Hydrogen Production by Immobilized Rhodopseudomonas sp. Cells in Calcium Alginate Beads
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algal Strain and Growth Conditions
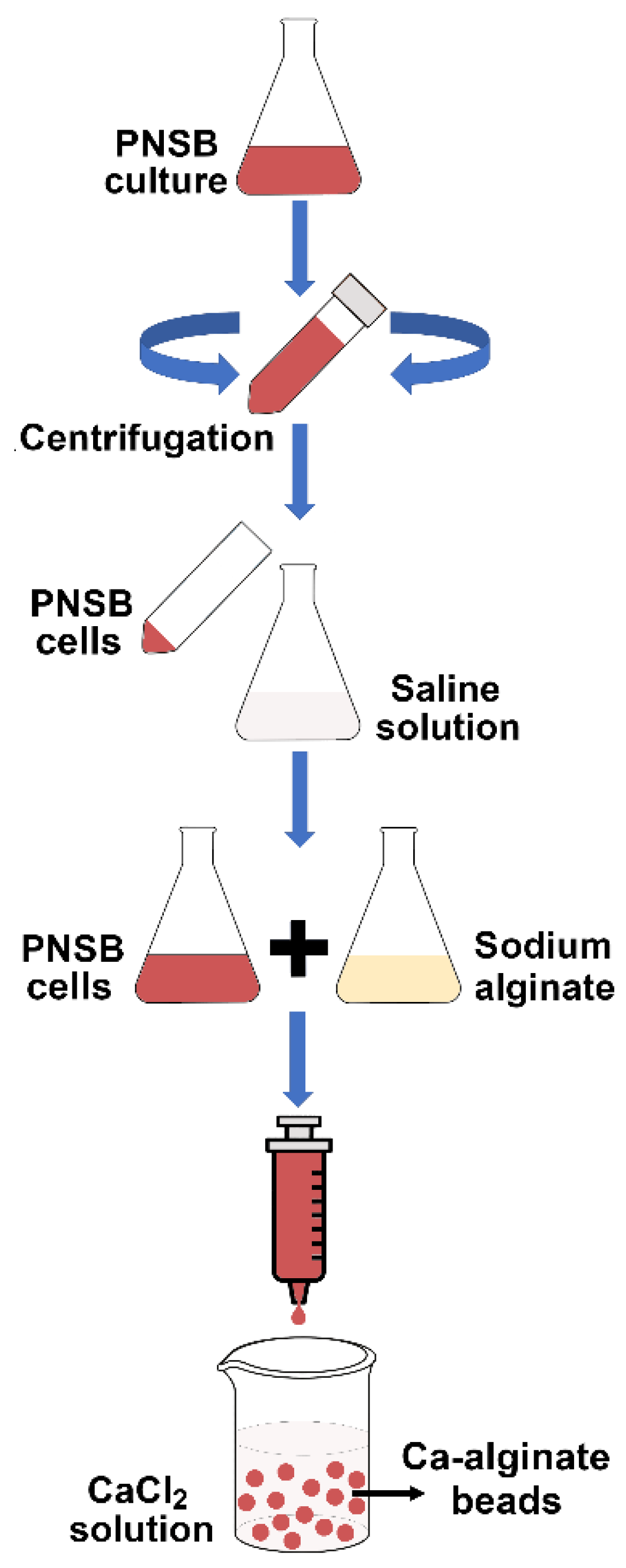
2.2. Immobilization
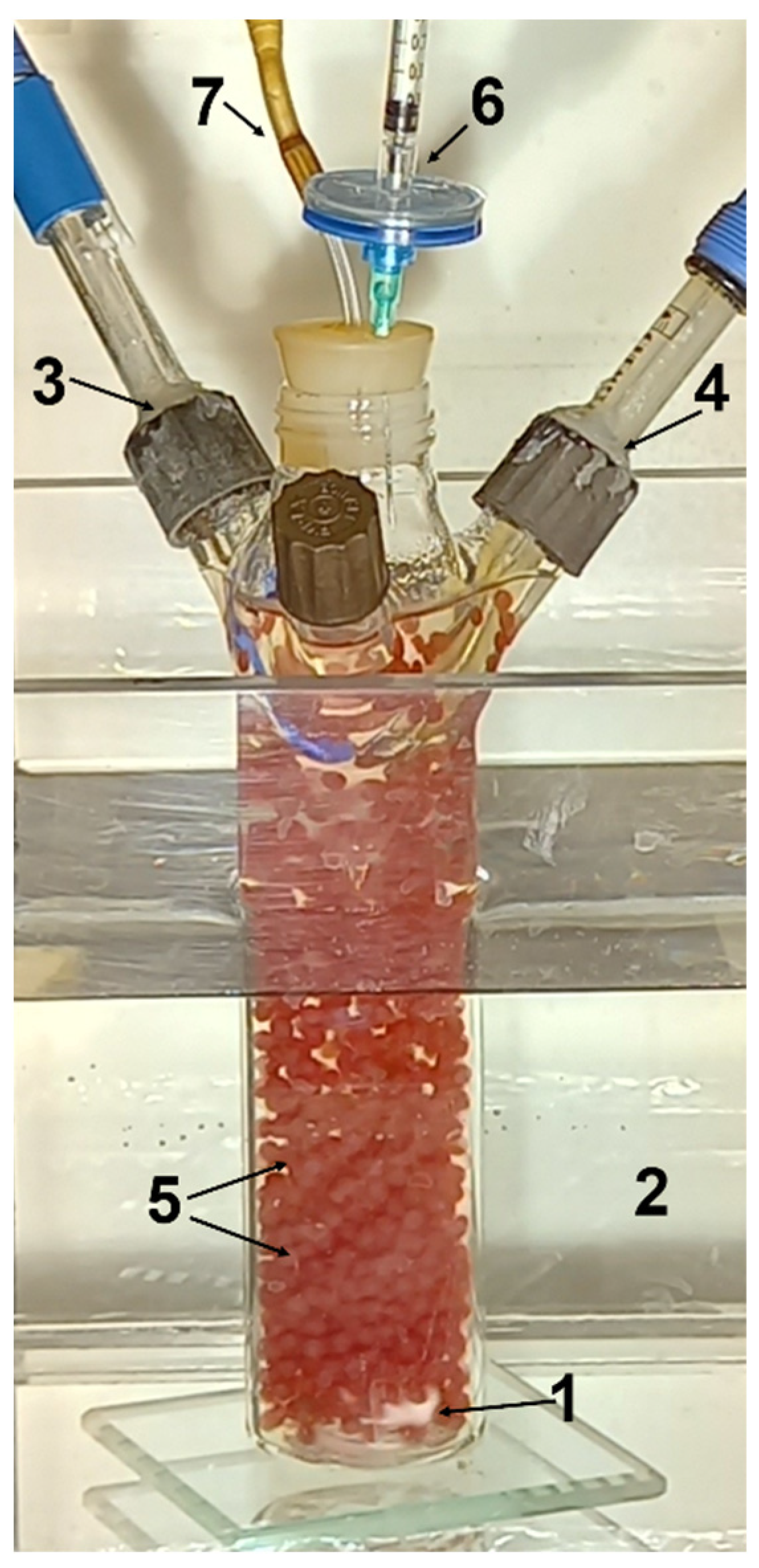
2.3. Hydrogen Production
2.4. Analytical Procedures
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- COM (2020) 301 Final, 08/07/2020. “Hydrogen Strategy for a Climate Neutral Europe”; European Economic and Social Committee and the Committee of the Regions: Bruxelles, Belgium, 2020.
- Qureshi, F.; Yusuf, M.; Kamyab, H.; Vo, D.-V.N.; Chelliapan, S.; Joo, S.-W.; Vasseghian, Y. Latest eco-friendly avenues on hydrogen production towards a circular bioeconomy: Currents challenges, innovative insights, and future perspectives. Renew. Sustain. Energy Rev. 2022, 168, 112916. [Google Scholar] [CrossRef]
- Qureshi, F.; Yusuf, M.; Pasha, A.A.; Khan, H.W.; Imteayaz, B.; Irshad, K. Sustainable and energy efficient hydrogen production via glycerol reforming techniques: A review. Int. J. Hydrogen Energy 2022, in press. [CrossRef]
- Qureshi, F.; Yusuf, M.; Kamyab, H.; Zaidi, S.; Khalil, M.J.; Khan, M.A.; Alam, M.A.; Masood, F.; Bazli, L.; Chelliapan, S.; et al. Current trends in hydrogen production, storage and applications in India: A review. Sustain. Energy Technol. Assess. 2022, 53 Part C, 102677. [Google Scholar] [CrossRef]
- Adessi, A.; De Philippis, R.; Hallenbeck, P.C. Combined systems for maximum substrate conversion. In Microbial Technologies in Advanced Biofuels Production; Hallenbeck, P.C., Ed.; Springer: New York, NY, USA, 2012; pp. 107–126. [Google Scholar]
- Redwood, M.D.; Paterson-Beedle, M.; Macaskie, L.E. Integrating dark and light biohydrogen production strategies: Towards the hydrogen economy. Rev. Environ. Sci. Biotechnol. 2009, 8, 149–185. [Google Scholar] [CrossRef] [Green Version]
- Baldi, F.; Pecorini, I.; Iannelli, R. Comparison of single-stage and two-stage anaerobic co-digestion of food waste and activated sludge for hydrogen and methane production. Renew. Energy 2019, 143, 1755–1765. [Google Scholar] [CrossRef]
- Touloupakis, E.; Faraloni, C.; Silva Benavides, A.M.; Torzillo, G. Recent achievements in microalgal photobiological hydrogen production. Energies 2021, 14, 7170. [Google Scholar] [CrossRef]
- Redding, K.E.; Appel, J.; Boehm, M.; Schuhmann, W.; Nowaczyk, M.M.; Yacoby, I.; Gutekunst, K. Advances and challenges in photosynthetic hydrogen production. Trends Biotechnol. 2022, 40, 1313–1325. [Google Scholar] [CrossRef]
- Chen, Y. Global potential of algae-based photobiological hydrogen production. Energy Environ. Sci. 2022, 15, 2843. [Google Scholar] [CrossRef]
- Carlozzi, P.; Touloupakis, E.; Filippi, S.; Cinelli, P.; Mezzetta, A.; Seggiani, M. Purple non-sulfur bacteria as cell factories to produce a copolymer as PHBV under light/dark cycle in a 4-L photobioreactor. J. Biotechnol. 2022, 356, 51–59. [Google Scholar] [CrossRef]
- Touloupakis, E.; Poloniataki, E.G.; Casciana, M.; Ghanotakis, D.F.; Carlozzi, P. Poly-β-hydroxybutyrate produc-tion by Rhodopseudomonas sp. grown in semi-continuous mode in a 4L photobioreactor. Symmetry 2021, 13, 1609. [Google Scholar] [CrossRef]
- Camacho, F.; Macedo, A.; Malcata, F. Potential industrial applications and commercialization of microalgae in the functional food and feed industries: A short review. Mar. Drugs 2019, 17, 53–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitnavare, R.; Bhattacharya, J.; Thongmee, S.; Ghosh, S. Photosynthetic microbes in nanobiotechnology: Applications and perspectives. Sci. Total Environ. 2022, 841, 156457. [Google Scholar] [CrossRef] [PubMed]
- Touloupakis, E.; Poloniataki, E.G.; Ghanotakis, D.F.; Carlozzi, P. Production of biohydrogen and/or poly-β-hydroxybutyrate by Rhodopseudomonas sp. using various carbon sources as substrate. Appl. Biochem. Biotechnol. 2021, 193, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Morsy, F.M.; Elbahloul, Y.; Elbadry, M. Photoheterotrophic growth of purple non-sulfur bacteria on tris acetate phosphate yeast extract (TAPY) medium and its hydrogen productivity in light under nitrogen deprivation. Int. J. Hydrogen Energy 2019, 44, 9282–9290. [Google Scholar] [CrossRef]
- Hakobyan, L.; Gabrielyan, L.; Trchounian, A. Biohydrogen by Rhodobacter sphaeroides during photo-fermentation: Mixed vs. sole carbon sources enhance bacterial growth and H2 production. Int. J. Hydrogen Energy 2019, 44, 674–679. [Google Scholar] [CrossRef]
- Boran, E.; Ozgur, E.; Van der Burg, J.; Yücel, M.; Gündüz, U.; Eroglu, I. Biological hydrogen production by Rhodobacter capsulatus in solar tubular photobioreactor. J. Clean. Prod. 2010, 18, S29–S35. [Google Scholar] [CrossRef]
- Markov, S.A.; Weaver, P.F. Bioreactors for H2 production by purple nonsulfur bacteria. Appl. Biochem. Biotechnol. 2008, 145, 79–86. [Google Scholar] [CrossRef]
- Oflaz, F.B.; Koku, H. Pilot-scale outdoor photofermentative hydrogen production from molasses using pH control. Int. J. Hydrogen Energy 2021, 46, 29160–29172. [Google Scholar] [CrossRef]
- Baeyens, J.; Zhang, H.; Nie, J.; Appels, L.; Dewil, R.; Ansart, R.; Deng, Y. Reviewing the potential of bio-hydrogen production by fermentation. Renew. Sustain. Energy Rev. 2020, 131, 110023. [Google Scholar] [CrossRef]
- Sagir, E.; Alipour, S. Photofermentative hydrogen production by immobilized photosynthetic bacteria: Current perspectives and challenges. Renew. Sustain. Energy Rev. 2021, 141, 110796. [Google Scholar] [CrossRef]
- Sharma, A.; Arya, S.K. Hydrogen from algal biomass: A review of production process. Biotechnol. Rep. 2017, 15, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Touloupakis, E.; Rontogiannis, G.; Silva Benavides, A.M.; Cicchi, B.; Ghanotakis, D.F.; Torzillo, G. Hydrogen production by immobilized Synechocystis sp. PCC 6803. Int. J. Hydrogen Energy 2016, 41, 15181–15186. [Google Scholar] [CrossRef]
- Sagir, E.; Hallenbeck, P.C. Photofermentative hydrogen production. Biomass. In Biohydrogen, Biofuels, Biochemicals, 2nd ed.; Pandey, A., Mohan, S.V., Chang, J.S., Hallenbeck, P., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 141–157. [Google Scholar]
- Moreno-Garrido, I. Microalgae immobilization: Current techniques and uses. Bioresour. Technol. 2008, 99, 3949–3964. [Google Scholar] [CrossRef]
- Song, W.; Rashid, N.; Choi, W.; Lee, K. Biohydrogen production by immobilized Chlorella sp. using cycles of oxygenic photosynthesis and anaerobiosis. Bioresour. Technol. 2011, 102, 8676–8681. [Google Scholar] [CrossRef] [PubMed]
- Shuba, E.S.; Kifle, D. Microalgae to biofuels: ‘Promising’ alternative and renewable energy, review. Renew. Sustain. Energy Rev. 2018, 81, 743755. [Google Scholar] [CrossRef]
- Carlozzi, P.; Seggiani, M.; Cinelli, P.; Mallegni, N.; Lazzeri, A. Photofermentative poly-3-hydroxybutyrate production by Rhodopseudomonas sp. S16-VOGS3 in a novel outdoor 70-L photobioreactor. Sustainability 2018, 10, 3133. [Google Scholar] [CrossRef] [Green Version]
- Touloupakis, E.; Silva Benavides, A.M.; Cicchi, B.; Torzillo, G. Growth and hydrogen production of outdoor cultures of Synechocystis PCC 6803. Algal Res. 2016, 16, 78–85. [Google Scholar] [CrossRef]
- Carlozzi, P.; Pushparaj, B.; Degl’Innocenti, A.; Capperucci, A. Growth characteristics of Rhodopseudomonas palustris cultured outdoors, in an underwater tubular photo-bioreactor, and investigation on photosynthetic efficiency. Appl. Microbiol. Biotechnol. 2006, 73, 789–795. [Google Scholar] [CrossRef]
- Carlozzi, P. The effect of irradiance growing on hydrogen photoevolution and on the kinetic growth in Rhodopseudomonas palustris, strain 42OL. Int. J. Hydrogen Energy 2009, 34, 7949–7958. [Google Scholar] [CrossRef]
- Chen, Y.T.; Wu, S.C.; Lee, C.M. Relationship between cell growth, hydrogen production and poly-β-hydroxybutyrate (PHB) accumulation by Rhodopseudomonas palustris WP3-5. Int. J. Hydrogen Energy 2012, 37, 13887–13894. [Google Scholar] [CrossRef]
- Kayahan, E.; Eroglu, I.; Koku, H. A compact tubular photobioreactor for outdoor hydrogen production from molasses. Int. J. Hydrogen Energy 2017, 42, 2575–2582. [Google Scholar] [CrossRef]
- Adessi, A.; De Philippis, R. Photobioreactor design and illumination systems for H2 production with anoxygenic photosynthetic bacteria: A review. Int. J. Hydrogen Energy 2014, 39, 3127–3141. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, S.; Zhang, Z.; Zhang, H.; Xia, C. Enhancement strategies for photo-fermentative biohydrogen production: A review. Bioresour. Technol. 2021, 340, 125601. [Google Scholar] [CrossRef] [PubMed]
- Elkahlout, K.; Alipour, S.; Eroglu, I.; Gunduz, U.; Yucel, M. Long-term biological production by agar immobilized Rhodobacter capsulatus in a sequential batch photobioreactor. Bioproc. Biosyst. Eng. 2016, 40, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Sagir, E.; Alipour, S.; Elkahlout, K.; Koku, H.; Gunduz, U.; Eroglu, I.; Yucel, M. Scale-up studies for stable, long-term indoor and outdoor production of hydrogen by immobilized Rhodobacter capsulatus. Int. J. Hydrogen Energy 2017, 42, 22743–22755. [Google Scholar] [CrossRef]
- Sagir, E.; Yucel, M.; Hallenbeck, P.C. Demonstration and optimization of sequential microaerobic dark- and photo-fermentation biohydrogen production by immobilized Rhodobacter capsulatus JP91. Bioresour. Technol. 2018, 250, 43–52. [Google Scholar] [CrossRef]
- Asada, Y.; Ohsawa, M.; Nagai, Y.; Ishimi, K.; Fukatsu, M.; Hideno, A.; Wakayama, T.; Miake, J. Re-evaluation of hydrogen productivity from acetate by some photosynthetic bacteria. Int. J. Hydrogen Energy 2008, 33, 5147–5150. [Google Scholar] [CrossRef]
- Basak, N.; Jana, A.K.; Das, D.; Saikia, D. Photofermentative molecular biohydrogen production by purple-non-sulfur (PNS) bacteria in various modes: The present progress and future perspective. Int. J. Hydrogen Energy 2014, 39, 6853–6871. [Google Scholar] [CrossRef]
- Zagrodnik, R.; Thiel, M.; Seifert, M.; Wlodarczak, M.; Laniecki, M. Application of immobilized Rhodobacter sphaeroides bacteria in hydrogen generation process under semi-continuous conditions. Int. J. Hydrogen Energy 2013, 38, 7632–7639. [Google Scholar] [CrossRef]
- Chen, C.Y.; Lee, C.M.; Chang, J.S. Feasibility study on bioreactor strategies for enhanced photohydrogen production from Rhodopseudomonas palustris WP3–5 using optical-fiber-assisted illumination systems. Int. J. Hydrogen Energy 2006, 31, 2345–2355. [Google Scholar] [CrossRef]
- Liao, Q.; Wang, Y.J.; Wang, Y.Z.; Zhu, X.; Tian, X.; Li, J. Formation and hydrogen production of photosynthetic bacterial biofilm under various illumination conditions. Bioresour. Technol. 2010, 101, 5315–5324. [Google Scholar] [CrossRef] [PubMed]
- Carlozzi, P. Hydrogen photoproduction by Rhodopseudomonas palustris 42OL cultured at high irradiance under a semicontinuous regime. J. Biomed. Biotechnol. 2012, 2012, 590693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Q.F.; Jin, Y.R.; Ma, C.; Wu, Y.N. Continuous hydrogen production in a novel photo-bioreactor with high light conversion efficiency. Adv. Mater. Res. 2014, 953–954, 970–973. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, X.; Liao, Q.; Wang, Y.Z.; Li, J.; Ding, Y.D.; Wang, H. Performance of a groove-type photobioreactor for hydrogen production by immobilized photosynthetic bacteria. Int. J. Hydrogen Energy 2010, 35, 5284–5292. [Google Scholar] [CrossRef]
- Wang, Y.; Tahir, N.; Cao, W.; Zhang, Q.; Lee, D.J. Grid columnar flat panel photobioreactor with immobilized photosynthetic bacteria for continuous photofermentative hydrogen production. Bioresour. Technol. 2019, 291, 121806. [Google Scholar] [CrossRef]
- Tsygankov, A.; Khusnutdinova, A. Hydrogen in metabolism of purple bacteria and prospects of practical application. Mikrobiologiia 2015, 84, 3–26. [Google Scholar] [CrossRef]
- Elkahlout, K.; Sagir, E.; Alipour, S.; Koku, H.; Gunduz, U.; Eroglu, I.; Yucel, M. Long-term stable hydrogen production from acetate using immobilized Rhodobacter capsulatus in a panel photobioreactor. Int. J. Hydrogen Energy 2018, 44, 18801–18810. [Google Scholar] [CrossRef]
- Xie, G.J.; Liu, B.F.; Ding, J.; Xing, D.F.; Ren, H.Y.; Guo, W.Q.; Ren, N.Q. Enhanced photo-H2 production by Rhodopseudomonas faecalis RLD-53 immobilization on activated carbon fibers. Biomass Bioenerg. 2012, 4, 122–129. [Google Scholar] [CrossRef]
- Zagrodnik, R.; Seifert, K.; Stodolny, M.; Laniecki, M. Continuous photofermentative production of hydrogen by immobilized Rhodobacter sphaeroides O.U.001. Int. J. Hydrogen Energy 2015, 40, 5062–5073. [Google Scholar] [CrossRef]
- Wen, H.-Q.; Du, J.; Xing, D.-F.; Ding, J.; Ren, N.-Q.; Liu, B.-F. Enhanced photo-fermentative hydrogen production of Rhodopseudomonas sp. nov. strain A7 by biofilm reactor. Int. J. Hydrogen Energy 2017, 42, 18288–18294. [Google Scholar] [CrossRef]
- Tian, X.; Liao, Q.A.; Zhu, X.; Wang, Y.Z.; Zhang, P.; Li, J. Characteristics of a biofilm photobioreactor as applied to photo-hydrogen production. Bioresour. Technol. 2009, 101, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.-L.; Zhu, X.; Liao, Q.; Wang, Y.Z.; Chen, R.; Lee, D.J. Enhancement of photo-hydrogen production in a biofilm photobioreactor using optical fiber with additional rough surface. Bioresour. Technol. 2011, 102, 8507–8513. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Z.; Duan, Y.; Wang, H. The effect of recycling culture medium after harvesting of Chlorella vulgaris biomass by flocculating bacteria on microalgal growth and the functionary mechanism. Bioresour. Technol. 2019, 280, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Park, J.Y.; Kim, Y.H.; Min, J. Evaluation of growth and utilization potential of Rhodobacter sphaeroides in reused medium. Mol. Biotechnol. 2022. [Google Scholar] [CrossRef]



| Organism | Immobilization Material | PBR (mL) | Light Intensity (W/m2) | Substrate (g/L) | H2 Production Rate (mL/L/h) | Reference |
|---|---|---|---|---|---|---|
| Rhodobacter capsulatus YO3 | Agar | 1400 | 200 | Acetic acid (3.6) | 31.2 | [50] |
| Rhodopseudomonas faecalis RLD-53 | Agar | 100 | 150 | Acetic acid (4.1) | 32.8 | [51] |
| Rhodobacter sphaeroides O.U.001 | Porous glass | 235 | 102 | Malic acid (2.0) | 12.7 | [52] |
| Rhodobacter sphaeroides | Porous glass | 200 | 64 | Malic acid (2.0) | 59 | [42] |
| Rhodopseudomonas sp. nov. strain A7 | Biofilm | 25 | 150 | Acetate (4.1) | 25 | [53] |
| Rhodopseudomonas palustris CQK 01 | Biofilm | 1200 | 39.5 | Glucose (21.6) | 38.9 | [54] |
| Rhodopseudomonas palustris CQK 01 | Biofilm | 125 | 12 | Glucose (9.0) | 39.2 | [55] |
| Rhodobacter capsulatus YO3 | Agar | 1400 | 200 | Sucrose (1.7) | 17.8 | [38] |
| Rhodopseudomonas sp. | Alginate | 200 | 80 | Acetate (4.0) | 22.2 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Touloupakis, E.; Chatziathanasiou, A.; Ghanotakis, D.F.; Carlozzi, P.; Pecorini, I. Hydrogen Production by Immobilized Rhodopseudomonas sp. Cells in Calcium Alginate Beads. Energies 2022, 15, 8355. https://doi.org/10.3390/en15228355
Touloupakis E, Chatziathanasiou A, Ghanotakis DF, Carlozzi P, Pecorini I. Hydrogen Production by Immobilized Rhodopseudomonas sp. Cells in Calcium Alginate Beads. Energies. 2022; 15(22):8355. https://doi.org/10.3390/en15228355
Chicago/Turabian StyleTouloupakis, Eleftherios, Angeliki Chatziathanasiou, Demetrios F. Ghanotakis, Pietro Carlozzi, and Isabella Pecorini. 2022. "Hydrogen Production by Immobilized Rhodopseudomonas sp. Cells in Calcium Alginate Beads" Energies 15, no. 22: 8355. https://doi.org/10.3390/en15228355
APA StyleTouloupakis, E., Chatziathanasiou, A., Ghanotakis, D. F., Carlozzi, P., & Pecorini, I. (2022). Hydrogen Production by Immobilized Rhodopseudomonas sp. Cells in Calcium Alginate Beads. Energies, 15(22), 8355. https://doi.org/10.3390/en15228355









