Shale Microstructure Characteristics under the Action of Supercritical Carbon Dioxide (Sc-CO2)
Abstract
1. Introduction
2. Materials and Methods
2.1. Shale Properties and Preparation
2.2. Experimental Method
2.2.1. Experimental Method of Shale Surface Structure
2.2.2. Experimental Method of Mineral Composition
3. Results and Discussion
3.1. Influence of Shale Surface Structure
3.2. Influence of Mineral Composition
3.3. Characteristics of Shale Fracture under the Action of Sc-CO2
- (1)
- The impact of temperature on the density of supercritical fluids: under isobaric conditions, temperature increases decrease the density of supercritical fluids, resulting in a decrease in the solubility of supercritical fluids;
- (2)
- Influence of temperature on vapor pressure: temperature increases increase vapor pressure, which increases solubility in supercritical fluids.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Q.; Chen, X.; Jha, A.N.; Rogers, H. Natural gas from shale formation—The evolution, evidences and challenges of shale gas revolution in United States. Renew. Sustain. Energy Rev. 2014, 30, 1–28. [Google Scholar] [CrossRef]
- Zhao, W.; Hu, S.; Hou, L.; Yang, T.; Li, X. Types and resource potential of continental shale oil in China and its boundary with tight oil. Pet. Explor. Dev. 2020, 47, 1–10. [Google Scholar] [CrossRef]
- Zhao, W.; Hu, S.; Hou, L. Connotation and strategic role of in-situ conversion processing of shale oil underground in the onshore China. Pet. Explor. Dev. 2018, 45, 537–545. [Google Scholar] [CrossRef]
- Kang, Z.; Zhao, Y.; Yang, D. Review of oil shale in-situ conversion technology. Appl. Energy 2020, 269, 115–121. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Sun, B.; Gao, Y.; Wang, X. Optimization design of hydraulic parameters for supercritical CO2 fracturing in unconventional gas reservoir. Fuel 2019, 235, 795–809. [Google Scholar] [CrossRef]
- Song, X.; Li, G.; Guo, B. Transport feasibility of proppant by supercritical carbon dioxide fracturing in reservoir fractures. J. Hydrodyn. 2018, 30, 507–513. [Google Scholar] [CrossRef]
- Li, X.; Feng, Z.; Han, G. Breakdown pressure and fracture surface morphology of hydraulic fracturing in shale with H2O, CO2 and N2. Geomech. Geophys. Geo-Energy Geo-Resour. 2016, 2, 63–76. [Google Scholar] [CrossRef]
- Song, Z.; Su, W.; Yang, Y. An experimental study on the CO2/sand dry-frac process. Nat. Gas Ind. B 2014, 1, 192–196. [Google Scholar]
- Middleton, R.S.; Carey, J.W.; Currier, R.P. Shale gas and non-aqueous fracturing fluids: Opportunities and challenges for supercritical CO2. Appl. Energy 2015, 147, 500–509. [Google Scholar] [CrossRef]
- Godec, M.; Koperna, G.; Petrusak, R.; Oudinot, A. Potential for enhanced gas recovery and CO2 storage in the Marcellus Shale in the Eastern United States. Int. J. Coal Geol. 2013, 118, 95–104. [Google Scholar] [CrossRef]
- Yuan, Z.; Liao, X.; Zhao, X.; Chen, Z. Influence of dissolution on reservoir physical properties during CO2 displacement in sandstone reservoirs. Pet. Geol. Recovery Effic. 2020, 27, 97–104. [Google Scholar]
- Ding, L.; Ni, H. Effects of Supercritical Carbon Dioxide Immersion on Mechanical Properties of Shale. Sci. Technol. Eng. 2019, 19, 122–127. [Google Scholar]
- Huang, J.; Moriyoshi, T.; Manabe, H.M. Patterning of fine particles by means of supercritical CO2. J. Mater. Sci. 2006, 41, 1605–1610. [Google Scholar] [CrossRef]
- Wang, H.; Shen, Z.H.; Li, G.S. Shale gas development with ScCO2. Pet. Drill. Tech. 2011, 3, 30–35. [Google Scholar]
- Wang, M.; Xie, W.; Huang, K.; Dai, X. Fine characterization of lithofacies and pore network structure of continental shale: Case study of the Shuinan Formation in the north Jiaolai Basin, China. J. Pet. Sci. Eng. 2019, 175, 948–960. [Google Scholar] [CrossRef]
- Gupta, A.; Langlinais, J. Feasibility of Supercritical Carbon Dioxide as a Drilling Fluid for Deep Underbalanced Drilling Operation. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 9–12 October 2005; Society of Petroleum Engineers: Dallas, TX, USA, 2005. [Google Scholar]
- Angeli, M.; Soldal, M.; Skurtveit, E.; Aker, E. Experimental percolation of supercritical CO2 through a caprock. Energy Procedia 2009, 1, 3351–3358. [Google Scholar] [CrossRef]
- Tang, J.R.; Lu, Y.Y.; Chen, Y.T. Experimental study of damage of shale mechanical properties under supercritical CO2. Rock Soil Mech. 2018, 39, 797–802. [Google Scholar]
- Lyu, Q.; Ranjith, P.G.; Long, X.; Ji, B. Experimental Investigation of Mechanical Properties of Black Shales after CO2-Water-Rock Interaction. Materials 2016, 9, 663. [Google Scholar] [CrossRef]
- Lyu, Q.; Long, X.; Ranjith, P.G.; Tan, J.; Kang, Y. Experimental investigation on the mechanical properties of a low-clay shale with different adsorption times in sub-/super-critical CO2. Energy 2018, 147, 1288–1298. [Google Scholar] [CrossRef]
- Jiang, Y.; Luo, Y.; Lu, Y.; Qin, C.; Liu, H. Effects of supercritical CO2 treatment time, pressure, and temperature on microstructure of shale. Energy 2016, 97, 173–181. [Google Scholar] [CrossRef]
- Yin, H.; Zhou, J.; Jiang, Y.; Xian, X.; Liu, Q. Physical and structural changes in shale associated with supercritical CO2 exposure. Fuel 2016, 184, 289–303. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, X.; Tang, J.; Li, H.; Zhou, L. Relationship between pore structure and mechanical properties of shale on supercritical carbon dioxide saturation. Energy 2019, 172, 270–285. [Google Scholar] [CrossRef]
- Cheng, Y.; Zeng, M.; Lu, Z.; Du, X. Effects of Supercritical CO2 Treatment Temperatures on Mineral Composition, Pore Structure and Functional Groups of Shale: Implications for CO2 Sequestration. Sustainability 2020, 12, 3927. [Google Scholar] [CrossRef]
- Dai, X.; Wang, M.; Wei, C.; Zhang, J. Factors affecting shale microscopic pore structure variation during interaction with supercritical CO2. J. CO2 Util. 2020, 38, 194–211. [Google Scholar] [CrossRef]
- Ma, L.; Fauchille, A.L.; Ansari, H.; Chandler, M.; Ashby, P. Linking multi-scale 3D microstructure to potential enhanced natural gas recovery and subsurface CO2 storage for Bowland shale, UK. Energy Environ. Sci. 2021, 14, 4481–4498. [Google Scholar] [CrossRef]
- Rochelle, C.A.; Czernichowski-Lauriol, I.; Milodowski, A.E. The impact of chemical reactions on CO2 storage in geological formations: A brief review. Geol. Soc. Lond. Spec. Publ. 2004, 233, 87–106. [Google Scholar] [CrossRef]
- Aksu, I.; Bazilevskaya, E.; Karpyn, Z.T. Swelling of clay minerals in unconsolidated porous media and its impact on permeability. GeoResJ 2015, 7, 1–13. [Google Scholar] [CrossRef]
- Fatah, A.; Bennour, Z.; Mahmud, H.; Gholami, R.; Hossain, M.M. A Review on the Influence of CO2/Shale Interaction on Shale Properties: Implications of CCS in Shales. Energies 2020, 13, 3200. [Google Scholar] [CrossRef]
- Romanov, V.; Myshakin, E.M. Experimental studies: Clay swelling. Green Energy Technol. 2018, 7, 125–145. [Google Scholar]
- Zhou, J.; Yang, K.; Zhou, L.; Jiang, Y. Microstructure and mechanical properties alterations in shale treated via CO2/CO2-water exposure. J. Pet. Sci. Eng. 2021, 196, 108088. [Google Scholar] [CrossRef]
- Sabegh, M.A.; Rajaei, H.; Esmaeilzadeh, F.; Lashkarbolooki, M. Solubility of ketoprofen in supercritical carbon dioxide. J. Supercrit. Fluids 2012, 72, 191–197. [Google Scholar] [CrossRef]
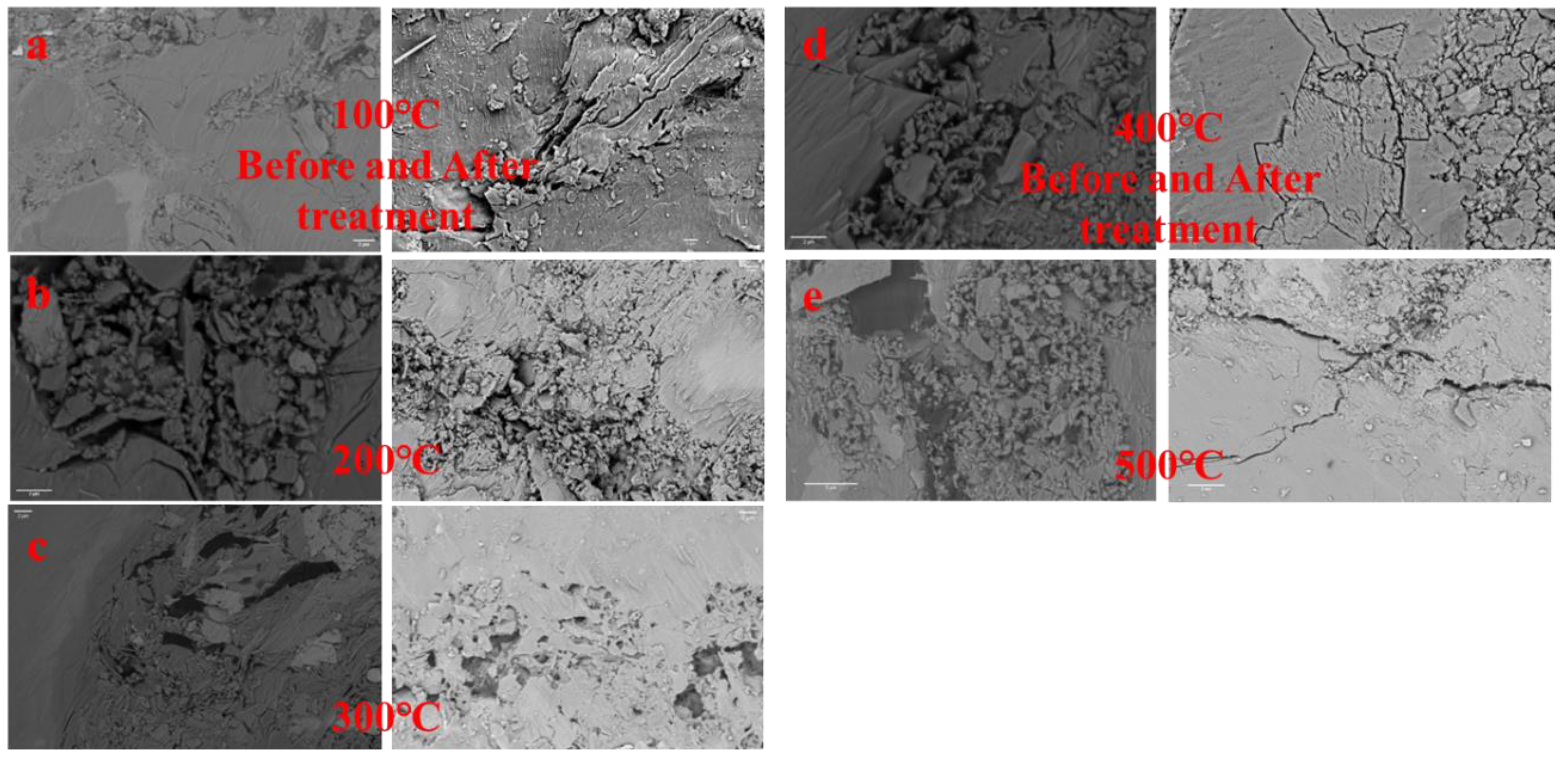
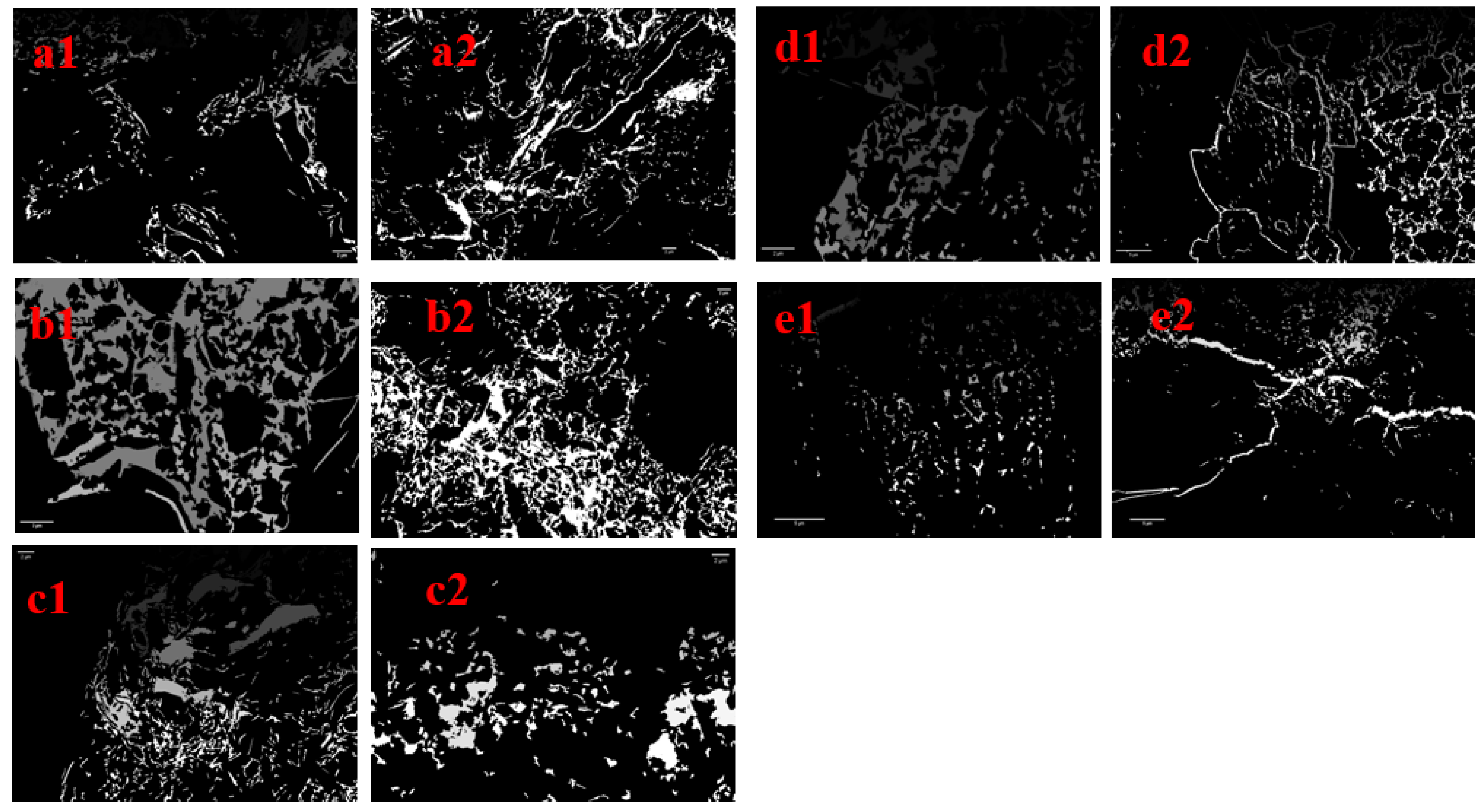
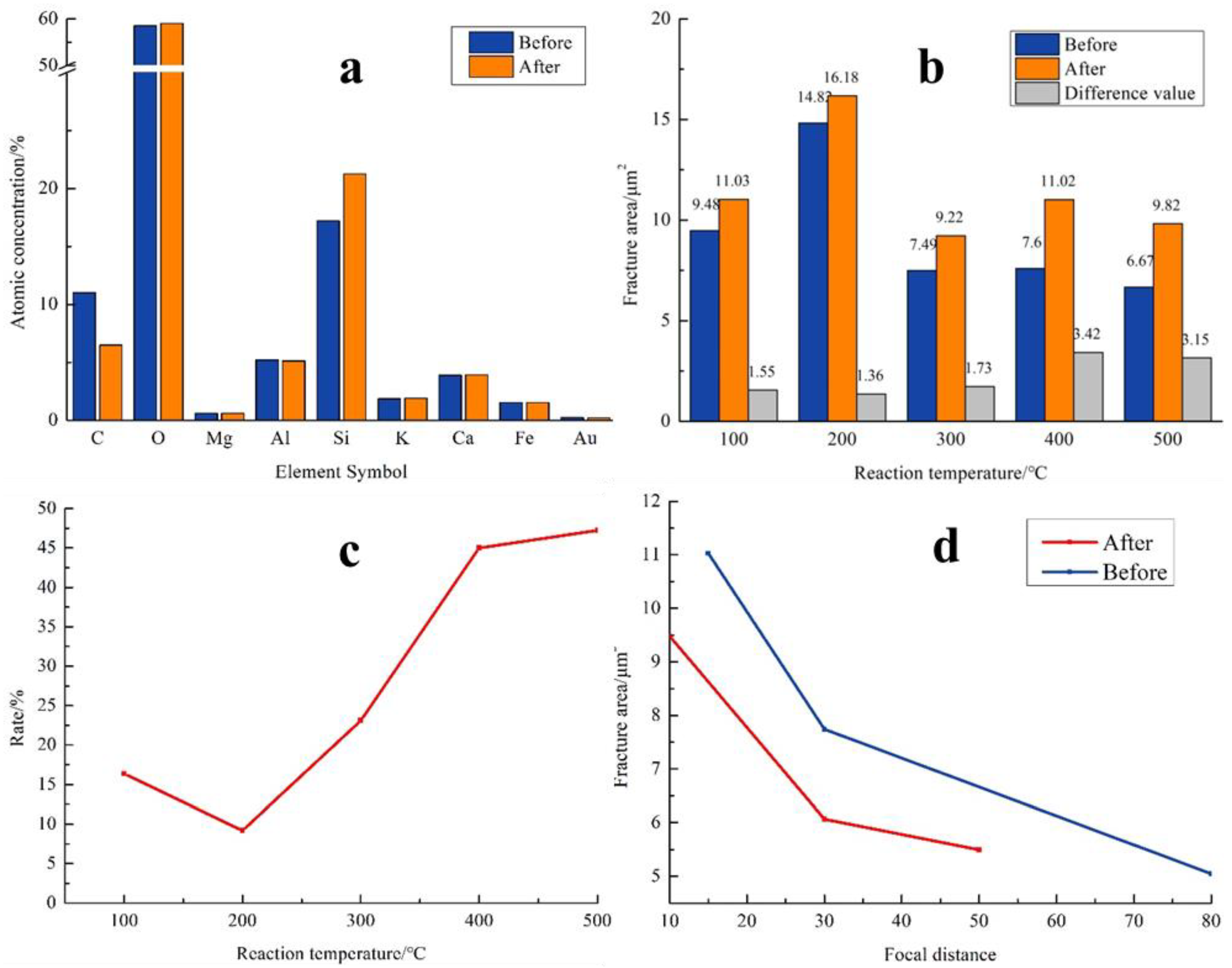

| T/°C | Before/μm2 | After/μm2 | Difference/μm2 | Rate/% |
|---|---|---|---|---|
| 100 | 9.48 | 11.03 | 1.55 | 16.35 |
| 200 | 14.82 | 16.18 | 1.36 | 9.18 |
| 300 | 7.49 | 9.22 | 1.73 | 23.10 |
| 400 | 7.60 | 11.02 | 3.42 | 45.00 |
| 500 | 6.67 | 9.82 | 3.15 | 47.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Zhao, X.; Jiang, Q.; Lin, X.; Gong, H.; Chen, X. Shale Microstructure Characteristics under the Action of Supercritical Carbon Dioxide (Sc-CO2). Energies 2022, 15, 8354. https://doi.org/10.3390/en15228354
Yu C, Zhao X, Jiang Q, Lin X, Gong H, Chen X. Shale Microstructure Characteristics under the Action of Supercritical Carbon Dioxide (Sc-CO2). Energies. 2022; 15(22):8354. https://doi.org/10.3390/en15228354
Chicago/Turabian StyleYu, Chunsheng, Xiao Zhao, Qi Jiang, Xiaosha Lin, Hengyuan Gong, and Xuanqing Chen. 2022. "Shale Microstructure Characteristics under the Action of Supercritical Carbon Dioxide (Sc-CO2)" Energies 15, no. 22: 8354. https://doi.org/10.3390/en15228354
APA StyleYu, C., Zhao, X., Jiang, Q., Lin, X., Gong, H., & Chen, X. (2022). Shale Microstructure Characteristics under the Action of Supercritical Carbon Dioxide (Sc-CO2). Energies, 15(22), 8354. https://doi.org/10.3390/en15228354






