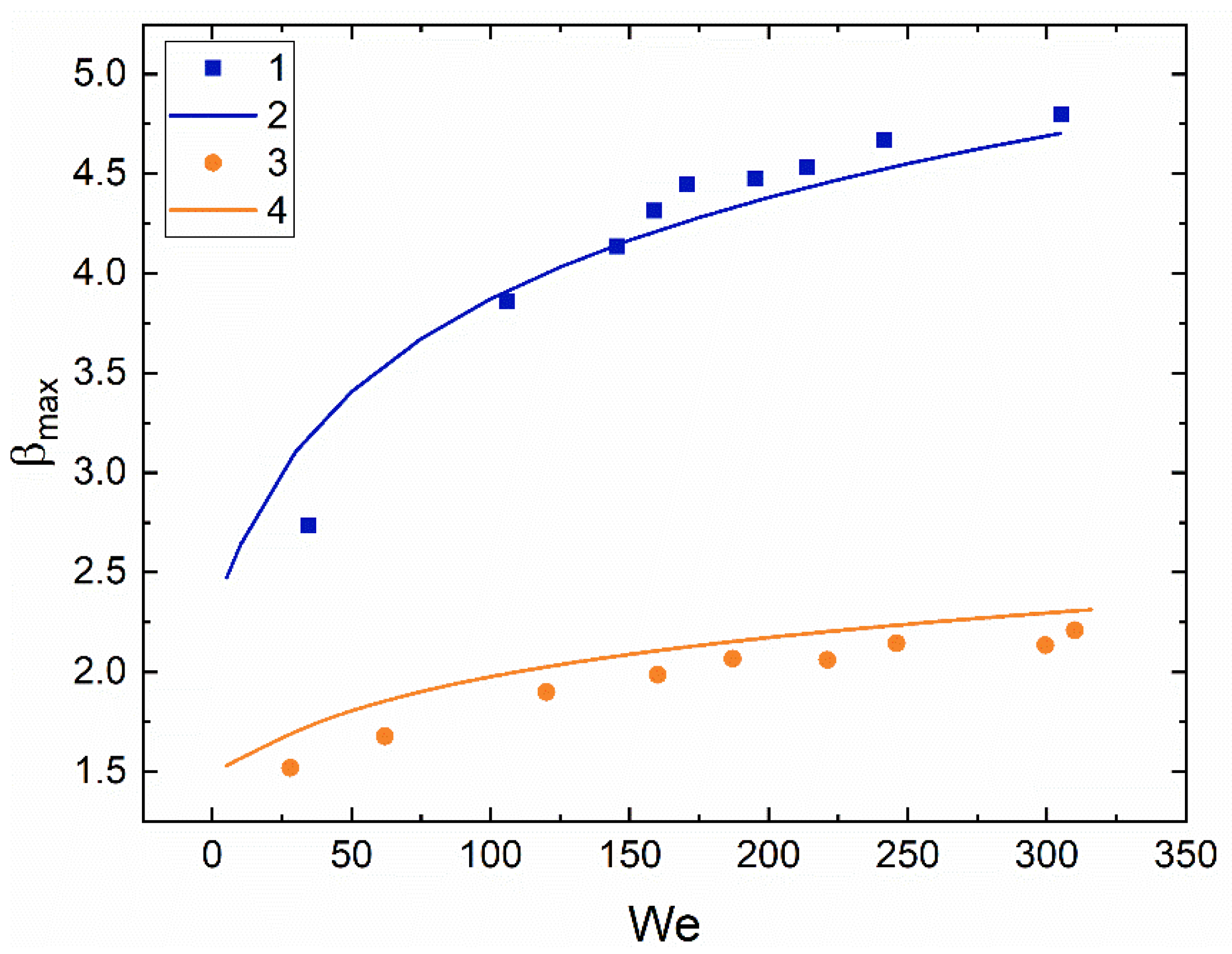
3.2. Collision Regime Maps for Liquid Droplets and Solid Particles
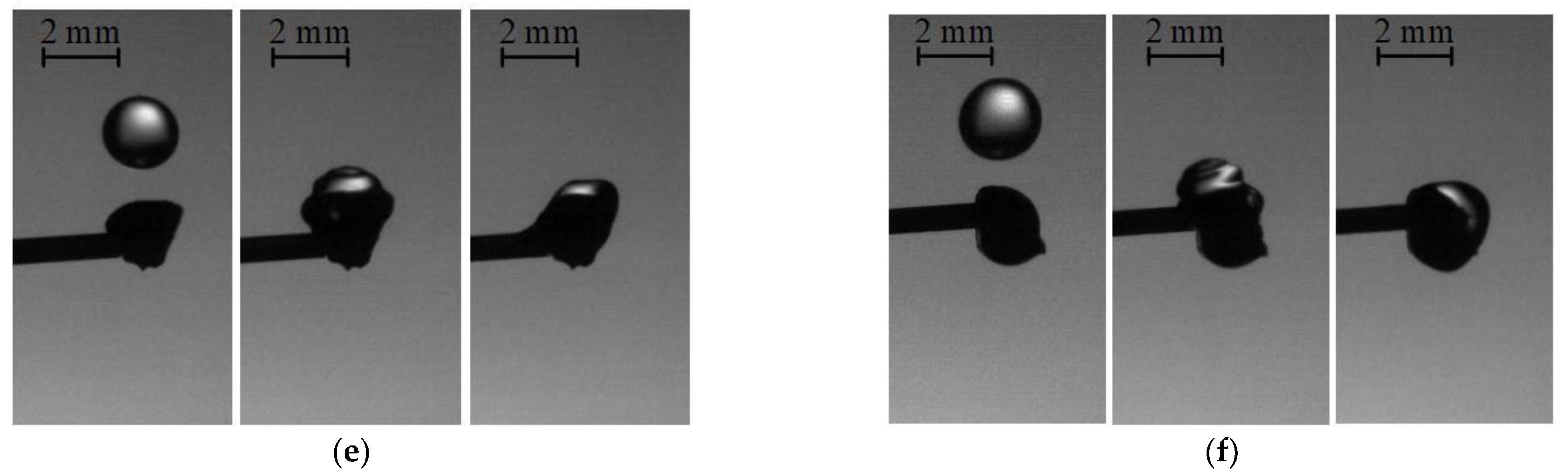
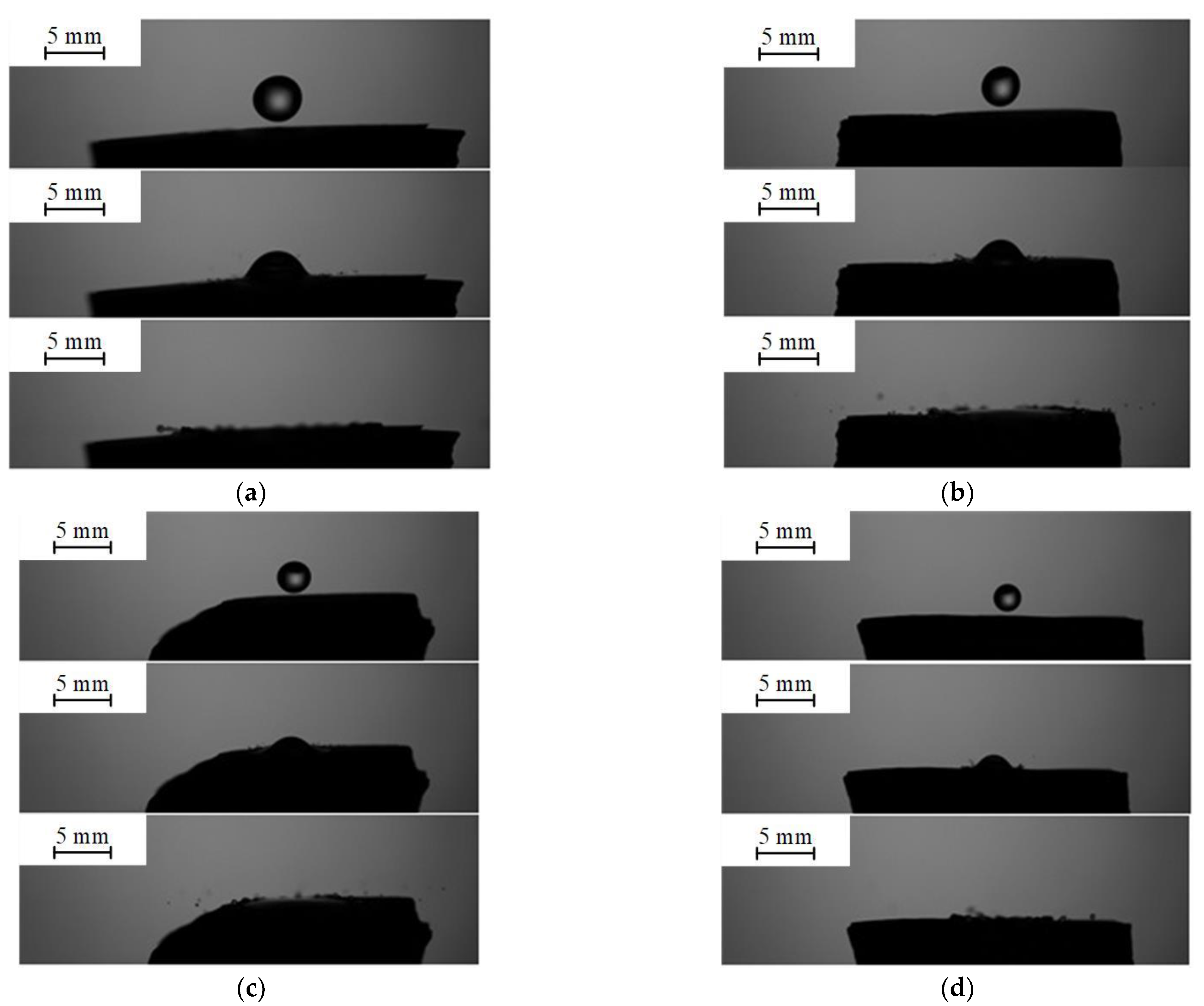
3.3. Collisions of Liquid Droplets with Solid Substrates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Ad | ash content, % |
| b | linear approach parameter, m |
| B | dimensionless linear interaction parameter |
| Dd | droplet diameter, m |
| Dmax | maximum spread diameter of the droplet, m |
| Ek1 | kinetic energy of the droplet before the collision, J |
| ES1, ES2 | surface energy of the droplet before and after the collision, J |
| Ed | energy lost to viscous dissipation, J |
| Nti | number of child droplets; |
| rd | radius of child droplets, m; |
| Rd, Rp | droplet and particle radii, m; |
| S0 | droplet surface area before interaction, m2; |
| S1 | surface area of post-collision secondary droplets, m2; |
| Ud | velocity, m/s |
| T* | dimensionless temperature; |
| T | temperature, °C; |
| V0 | total volume of the droplet before interaction, m3 |
| V1 | total volume of droplets after interaction, m3 |
| Re | Reynolds number |
| We | Weber number |
| Wecr | critical Weber number |
| Wa | moisture content, % |
| Oh | Ohnesorge number |
| n, k, m | empirical coefficients |
| Greek symbols | |
| αd | impact angle |
| βmax | maximum spread factor |
| θa | dynamic contact angle |
| Δ | Particle–droplet size ratio |
| µ | dynamic viscosity, Pa∙s |
| ρ | liquid density, kg/m3 |
| σ | surface tension of liquid, N/m |
| Abbreviations | |
| A | agglomeration |
| S | separation |
| SS | stretching separation |
References
- Wang, G.; Deng, J.; Zhang, Y.; Zhang, Q.; Duan, L.; Hao, J.; Jiang, J. Air Pollutant Emissions from Coal-Fired Power Plants in China over the Past Two Decades. Sci. Total Environ. 2020, 741, 140326. [Google Scholar] [CrossRef] [PubMed]
- Nyashina, G.; Legros, J.C.; Strizhak, P. Environmental Potential of Using Coal-Processing Waste as the Primary and Secondary Fuel for Energy Providers. Energies 2017, 10, 405. [Google Scholar] [CrossRef] [Green Version]
- Munawer, M.E. Human Health and Environmental Impacts of Coal Combustion and Post-Combustion Wastes. J. Sustain. Min. 2018, 17, 87–96. [Google Scholar] [CrossRef]
- Dudley, B. BP Statistical Review of World Energy. BP Stat. Rev. 2018, 6, 00116. [Google Scholar]
- Ahmad, T.; Zhang, D. A Critical Review of Comparative Global Historical Energy Consumption and Future Demand: The Story Told so Far. Energy Rep. 2020, 6, 1973–1991. [Google Scholar] [CrossRef]
- Miller, B.G. Clean Coal Engineering Technology; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Cansino, J.M.; Román-Collado, R.; Merchán, J. Do Spanish Energy Efficiency Actions Trigger JEVON’S Paradox? Energy 2019, 181, 760–770. [Google Scholar] [CrossRef]
- Zaitsev, A.S.; Taburchinov, R.I.; Ozerova, I.P.; Pereira, A.O.; Egorov, R.I. Allothermal Gasification of Peat and Lignite by a Focused Light Flow. Appl. Sci. 2020, 10, 2640. [Google Scholar] [CrossRef]
- Feng, Z.; Bai, Z.; Hou, R.; Guo, Z.; Kong, L.; Bai, J.; Li, W. Co-Pyrolysis of Mild Liquefaction Solid Product and Low Rank Coals: Products Distributions, Products Properties and Interactions. Fuel 2021, 306, 121719. [Google Scholar] [CrossRef]
- Spiegl, N.; Long, X.; Berrueco, C.; Paterson, N.; Millan, M. Oxy-Fuel Co-Gasification of Coal and Biomass for Negative CO2 Emissions. Fuel 2021, 306, 121671. [Google Scholar] [CrossRef]
- Bielecki, Z.; Ochowiak, M.; Włodarczak, S.; Krupińska, A.; Matuszak, M.; Jagiełło, K.; Dziuba, J.; Szajna, E.; Choiński, D.; Odziomek, M.; et al. The Optimal Diameter of the Droplets of a High-Viscosity Liquid Containing Solid State Catalyst Particles. Energies 2022, 15, 3937. [Google Scholar] [CrossRef]
- Jiang, P.; Xie, C.; Luo, C.; Meng, W.; Yang, G.; Yu, G.; Gong, Y.; Xu, M.; Wu, T. Distribution and Modes of Occurrence of Heavy Metals in Opposed Multi-Burner Coal-Water-Slurry Gasification Plants. Fuel 2021, 303, 121163. [Google Scholar] [CrossRef]
- Chu, R.; Li, Y.; Meng, X.; Fan, L.; Wu, G.; Li, X.; Jiang, X.; Yu, S.; Hu, Y. Research on the Slurrying Performance of Coal and Alkali-Modified Sludge. Fuel 2021, 294, 120548. [Google Scholar] [CrossRef]
- Adnan, M.A.; Hidayat, A.; Hossain, M.M.; Muraza, O. Transformation of Low-Rank Coal to Clean Syngas and Power via Thermochemical Route. Energy 2021, 236, 121505. [Google Scholar] [CrossRef]
- Adnan, M.A.; Xiong, Q.; Hidayat, A.; Hossain, M.M. Gasification Performance of Spirulina Microalgae–A Thermodynamic Study with Tar Formation. Fuel 2019, 241, 372–381. [Google Scholar] [CrossRef]
- Zeng, S.; Zhou, H.; Qian, Y. Review and Techno-Economic Analysis of Coal Pyrolysis to Liquid and Oil Shale to Liquid Processes. CIESC J. 2017, 68, 3658–3668. [Google Scholar] [CrossRef]
- Shadrin, E.Y.; Anufriev, I.S.; Butakov, E.B.; Kopyev, E.P.; Alekseenko, S.V.; Maltsev, L.I.; Sharypov, O. V Coal-Water Slurry Atomization in a New Pneumatic Nozzle and Combustion in a Low-Power Industrial Burner. Fuel 2021, 303, 121182. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, Z.; Zhang, Y.; Liu, P.; Zhang, D. An Experimental Investigation into the Ignition and Combustion Characteristics of Single Droplets of Biochar Water Slurry Fuels in Air. Appl. Energy 2017, 185, 2160–2167. [Google Scholar] [CrossRef]
- Yan, M.; Shi, Y. Thermal and Economic Analysis of Multi-Effect Concentration System by Utilizing Waste Heat of Flue Gas for Magnesium Desulfurization Wastewater. Energies 2020, 13, 5384. [Google Scholar] [CrossRef]
- Staroń, A.; Kowalski, Z.; Staroń, P.; Banach, M. Studies on CWL with Glycerol for Combustion Process. Environ. Sci. Pollut. Res. 2019, 26, 2835–2844. [Google Scholar] [CrossRef] [Green Version]
- Niu, J.; Liu, S.; Xu, J. Investigation into Atomization Spray Blending Property in Heavy Crude Oil Extraction under Laboratory Conditions. J. Pet. Sci. Eng. 2020, 184, 106494. [Google Scholar] [CrossRef]
- Yuan, N.; Zhao, A.; Hu, Z.; Tan, K.; Zhang, J. Preparation and Application of Porous Materials from Coal Gasification Slag for Wastewater Treatment: A Review. Chemosphere 2022, 287, 132227. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Luo, X.; Li, Q.; Xu, K.; Liu, J. Operation Optimization and Costs Analysis of the Wet Desulfurization System in an Ultra-Supercritical Coal-Fired Power Plants. Environ. Prog. Sustain. Energy 2021, 40, e13527. [Google Scholar] [CrossRef]
- Zheng, J.; Xu, Y.; Wang, Q.; He, H. Characteristics of Particle Size and Velocity of Droplets of Coal Water Slurry Subjected to Air-Blast Electrostatic Atomization Using a Phase Doppler Particle Analyzer. J. Electrostat. 2019, 98, 40–48. [Google Scholar] [CrossRef]
- Wu, X.; Gong, Y.; Guo, Q.; Xue, Z.; Yu, G. Experimental Study on the Atomization and Particle Evolution Characteristics in an Impinging Entrained-Flow Gasifier. Chem. Eng. Sci. 2019, 207, 542–555. [Google Scholar] [CrossRef]
- Wu, X.; Guo, Q.; Gong, Y.; Liu, J.; Luo, X.; Wu, T.; Yu, G. Influence of Burner Geometry on Atomization of Coal Water Slurry in an Entrained-Flow Gasifier. Chem. Eng. Sci. 2022, 247, 117088. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, H.-F.; Xu, J.-L.; Li, W.-F.; Cheng, W. Breakup and Atomization of a Round Coal Water Slurry Jet by an Annular Air Jet. Chem. Eng. Sci. 2012, 78, 63–74. [Google Scholar] [CrossRef]
- Khojasteh, D.; Kazerooni, N.M.; Marengo, M. A Review of Liquid Droplet Impacting onto Solid Spherical Particles: A Physical Pathway to Encapsulation Mechanisms. J. Ind. Eng. Chem. 2019, 71, 50–64. [Google Scholar] [CrossRef]
- Gvozdyakov, D.; Zenkov, A. Improvement of Atomization Characteristics of Coal-Water Slurries. Energy 2021, 230, 120900. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, Z.; Zhang, Y.; Setyawan, H.; Liu, P.; Zhang, D. An Experimental Study of the Ignition and Combustion Characteristics of Single Droplets of Biochar-Glycerol-Water Slurry Fuels. Proc. Combust. Inst. 2017, 36, 2475–2482. [Google Scholar] [CrossRef]
- Meng, Z.; Yang, Z.; Yin, Z.; Li, Y.; Song, X.; Zhao, J.; Wu, W. Effects of Coal Slime on the Slurry Ability of a Semi-Coke Water Slurry. Powder Technol. 2020, 359, 261–267. [Google Scholar] [CrossRef]
- Solomatin, Y.; Shlegel, N.E.E.; Strizhak, P.A.A. Atomization of Promising Multicomponent Fuel Droplets by Their Collisions. Fuel 2019, 255, 115751. [Google Scholar] [CrossRef]
- Wang, S.; Liu, J.; Pisupati, S.V.; Li, D.; Wang, Z.; Cheng, J. Dispersion Mechanism of Coal Water Slurry Prepared by Mixing Various High-Concentration Organic Waste Liquids. Fuel 2021, 287, 119340. [Google Scholar] [CrossRef]
- Dremicheva, E.S.; Laptev, A.G. Viscous Properties of Petroleum-Containing Waste Waters from Industrial Enterprises. J. Eng. Phys. Thermophys. 2021, 94, 1326–1330. [Google Scholar] [CrossRef]
- Pawar, S.K.; Henrikson, F.; Finotello, G.; Padding, J.T.; Deen, N.G.; Jongsma, A.; Innings, F.; Kuipers, J.A.M.H. An Experimental Study of Droplet-Particle Collisions. Powder Technol. 2016, 300, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Lee, S.; Tahmasebi, A.; Jeon, C.-H.; Yu, J. A Review of the Numerical Modeling of Pulverized Coal Combustion for High-Efficiency, Low-Emissions (HELE) Power Generation. Energy Fuels 2021, 35, 7434–7466. [Google Scholar] [CrossRef]
- Kuznetsov, G.V.; Syrodoy, S.V.; Gutareva, N.Y.; Nigay, N.A. Mathematical Modeling of the Thermochemical Processes of Nitrogen Oxides Sequestration during Combustion of Wood-Coal Mixture Particles. J. Energy Inst. 2021, 96, 280–293. [Google Scholar] [CrossRef]
- Egorov, R.I.; Taburchinov, R.I. The Numerical Study of Allothermal Gasification of the Peat by the Focused Light Flow. Appl. Therm. Eng. 2021, 195, 117253. [Google Scholar] [CrossRef]
- Kajitani, S.; Tay, H.-L.; Zhang, S.; Li, C.-Z. Mechanisms and Kinetic Modelling of Steam Gasification of Brown Coal in the Presence of Volatile–Char Interactions. Fuel 2013, 103, 7–13. [Google Scholar] [CrossRef]
- Vershinina, K.Y.; Dorokhov, V.V.; Nyashina, G.S.; Romanov, D.S. Environmental Aspects and Energy Characteristics of the Combustion of Composite Fuels Based on Peat, Oil, and Water. Solid Fuel Chem. 2019, 53, 294–302. [Google Scholar] [CrossRef]
- Gaber, C.; Wachter, P.; Demuth, M.; Hochenauer, C. Experimental Investigation and Demonstration of Pilot-Scale Combustion of Oil-Water Emulsions and Coal-Water Slurry with Pronounced Water Contents at Elevated Temperatures with the Use of Pure Oxygen. Fuel 2020, 282, 118692. [Google Scholar] [CrossRef]
- Yi, S.; Hao, L.; Li, S.; Song, W. The Influence of Water Content in Rice Husk Bio-Oil on the Rheological Properties of Coal Bio-Oil Slurries. Energy 2019, 189, 116307. [Google Scholar] [CrossRef]
- Chen, X.; Wang, C.; Wang, Z.; Zhao, H.; Liu, H. Preparation of High Concentration Coal Water Slurry of Lignite Based on Surface Modification Using the Second Fluid and the Second Particle. Fuel 2019, 242, 788–793. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, F.; Wang, J.; Li, B.; Xu, H.; Yao, E.; Zhao, L. Influence of Nanoemulsion Droplet Size of Removing Water Blocking Damage in Tight Gas Reservoir. Energies 2022, 15, 5283. [Google Scholar] [CrossRef]
- Wang, X.; Dou, L.; Hossain, M.; Rezaee, R.; Zhao, F.; Dong, Z.; Wang, C.; Zhang, W.; Yu, R. Pore Connectivity Characteristics and Controlling Factors for Black Shales in the Wufeng-Longmaxi Formation, Southeastern Sichuan Basin, China. Energies 2022, 15, 2909. [Google Scholar] [CrossRef]
- Kuznetsov, G.V.; Islamova, A.G.; Orlova, E.G.; Strizhak, P.A.; Feoktistov, D.V. Physicochemical Features of the Effect of Special Water-Based Fire Retardants on Forest Materials. Fire Saf. J. 2021, 123, 103371. [Google Scholar] [CrossRef]
- Egorov, R.I.; Tkachenko, P.P.; Taburchinov, R.I.; Chulkov, A.O. The Propagation and Ignition of the Finely Dispersed Coal-Water Aerosol. Fuel 2020, 263, 116767. [Google Scholar] [CrossRef]
- Lin, Z.; Chi, S.; Ye, J.; Zhu, Z.; Li, Y.; Jin, Y. Effect of Liquid Layer on the Motion of Particle during Oblique Wet Collision. Adv. Powder Technol. 2021, 32, 3259–3267. [Google Scholar] [CrossRef]
- Islamova, A.G.; Kerimbekova, S.A.; Shlegel, N.E.; Strizhak, P.A. Droplet-Droplet, Droplet-Particle, and Droplet-Substrate Collision Behavior. Powder Technol. 2022, 403, 117371. [Google Scholar] [CrossRef]
- Tkachenko, P.P.; Shlegel, N.E.; Strizhak, P.A. Experimental Research of Liquid Droplets Colliding with Solid Particles in a Gaseous Medium. Chem. Eng. Res. Des. 2022, 177, 200–209. [Google Scholar] [CrossRef]
- Shlegel, N.E.; Tkachenko, P.P.; Strizhak, P.A. Influence of Viscosity, Surface and Interfacial Tensions on the Liquid Droplet Collisions. Chem. Eng. Sci. 2020, 220, 115639. [Google Scholar] [CrossRef]
- Biance, A.L.; Chevy, F.; Clanet, C.; Lagubeau, G.; Quéré, D. On the Elasticity of an Inertial Liquid Shock. J. Fluid Mech. 2006, 554, 47–66. [Google Scholar] [CrossRef]
- Lastakowski, H.; Boyer, F.; Biance, A.L.; Pirat, C.; Ybert, C. Bridging Local to Global Dynamics of Drop Impact onto Solid Substrates. J. Fluid Mech. 2014, 747, 103–118. [Google Scholar] [CrossRef]
- Tran, T.; Staat, H.J.J.; Prosperetti, A.; Sun, C.; Lohse, D. Drop Impact on Superheated Surfaces. Phys. Rev. Lett. 2012, 108, 036101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, G.; Shen, S.; Guo, Y.; Zhang, J. Boiling from Liquid Drops Impact on a Heated Wall. Int. J. Heat Mass Transf. 2016, 100, 48–57. [Google Scholar] [CrossRef]
- Negeed, E.S.R.; Hidaka, S.; Kohno, M.; Takata, Y. High Speed Camera Investigation of the Impingement of Single Water Droplets on Oxidized High Temperature Surfaces. Int. J. Therm. Sci. 2013, 63, 1–14. [Google Scholar] [CrossRef]
- Roisman, I.V. Inertia Dominated Drop Collisions. II. An Analytical Solution of the Navier–Stokes Equations for a Spreading Viscous Film. Phys. Fluids 2009, 21, 052104. [Google Scholar] [CrossRef]
- Negeed, E.-S.R.; Ishihara, N.; Tagashira, K.; Hidaka, S.; Kohno, M.; Takata, Y. Experimental Study on the Effect of Surface Conditions on Evaporation of Sprayed Liquid Droplet. Int. J. Therm. Sci. 2010, 49, 2250–2271. [Google Scholar] [CrossRef]
- Bayer, I.S.; Megaridis, C.M. Contact Angle Dynamics in Droplets Impacting on Flat Surfaces with Different Wetting Characteristics. J. Fluid Mech 2006, 558, 415–449. [Google Scholar] [CrossRef]
- Pasandideh-Fard, M.; Qiao, Y.M.; Chandra, S.; Mostaghimi, J. Capillary Effects during Droplet Impact on a Solid Surface. Phys. Fluids 1998, 8, 650. [Google Scholar] [CrossRef]
- Mundo, C.; Sommerfeld, M.; Tropea, C. Droplet-Wall Collisions: Experimental Studies of the Deformation and Breakup Process. Int. J. Multiph. Flow 1995, 21, 151–173. [Google Scholar] [CrossRef]
- Ribeiro, D.F.S.; Silva, A.R.R.; Panão, M.R.O. Insights into Single Droplet Impact Models upon Liquid Films Using Alternative Fuels for Aero-Engines. Appl. Sci. 2020, 10, 6698. [Google Scholar] [CrossRef]
- Range, K.; Feuillebois, F. Influence of Surface Roughness on Liquid Drop Impact. J. Colloid Interface Sci. 1998, 203, 16–30. [Google Scholar] [CrossRef]
- Vander Wal, R.L.; Berger, G.M.; Mozes, S.D. The Splash/Non-Splash Boundary upon a Dry Surface and Thin Fluid Film. Exp. Fluids 2006, 40, 53–59. [Google Scholar] [CrossRef]
- Cossali, G.E.; Coghe, A.; Marengo, M. The Impact of a Single Drop on a Wetted Solid Surface. Exp. Fluids 1997, 22, 463–472. [Google Scholar] [CrossRef]
- He, X.Y.; Zhu, X.; Wang, H.; Tan, Y.; Ding, B.; Lv, Y.W.; Liao, Q. Dynamic Behaviors and Regime Map of a Molten Blast Furnace Slag Droplet Impacting a Solid Surface. Fuel 2020, 279, 118451. [Google Scholar] [CrossRef]
- Jadidbonab, H.; Malgarinos, I.; Karathanassis, I.; Mitroglou, N.; Gavaises, M. We-T Classification of Diesel Fuel Droplet Impact Regimes. Proc. R. Soc. A Math. Phys. Eng. Sci. 2018, 474, 20170759. [Google Scholar] [CrossRef]
- Stow, C.D.; Hadfield, M.G. An experimental investigation of fluid flow resulting from the impact of a water drop with an unyielding dry surface. Proc. R. Soc. London. A. Math. Phys. Sci. 1981, 373, 419–441. [Google Scholar] [CrossRef]
- Jadidbonab, H.; Mitroglou, N.; Karathanassis, I.; Gavaises, M. Experimental Study of Diesel-Fuel Droplet Impact on a Similarly Sized Polished Spherical Heated Solid Particle. Langmuir 2017, 34, 36–49. [Google Scholar] [CrossRef]
- Mitra, S.; Sathe, M.J.; Doroodchi, E.; Utikar, R.; Shah, M.K.; Pareek, V.; Joshi, J.B.; Evans, G.M. Droplet Impact Dynamics on a Spherical Particle. Chem. Eng. Sci. 2013, 100, 105–119. [Google Scholar] [CrossRef]
- Mitra, S.; Nguyen, T.B.T.; Doroodchi, E.; Pareek, V.; Joshi, J.B.; Evans, G.M. On Wetting Characteristics of Droplet on a Spherical Particle in Film Boiling Regime. Chem. Eng. Sci. 2016, 149, 181–203. [Google Scholar] [CrossRef]
- Mitra, S.; Evans, G. Dynamic Surface Wetting and Heat Transfer in a Droplet-Particle System of Less Than Unity Size Ratio. Front. Chem. 2018, 6, 259. [Google Scholar] [CrossRef] [PubMed]
- Charalampous, G.; Hardalupas, Y. Collisions of Droplets on Spherical Particles. Phys. Fluids 2017, 29, 103305. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.; Li, Y. A Comprehensive Study of Dynamic and Heat Transfer Characteristics of Droplet Impact on Micro-Scale Rectangular Grooved Surface. Energies 2018, 11, 1390. [Google Scholar] [CrossRef] [Green Version]
- Rein, M. Phenomena of Liquid Drop Impact on Solid and Liquid Surfaces. Fluid Dyn. Res. 1993, 12, 61–93. [Google Scholar] [CrossRef]







| Liquid | Temperature, °С | Viscosity, mPa∙s | Density, kg/m3 | Surface Tension, mN/m |
|---|---|---|---|---|
| Distilled water | 20 | 1 | 998 | 72.69 |
| Used motor oil | 20 | 97.92 | 832 | 28.85 |
| Coal Rank | Contact Angle, ° | |
|---|---|---|
| Pressed Pellet | Monolithic Fragment | |
| Coking coal filter cake | 69.3 | – |
| Brown coal | 77.2 | 104 |
| Coking bituminous coal | 72.2 | 102.3 |
| Anthracite | 71.3 | 97.8 |
| Coal Rank/Liquid | Distilled Water | Used Motor Oil | ||
|---|---|---|---|---|
| n | k | n | k | |
| Coking coal filter cake | 4.349 | −1.1 | 4.385 | −0.79 |
| Wetted particle of coking coal filter cake | 5.907 | 7.828 | ||
| Brown coal | 3.323 | 5.546 | ||
| Coking coal | 3.535 | 4.734 | ||
| Anthracite | 2.893 | 5.193 | ||
| Wetted particle of anthracite | 8.432 | 6.261 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islamova, A.; Tkachenko, P.; Pavlova, K.; Strizhak, P. Interaction between Droplets and Particles as Oil–Water Slurry Components. Energies 2022, 15, 8288. https://doi.org/10.3390/en15218288
Islamova A, Tkachenko P, Pavlova K, Strizhak P. Interaction between Droplets and Particles as Oil–Water Slurry Components. Energies. 2022; 15(21):8288. https://doi.org/10.3390/en15218288
Chicago/Turabian StyleIslamova, Anastasia, Pavel Tkachenko, Kristina Pavlova, and Pavel Strizhak. 2022. "Interaction between Droplets and Particles as Oil–Water Slurry Components" Energies 15, no. 21: 8288. https://doi.org/10.3390/en15218288





