Effects of Different Heat Transfer Conditions on the Hydrogen Desorption Performance of a Metal Hydride Hydrogen Storage Tank
Abstract
:1. Introduction
2. Numerical Model of Hydrogen Desorption Reaction of Hydrogen Storage Alloy
2.1. Model Assumptions
2.2. Conservation Equations of the Reaction Zone
2.3. Kinetic Model of the Reaction Zone
2.4. Conservation Equations of the Heat Transfer Zone
2.5. Initial and Boundary Conditions
2.5.1. Initial Conditions
2.5.2. Boundary Conditions
3. Results and Discussion
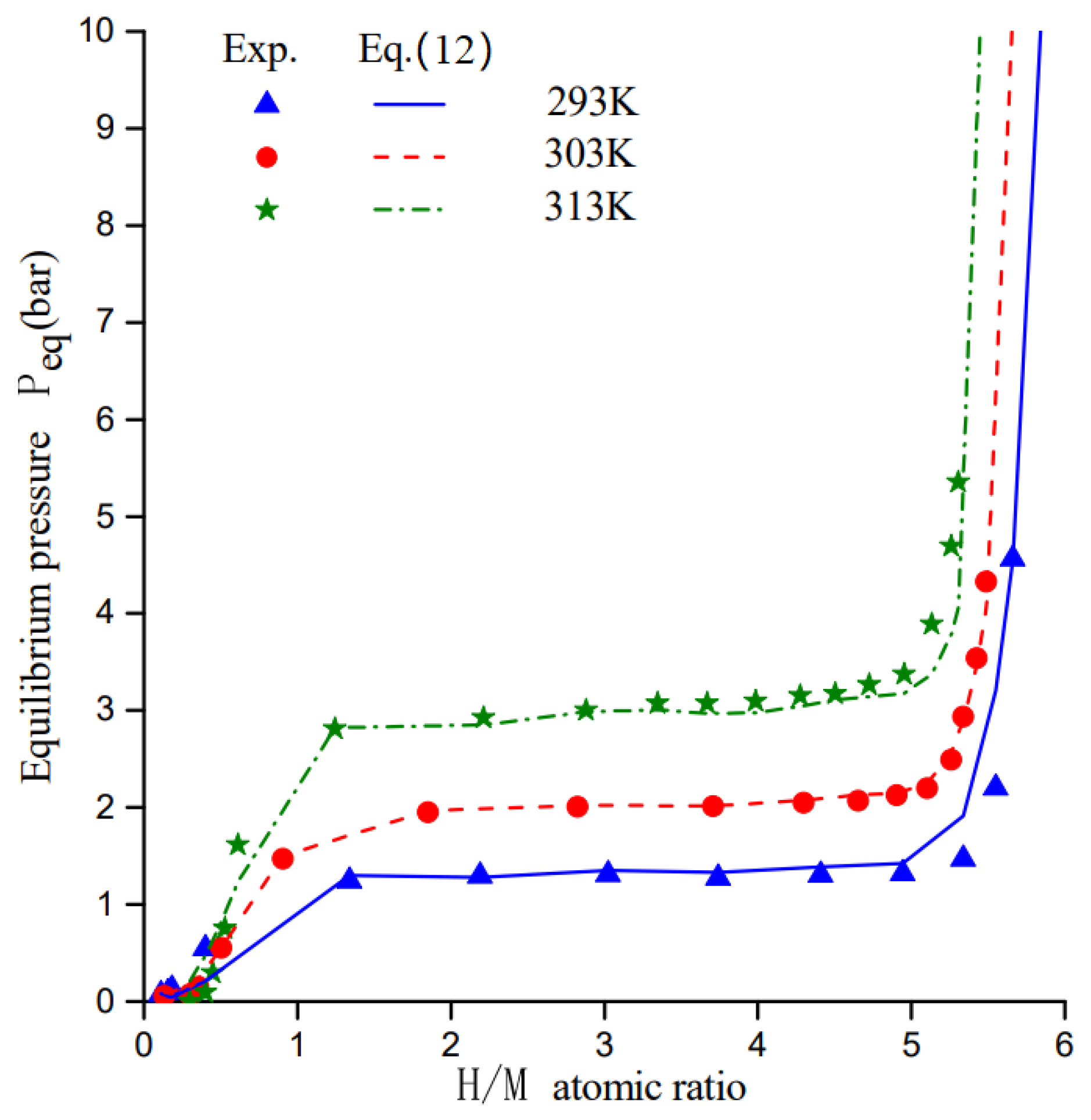
3.1. Model Validation
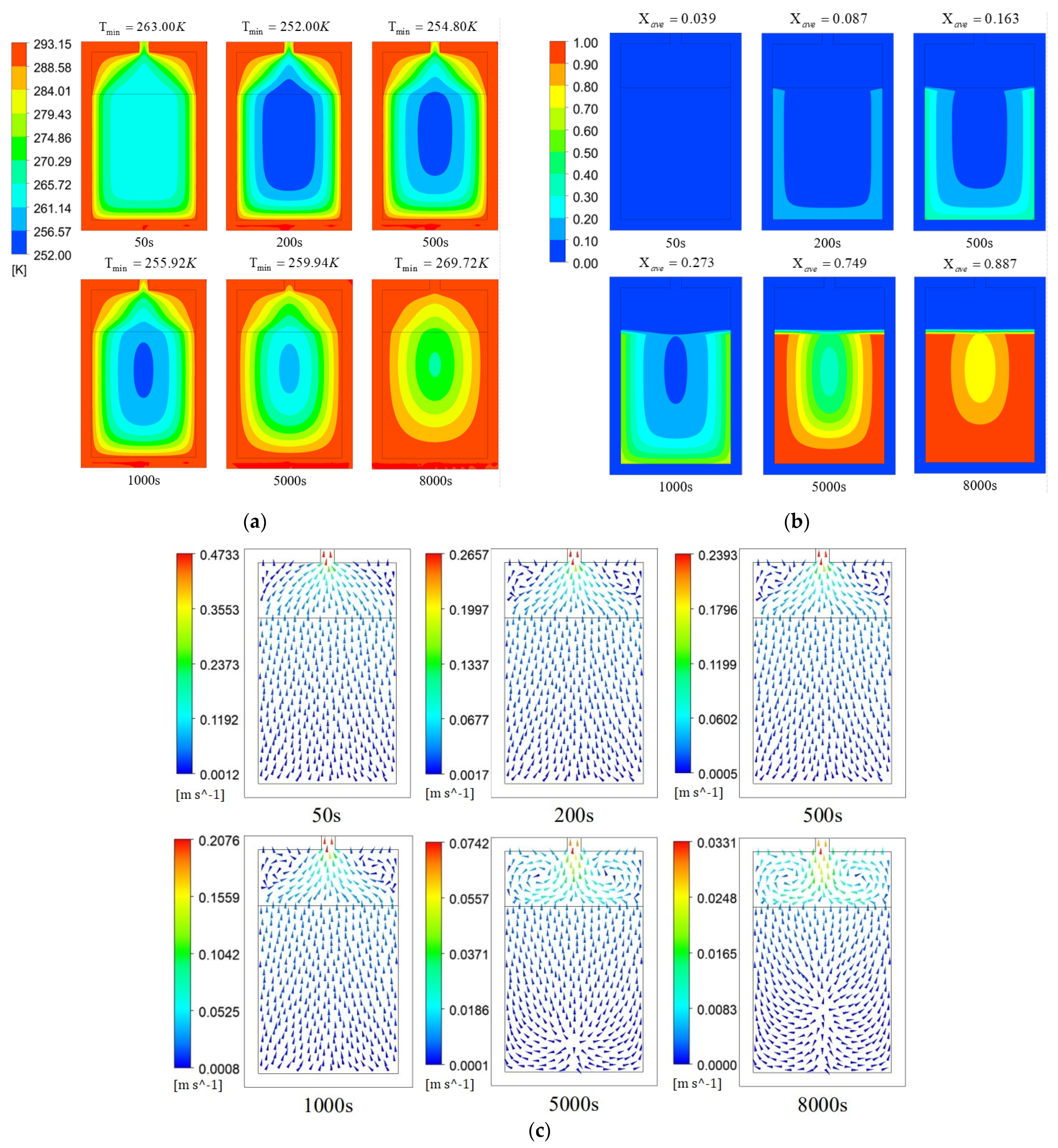
3.2. Analysis of Reactor Temperature, Reaction Fraction, and Flow Rate Distribution
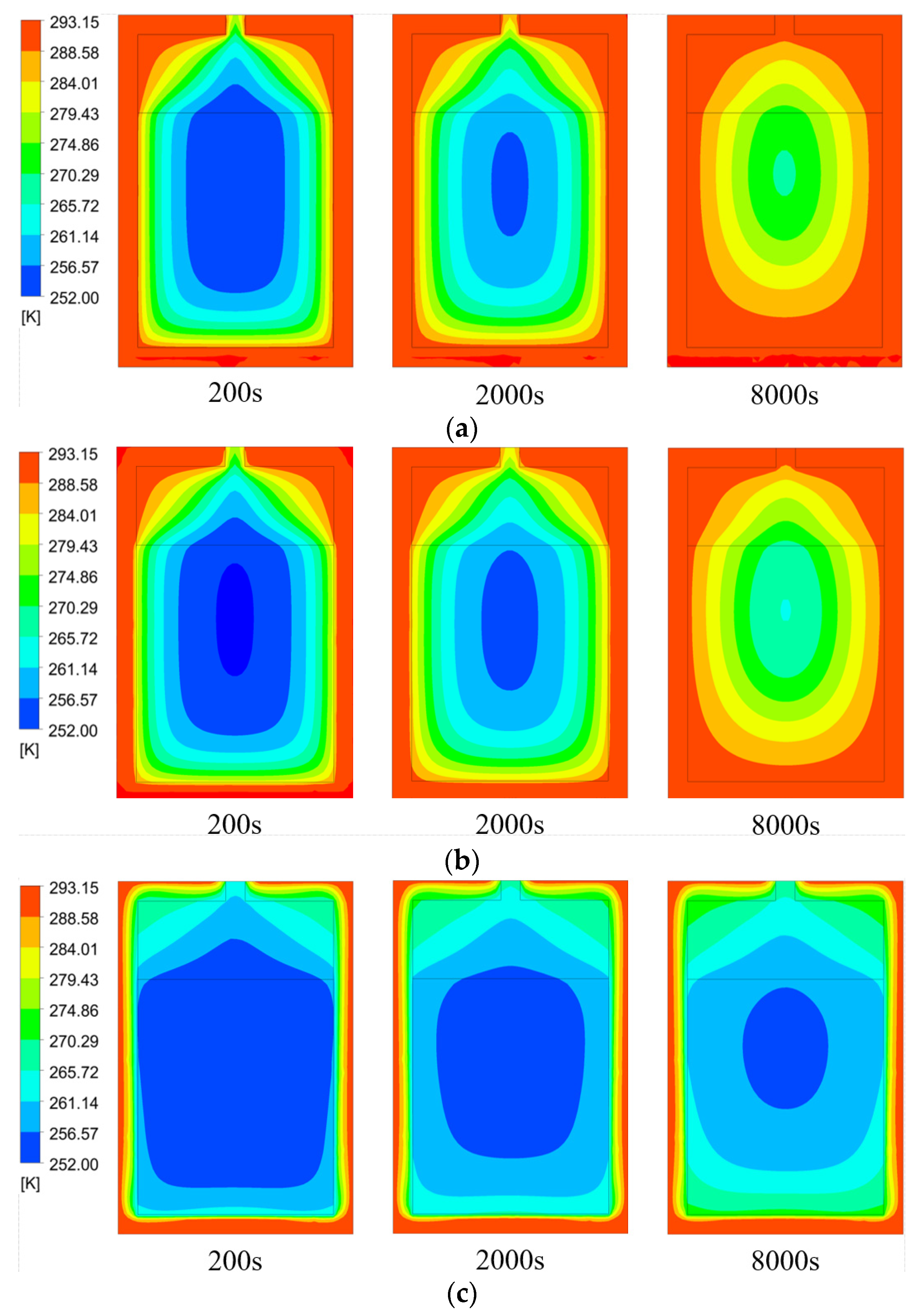
3.3. Effects of Temperature and Type of Heat Transfer Medium
4. Conclusions
- (1)
- In the initial stage of the hydrogen desorption reaction, the reaction rate is high, the reaction absorbs a lot of heat, and the temperature in the alloy zone drops sharply. As the temperature in the alloy zone continues to decrease, the reaction rate gradually decreases, the temperature in the tank reaches its minimum, and the hydrogen desorption reaction almost stops.
- (2)
- Under heat transfer water flow, the tank wall temperature gradually rises, and the reaction rate increases. While increasing the temperature of the water flow can shorten the hydrogen desorption time within a certain range, the tank wall first reaches hydrogen desorption saturation, and the hydrogen desorption reaction begins on the tank wall and progresses to the center of the tank. However, as the water flow temperature continues to increase, the effect of heat and mass transfer performance in the hydrogen storage tank gradually diminishes.
- (3)
- When the heat transfer medium is water and oil, the temperature and hydrogen storage capacity of the reactor during hydrogen desorption have similar characteristics, but the heat transfer time of water is about 10% shorter than that of oil, and the corresponding hydrogen desorption time is about 5% shorter. When the heat transfer medium is air, the heat transfer effect of the hydrogen storage tank decreases significantly at the same flow rate, the reaction rate difference between the tank wall and the center of the tank is small, the minimum temperature of the hydrogen desorption reaction decreases, and the hydrogen desorption time increases significantly.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thomas, K.M. Hydrogen adsorption and storage on porous materials. Catal. Today 2007, 120, 389–398. [Google Scholar] [CrossRef]
- Rusman, N.; Dahari, M. A review on the current progress of metal hydrides material for solid-state hydrogen storage applications. Int. J. Hydrog. Energy 2016, 41, 12108–12126. [Google Scholar] [CrossRef]
- Barthelemy, H.; Weber, F.; Barbier, F. Hydrogen storage: Recent improvements and industrial perspectives. Int. J. Hydrog. Energy 2017, 42, 7254–7262. [Google Scholar] [CrossRef]
- Song, J.; Wang, Y.; Li, S.; He, C.; Wang, D.; Yang, F.; Wu, Z.; Zheng, L.; Zhang, Z. Numerical and experimental study of La-Ni hydriding kinetics based on the varying-size model. Chem. Eng. Sci. 2018, 176, 580–599. [Google Scholar] [CrossRef]
- Chung, C.A.; Ho, C.J. Thermal–fluid behavior of the hydriding and dehydriding processes in a metal hydride hydrogen storage canister. Int. J. Hydrog. Energy 2009, 34, 4351–4364. [Google Scholar] [CrossRef]
- Baykara, S.Z. Hydrogen: A brief overview on its sources, production and environmental impact. Int. J. Hydrog. Energy 2018, 43, 10605–10614. [Google Scholar] [CrossRef]
- Marty, P.; Rango, P.; Delhomme, B.; Garrier, S. Various tools for optimizing large scale magnesium hydride storage. J. Alloy. Compd. 2013, 580 (Suppl. S1), S324–S328. [Google Scholar] [CrossRef]
- Nam, J.; Ko, J.; Ju, H. Three-dimensional modeling and simulation of hydrogen absorption in metal hydride hydrogen storage vessels. Appl. Energy 2012, 89, 164–175. [Google Scholar] [CrossRef]
- Chippar, P.; Lewis, S.D.; Rai, S.; Sircar, A. Numerical investigation of hydrogen absorption in a stackable metal hydride reactor utilizing compartmentalization. Int. J. Hydrog. Energy 2018, 43, 8007–8017. [Google Scholar] [CrossRef]
- Wang, Y.; Adroher, X.C.; Chen, J.; Yang, X.G.; Miller, T. Three-dimensional modeling of hydrogen sorption in metal hydride hydrogen storage beds. J. Power Sources 2009, 194, 997–1006. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Huang, Z.; Yang, F.; Wu, Z.; Zheng, L.; Wu, L.; Zhang, Z. Design optimization and sensitivity analysis of the radiation mini-channel metal hydride reactor. Energy 2019, 173, 443–456. [Google Scholar] [CrossRef]
- Agostini, A.; Belmonte, N.; Masala, A.; Hu, J.; Rizzi, P.; Fichtner, M.; Moretto, P.; Luetto, C.; Sgroi, M.; Baricco, M. Role of hydrogen tanks in the life cycle assessment of fuel cell-based auxiliary power units. Appl. Energy 2018, 215, 1–12. [Google Scholar] [CrossRef]
- Mghari, H.E.; Huot, J.; Xiao, J. Analysis of hydrogen storage performance of metal hydride reactor with phase change materials. Int. J. Hydrog. Energy 2019, 44, 28893–28908. [Google Scholar] [CrossRef]
- Kyoung, S.; Ferekh, S.; Gwak, G.; Jo, A.; Ju, H. Three-dimensional modeling and simulation of hydrogen desorption in metal hydride hydrogen storage vessels. Int. J. Hydrog. Energy 2015, 40, 14322–14330. [Google Scholar] [CrossRef]
- Corgnale, C.; Hardy, B.; Chahine, R.; Cossement, D. Hydrogen desorption using honeycomb finned heat exchangers integrated in adsorbent storage systems. Appl. Energy 2018, 213, 426–434. [Google Scholar] [CrossRef]
- Jemni, A.; Nasrallah, S.B.; Lamloumi, J. Experimental and theoretical study of ametal–hydrogen reactor. Int. J. Hydrog. Energy 1999, 24, 631–644. [Google Scholar] [CrossRef]
- Gkanas, E.I.; Grant, D.M.; Khzouz, M.; Stuart, A.D.; Manickam, K.; Walker, G.S. Efficient hydrogen storage in up-scale metal hydride tanks as possible metal hydride compression agents equipped with aluminium extended surfaces. Int. J. Hydrog. Energy 2016, 41, 10795–10810. [Google Scholar] [CrossRef]
- Askri, F.; Jemni, A.; Nasrallah, S.B. Dynamic behavior of metal–hydrogen reactor during hydriding process. Int. J. Hydrog. Energy 2004, 29, 635–647. [Google Scholar] [CrossRef]
- Dhaou, H.; Askri, F.; Salah, M.B.; Jemni, A.; Nasrallah, S.B.; Lamloumi, J. Measurement and modelling of kinetics of hydrogen sorption by LaNi and two related pseudobinary compounds. Int. J. Hydrog. Energy 2007, 32, 576–587. [Google Scholar] [CrossRef]
- Bao, Z.; Wu, Z.; Nyamsi, S.N.; Yang, F.; Zhang, Z. Three-dimensional modeling and sensitivity analysis of multi-tubular metal hydride reactors. Appl. Therm. Eng. 2013, 52, 97–108. [Google Scholar] [CrossRef]
- Du, T.; Chen, Q.; Du, W.; Cheng, L. Performance of continuous helical baffled heat exchanger with varying elliptical tube layouts. Int. J. Heat Mass Transf. 2019, 133, 1165–1175. [Google Scholar] [CrossRef]





| d0 | d1 | d2 | d3 | d4 | d5 | d6 | d7 | d8 | d9 |
|---|---|---|---|---|---|---|---|---|---|
| 0.426 | −4.114 | 14.180 | −13.109 | 4.557 | 0.166 | −0.592 | 0.179 | −0.023 | 0.00113 |
| Parameter | Unit | Value |
|---|---|---|
| Initial temperature, | °C | 20 |
| Reference temperature, | °C | 30 |
| Hydrogen desorption rate constant, | 9.57 | |
| Activation energy, | 16473 | |
| Enthalpy change, | −30,478.0 | |
| Hydrogen heat capacity, | 1489 | |
| Heat capacity of hydrogen storage alloy, | 419 | |
| Heat conductivity coefficient of hydrogen, | 0.167 | |
| Heat conductivity coefficient of alloy, | 1.3 | |
| Porosity, | -- | 0.63 |
| Permeability of the pore area, | ||
| Alloy density, | 8300 | |
| Saturated alloy density, | 8428 |
| Heat Transfer Media | Water | Oil | Air |
|---|---|---|---|
| Heat capacity | 4182 | 1845 | 1006.43 |
| Heat conductivity coefficient | 0.6 | 0.145 | 0.0242 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, M.; Tan, J.; Gao, L.; Liu, Z.; Chen, Y.; He, K.; Jiang, M. Effects of Different Heat Transfer Conditions on the Hydrogen Desorption Performance of a Metal Hydride Hydrogen Storage Tank. Energies 2022, 15, 8375. https://doi.org/10.3390/en15228375
Chai M, Tan J, Gao L, Liu Z, Chen Y, He K, Jiang M. Effects of Different Heat Transfer Conditions on the Hydrogen Desorption Performance of a Metal Hydride Hydrogen Storage Tank. Energies. 2022; 15(22):8375. https://doi.org/10.3390/en15228375
Chicago/Turabian StyleChai, Mu, Jiahui Tan, Lingwei Gao, Zhenan Liu, Yong Chen, Kuanfang He, and Mian Jiang. 2022. "Effects of Different Heat Transfer Conditions on the Hydrogen Desorption Performance of a Metal Hydride Hydrogen Storage Tank" Energies 15, no. 22: 8375. https://doi.org/10.3390/en15228375






