Experimental and Theoretical Investigation of the Synthesis, Electronic and Magnetic Properties of MnFe2O4 Spinel Ferrite
Abstract
:1. Introduction
2. Experimental Methods
2.1. Synthesis of MnFe2O4 Nanoparticles
2.2. Characterization Techniques
2.3. Simulation and Computational Analysis
3. Results and Discussion
3.1. Experimental Results: X-ray Diffraction and SEM Analysis
3.2. Simulation Results: DFT and Monte Carlo Simulations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goumrhar, F.; Bahmad, L.; Mounkachi, O.; Benyoussef, A. Magnetic Properties of Vanadium Doped CdTe: Ab Initio Calculations. J. Magn. Magn. Mater. 2017, 428, 368–371. [Google Scholar] [CrossRef]
- Mediane, N.; Goumrhar, F.; Drissi, L.B.; Laghrissi, M.; Laamara, R.A. High Curie Temperatures and Ferromagnetism Formation in Cr-Substituted Oxide Lithium Compound. J. Supercond. Nov. Magn. 2022, 35, 463–471. [Google Scholar] [CrossRef]
- Mamouni, N.; Goumrhar, F.; Salmani, E.; Benyoussef, A.; Ez-Zahraouy, H.; Mounkachi, O. Spin-Orbit Interaction in SnO2 Based Diluted Magnetic Semiconductor: Ab-Initio Calculations. J. Magn. Magn. Mater. 2021, 535, 168084. [Google Scholar] [CrossRef]
- Zhao, G.L.; Callaway, J.; Hayashibara, M. Electronic Structures of Iron and Cobalt Pyrites. Phys. Rev. B 1993, 48, 15781–15786. [Google Scholar] [CrossRef] [PubMed]
- Ennaoui, A.; Fiechter, S.; Jaegermann, W.; Tributsch, H. Photoelectrochemistry of Highly Quantum Efficient Single-Crystalline n-FeS2 (Pyrite). J. Electrochem. Soc. 1986, 133, 97. [Google Scholar] [CrossRef]
- Mouatassime, M.; Selmani, Y.; Idrissi, S.; Bahmad, L.; Goumrhar, F.; Labrim, H.; Benyoussef, A. Magnetic Properties and Half Metallic Behavior of the Full-Heusler Co2FeGe Alloy: DFT and Monte Carlo Studies. J. Solid State Chem. 2021, 304, 122534. [Google Scholar] [CrossRef]
- Güler, E.; Güler, M.; Uğur, Ş.; Uğur, G. First Principles Study of Electronic, Elastic, Optical and Magnetic Properties of Rh2MnX (X = Ti, Hf, Sc, Zr, Zn) Heusler Alloys. Quantum Chem. 2021, 121, e26606. [Google Scholar] [CrossRef]
- Bouachraoui, R.; Ziat, Y.; Sbai, Y.; El Rhazouani, O.; Goumrhar, F.; Bahmad, L. The Magnetocaloric and Magnetic Properties of the MnFe4Si3: Monte Carlo Investigation. J. Alloy. Compd. 2019, 809, 151785. [Google Scholar] [CrossRef]
- Selmani, Y.; Mouatassime, M.; Goumrhar, F.; Labrim, H.; Bahmad, L.; Benyoussef, A. Structural, Electronic and Magnetic Properties of the Perovskite Ymno3. Solid State Commun. 2021, 328, 114254. [Google Scholar] [CrossRef]
- Jonathan, L.; Diguna, L.J.; Samy, O.; Muqoyyanah, M.; Abu Bakar, S.; Birowosuto, M.D.; El Moutaouakil, A. Hybrid Organic–Inorganic Perovskite Halide Materials for Photovoltaics towards Their Commercialization. Polymers 2022, 14, 1059. [Google Scholar] [CrossRef]
- Sahdane, T.; Mtougui, S.; Goumrhar, F.; Mamouni, N.; Salmani, E.; Ez-Zahraouy, H.; Benyoussef, A.; Mounkachi, O. Magnetic Phase Transitions of Phosphorene-like Nano-Structure: Monte Carlo Study. Philos. Mag. 2021, 101, 1836–1848. [Google Scholar] [CrossRef]
- Tiouitchi, G.; Ali, M.A.; Benyoussef, A.; Hamedoun, M.; Lachgar, A.; Kara, A.; Ennaoui, A.; Mahmoud, A.; Boschini, F.; Oughaddou, H.; et al. Efficient Production of Few-Layer Black Phosphorus by Liquid-Phase Exfoliation. R. Soc. Open Sci. 2020, 7, 201210. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Matte, H.S.S.R.; Subrahmanyam, K.S.; Maitra, U. Unusual Magnetic Properties of Graphene and Related Materials. Chem. Sci. 2011, 3, 45–52. [Google Scholar] [CrossRef]
- Panda, J.; Ramu, M.; Karis, O.; Sarkar, T.; Kamalakar, M.V. Ultimate Spin Currents in Commercial Chemical Vapor Deposited Graphene. ACS Nano 2020, 14, 12771–12780. [Google Scholar] [CrossRef]
- Schulz, N.; Chanda, A.; Datt, G.; Kamalakar, M.V.; Sarkar, T.; Phan, M.H.; Srikanth, H. Proximity Enhanced Magnetism at NiFe2O4/Graphene Interface. AIP Adv. 2022, 12, 035132. [Google Scholar] [CrossRef]
- Moutaouakil, A.E.; Kang, H.-C.; Handa, H.; Fukidome, H.; Suemitsu, T.; Sano, E.; Suemitsu, M.; Otsuji, T. Room Temperature Logic Inverter on Epitaxial Graphene-on-Silicon Device. Jpn. J. Appl. Phys. 2011, 50, 070113. [Google Scholar] [CrossRef] [Green Version]
- Hijazi, A.; Moutaouakil, A.E. Graphene and MoS2 Structures for THz Applications. In Proceedings of the 2019 44th International Conference on Infrared, Millimeter, and Terahertz Waves (IRMMW-THz), Paris, France, 1–6 September 2019; pp. 1–2. [Google Scholar]
- Samy, O.; El Moutaouakil, A. A Review on MoS2 Energy Applications: Recent Developments and Challenges. Energies 2021, 14, 4586. [Google Scholar] [CrossRef]
- Samy, O.; Zeng, S.; Birowosuto, M.D.; El Moutaouakil, A. A Review on MoS2 Properties, Synthesis, Sensing Applications and Challenges. Crystals 2021, 11, 355. [Google Scholar] [CrossRef]
- Moutaouakil, A.E. Two-Dimensional Electronic Materials for Terahertz Applications: Linking the Physical Properties with Engineering Expertise. In Proceedings of the 2018 6th International Renewable and Sustainable Energy Conference (IRSEC), Rabat, Morocco, 5–8 December 2018; pp. 1–4. [Google Scholar]
- El Moutaouakil, A.; Suemitsu, T.; Otsuji, T.; Videlier, H.; Boubanga-Tombet, S.-A.; Coquillat, D.; Knap, W. Device Loading Effect on Nonresonant Detection of Terahertz Radiation in Dual Grating Gate Plasmon-Resonant Structure Using InGaP/InGaAs/GaAs Material Systems. Phys. Status Solidi (c) 2011, 8, 346–348. [Google Scholar] [CrossRef]
- Moutaouakil, A.E.; Suemitsu, T.; Otsuji, T.; Coquillat, D.; Knap, W. Nonresonant Detection of Terahertz Radiation in High-Electron-Mobility Transistor Structure Using InAlAs/InGaAs/InP Material Systems at Room Temperature. J. Nanosci. Nanotechnol. 2012, 12, 6737–6740. [Google Scholar] [CrossRef]
- Moutaouakil, A.E.; Watanabe, T.; Haibo, C.; Komori, T.; Nishimura, T.; Suemitsu, T.; Otsuji, T. Spectral Narrowing of Terahertz Emission from Super-Grating Dual-Gate Plasmon-Resonant High-Electron Mobility Transistors. J. Phys. Conf. Ser. 2009, 193, 012068. [Google Scholar] [CrossRef]
- Meziani, Y.M.; Garcia, E.; Velazquez, E.; Diez, E.; El Moutaouakil, A.; Otsuji, T.; Fobelets, K. Strained Silicon Modulation Field-Effect Transistor as a New Sensor of Terahertz Radiation. Semicond. Sci. Technol. 2011, 26, 105006. [Google Scholar] [CrossRef]
- Rabias, I.; Tsitrouli, D.; Karakosta, E.; Kehagias, T.; Diamantopoulos, G.; Fardis, M.; Stamopoulos, D.; Maris, T.G.; Falaras, P.; Zouridakis, N.; et al. Rapid Magnetic Heating Treatment by Highly Charged Maghemite Nanoparticles on Wistar Rats Exocranial Glioma Tumors at Microliter Volume. Biomicrofluidics 2010, 4, 024111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, O.S.; Lee, S.H.; Park, S.J.; An, J.H.; Song, H.S.; Kim, T.; Oh, J.H.; Bae, J.; Yoon, H.; Park, T.H.; et al. Large-Scale Graphene Micropattern Nano-Biohybrids: High-Performance Transducers for FET-Type Flexible Fluidic HIV Immunoassays. Adv. Mater. 2013, 25, 4177–4185. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhao, F.; Zeng, B. Magnetic Entrapment for Fast and Sensitive Determination of Metronidazole with a Novel Magnet-Controlled Glassy Carbon Electrode. Electrochim. Acta 2014, 135, 154–160. [Google Scholar] [CrossRef]
- Schätz, A.; Reiser, O.; Stark, W.J. Nanoparticles as Semi-Heterogeneous Catalyst Supports. Chem. Eur. J. 2010, 16, 8950–8967. [Google Scholar] [CrossRef]
- Yoon, T.-J.; Lee, W.; Oh, Y.-S.; Lee, J.-K. Magnetic Nanoparticles as a Catalyst Vehicle for Simple and Easy Recycling. New J. Chem. 2003, 27, 227–229. [Google Scholar] [CrossRef]
- Sun, X.; Huang, Y.; Nikles, D.E. FePt and CoPt Magnetic Nanoparticles Film for Future High Density Data Storage Media. Int. J. Nanotechnol. 2004, 1, 328–346. [Google Scholar] [CrossRef]
- Harris, V.G. Modern Microwave Ferrites. IEEE Trans. Magn. 2012, 48, 1075–1104. [Google Scholar] [CrossRef]
- Datt, G.; Bishwas, M.S.; Raja, M.M.; Abhyankar, A.C. Observation of Magnetic Anomalies in One-Step Solvothermally Synthesized Nickel–Cobalt Ferrite Nanoparticles. Nanoscale 2016, 8, 5200–5213. [Google Scholar] [CrossRef]
- López-Ortega, A.; Lottini, E.; de Julián Fernández, C.; Sangregorio, C. Exploring the Magnetic Properties of Cobalt-Ferrite Nanoparticles for the Development of a Rare-Earth-Free Permanent Magnet. Chem. Mater. 2015, 27, 4048–4056. [Google Scholar] [CrossRef]
- Sivakumar, P.; Ramesh, R.; Ramanand, A.; Ponnusamy, S.; Muthamizhchelvan, C. Synthesis and Characterization of Nickel Ferrite Magnetic Nanoparticles. Mater. Res. Bull. 2011, 46, 2208–2211. [Google Scholar] [CrossRef]
- Granone, L.I.; Ulpe, A.C.; Robben, L.; Klimke, S.; Jahns, M.; Renz, F.; Gesing, T.M.; Bredow, T.; Dillert, R.; Bahnemann, D.W. Effect of the Degree of Inversion on Optical Properties of Spinel ZnFe2O4. Phys. Chem. Chem. Phys. 2018, 20, 28267–28278. [Google Scholar] [CrossRef] [Green Version]
- Soufi, A.; Hajjaoui, H.; Elmoubarki, R.; Abdennouri, M.; Qourzal, S.; Barka, N. Spinel Ferrites Nanoparticles: Synthesis Methods and Application in Heterogeneous Fenton Oxidation of Organic Pollutants—A Review. Appl. Surf. Sci. Adv. 2021, 6, 100145. [Google Scholar] [CrossRef]
- Dippong, T.; Levei, E.A.; Cadar, O. Recent Advances in Synthesis and Applications of MFe2O4 (M = Co, Cu, Mn, Ni, Zn) Nanoparticles. Nanomaterials 2021, 11, 1560. [Google Scholar] [CrossRef]
- Abraime, B.; El Maalam, K.; Fkhar, L.; Mahmoud, A.; Boschini, F.; Ait Tamerd, M.; Benyoussef, A.; Hamedoun, M.; Hlil, E.K.; Ait Ali, M.; et al. Influence of Synthesis Methods with Low Annealing Temperature on the Structural and Magnetic Properties of CoFe2O4 Nanopowders for Permanent Magnet Application. J. Magn. Magn. Mater. 2020, 500, 166416. [Google Scholar] [CrossRef]
- Mounkachi, O.; Lamouri, R.; Salmani, E.; Hamedoun, M.; Benyoussef, A.; Ez-Zahraouy, H. Origin of the Magnetic Properties of MnFe2O4 Spinel Ferrite: Ab Initio and Monte Carlo Simulation. J. Magn. Magn. Mater. 2021, 533, 168016. [Google Scholar] [CrossRef]
- Lamouri, R.; Mounkachi, O.; Salmani, E.; Hamedoun, M.; Benyoussef, A.; Ez-Zahraouy, H. Size Effect on the Magnetic Properties of CoFe2O4 Nanoparticles: A Monte Carlo Study. Ceram. Int. 2020, 46, 8092–8096. [Google Scholar] [CrossRef]
- Srivastava, C.M.; Srinivasan, G.; Nanadikar, N.G. Exchange Constants in Spinel Ferrites. Phys. Rev. B 1979, 19, 499–508. [Google Scholar] [CrossRef] [Green Version]
- Bercoff, P.G.; Bertorello, H.R. Exchange Constants and Transfer Integrals of Spinel Ferrites. J. Magn. Magn. Mater. 1997, 169, 314–322. [Google Scholar] [CrossRef]
- Nasri, M.; Henchiri, C.; Dhahri, R.; Khelifi, J.; Dhahri, E.; Mariano, J.F.M.L. Study of Structural, Magnetic, Magnetocaloric Properties and Critical Behavior of CoFeCuO4 Spinel Ferrite. Inorg. Chem. Commun. 2021, 133, 108933. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. (Eds.) Sol-Gel Science; Academic Press: San Diego, CA, USA, 1990; p. iv. ISBN 978-0-08-057103-4. [Google Scholar]
- Schwarz, K. DFT Calculations of Solids with LAPW and WIEN2k. J. Solid State Chem. 2003, 176, 319–328. [Google Scholar] [CrossRef]
- Ong, K.P.; Bai, K.; Blaha, P.; Wu, P. Electronic Structure and Optical Properties of AFeO2 (A = Ag, Cu) within GGA Calculations. Chem. Mater. 2007, 19, 634–640. [Google Scholar] [CrossRef]
- Tang, S.; Zhao, M.; Yuan, D.; Li, X.; Zhang, X.; Wang, Z.; Jiao, T.; Wang, K. MnFe2O4 Nanoparticles Promoted Electrochemical Oxidation Coupling with Persulfate Activation for Tetracycline Degradation. Sep. Purif. Technol. 2021, 255, 117690. [Google Scholar] [CrossRef]
- Baig, M.M.; Zulfiqar, S.; Yousuf, M.A.; Touqeer, M.; Ullah, S.; Agboola, P.; Warsi, M.F.; Shakir, I. Structural and Photocatalytic Properties of New Rare Earth La3+ Substituted MnFe2O4 Ferrite Nanoparticles. Ceram. Int. 2020, 46, 23208–23217. [Google Scholar] [CrossRef]
- Sharifi, S.; Rahimi, K.; Yazdani, A. Highly Improved Supercapacitance Properties of MnFe2O4 Nanoparticles by MoS2 Nanosheets. Sci. Rep. 2021, 11, 8378. [Google Scholar] [CrossRef] [PubMed]
- Datt, G.; Raja, M.M.; Abhyankar, A.C. Steering of Magnetic Interactions in Ni0.5Zn0.5Fe2–x(Mn)XO4 Nanoferrites via Substitution-Induced Cationic Redistribution. J. Phys. Chem. C 2021, 125, 10693–10707. [Google Scholar] [CrossRef]
- Mary Jacintha, A.; Umapathy, V.; Neeraja, P.; Rex Jeya Rajkumar, S. Synthesis and Comparative Studies of MnFe2O4 Nanoparticles with Different Natural Polymers by Sol–Gel Method: Structural, Morphological, Optical, Magnetic, Catalytic and Biological Activities. J. Nanostruct. Chem. 2017, 7, 375–387. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.P.; Sorensen, C.M.; Klabunde, K.J.; Hadjipanayis, G.C.; Devlin, E.; Kostikas, A. Size-Dependent Magnetic Properties of MnFe2O4 Fine Particles Synthesized by Coprecipitation. Phys. Rev. B 1996, 54, 9288–9296. [Google Scholar] [CrossRef] [PubMed]
- Ostler, T.A.; Barker, J.; Evans, R.F.L.; Chantrell, R.W.; Atxitia, U.; Chubykalo-Fesenko, O.; El Moussaoui, S.; Le Guyader, L.; Mengotti, E.; Heyderman, L.J.; et al. Ultrafast Heating as a Sufficient Stimulus for Magnetization Reversal in a Ferrimagnet. Nat. Commun. 2012, 3, 666. [Google Scholar] [CrossRef]
- Amighian, J.; Mozaffari, M.; Nasr, B. Preparation of Nano-Sized Manganese Ferrite (MnFe2O4) via Coprecipitation Method. Phys. Status solidi (c) 2006, 3, 3188–3192. [Google Scholar] [CrossRef]
- Bas, J.A.; Calero, J.A.; Dougan, M.J. Sintered Soft Magnetic Materials. Properties and Applications. J. Magn. Magn. Mater. 2003, 254–255, 391–398. [Google Scholar] [CrossRef]
- Aghrich, K.; Abdellaoui, M.; Mamouni, N.; Bellaouchou, A.; Fekhaoui, M.; Hlil, E.K.; Mounkachi, O. Experimental and First-Principles Study of the Origin of the Magnetic Properties of CoFe2O4 Spinel Ferrite. Appl. Phys. A 2020, 126, 940. [Google Scholar] [CrossRef]
- Kumar, V.; Rana, A.; Yadav, M.S.; Pant, R.P. Size-Induced Effect on Nano-Crystalline CoFe2O4. J. Magn. Magn. Mater. 2008, 320, 1729–1734. [Google Scholar] [CrossRef]
- Biswas, A.; Zarkevich, N.A.; Pathak, A.K.; Dolotko, O.; Hlova, I.Z.; Smirnov, A.V.; Mudryk, Y.; Johnson, D.D.; Pecharsky, V.K. First-Order Magnetic Phase Transition in Pr2In with Negligible Thermomagnetic Hysteresis. Phys. Rev. B 2020, 101, 224402. [Google Scholar] [CrossRef]
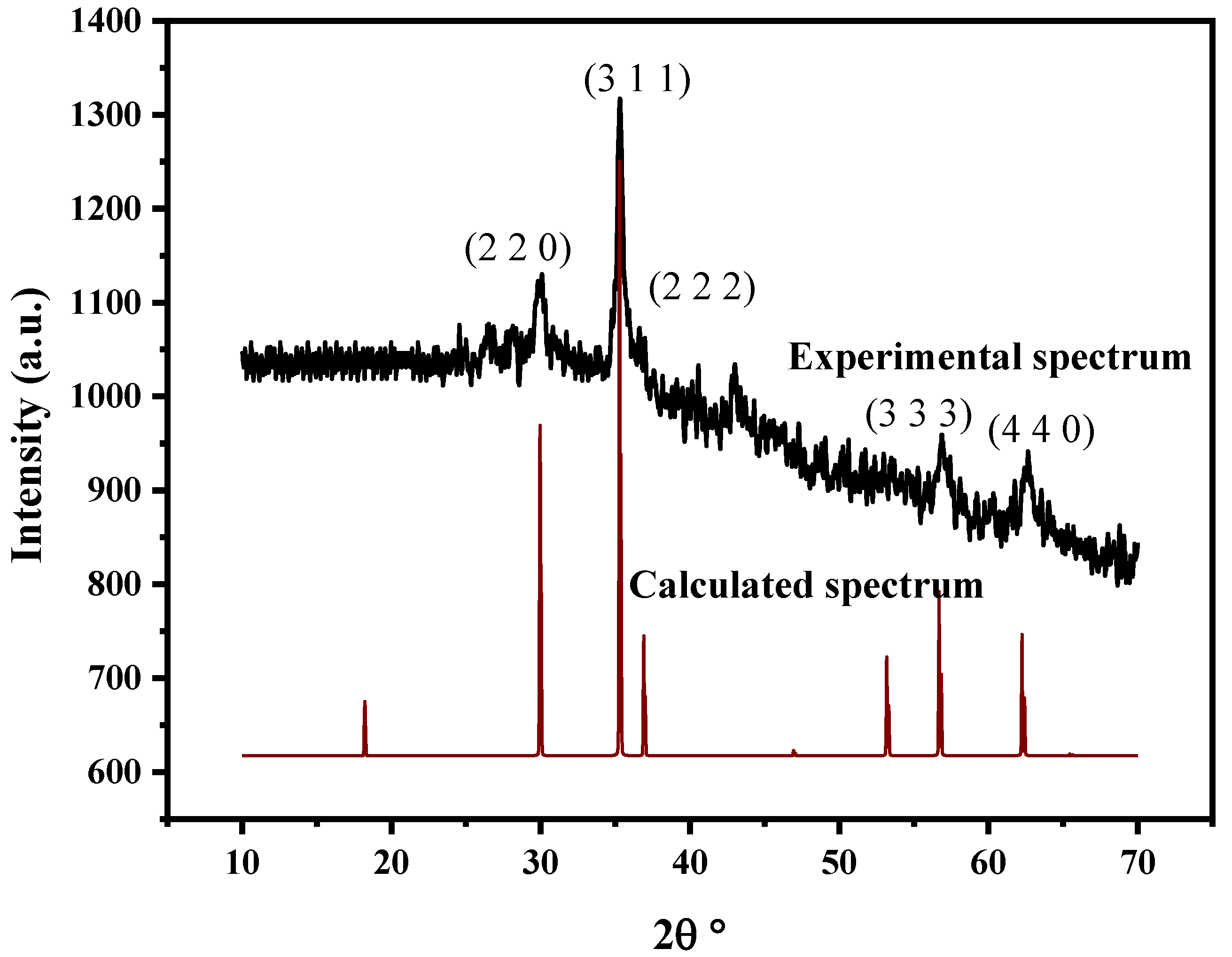
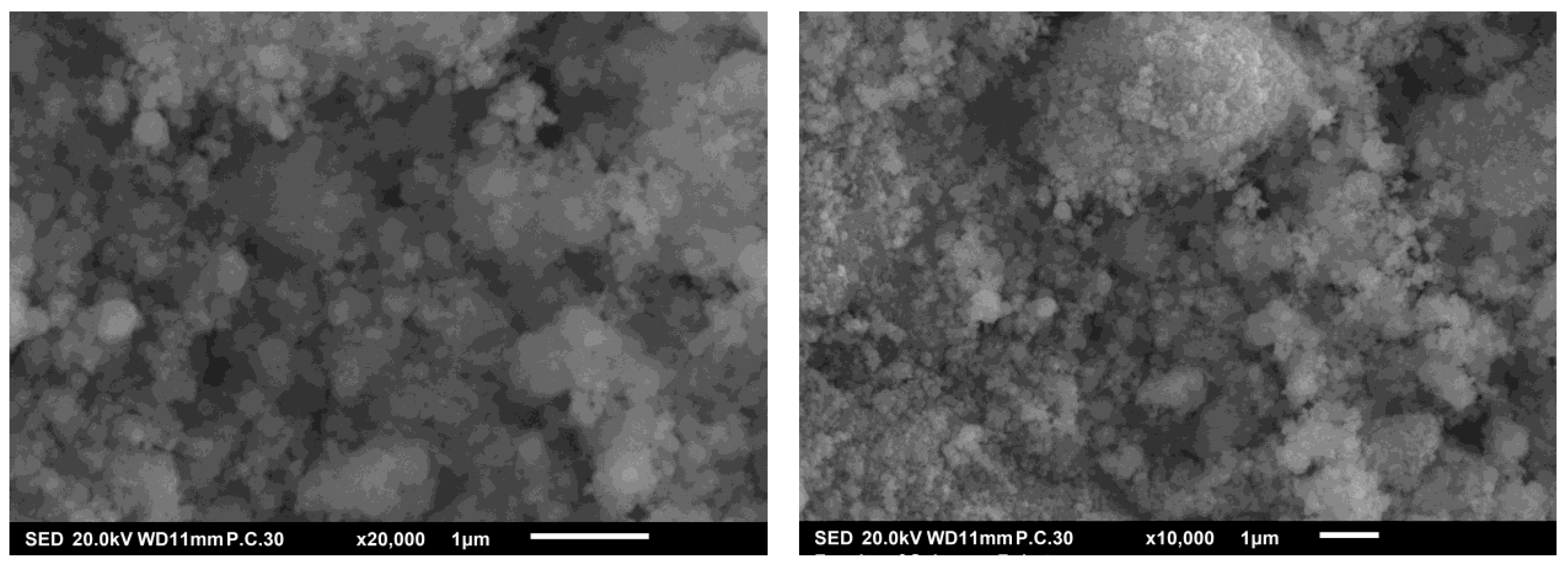



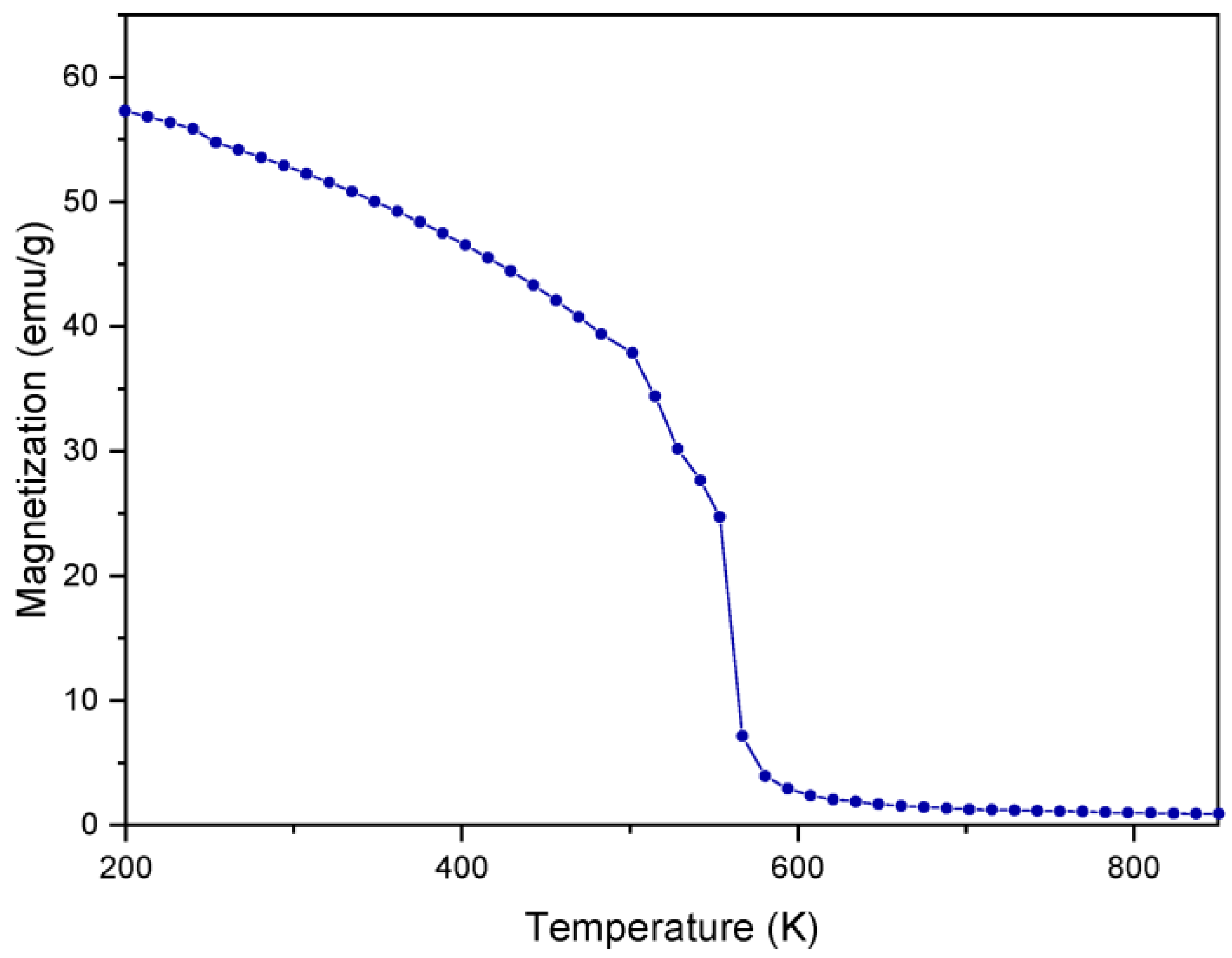

| Samples | Crystallite Size dDRX (nm) | Lattice Parameter a (Å) |
|---|---|---|
| Our work | 20.00 | 8.4043 |
| Sample(sol-gel-wheat flour /MnFe2O4) [51] | 16.87 | 8.4960 |
| Sample (sol-gel-potato flour/MnFe2O4) [52] | 23.12 | 8.4920 |
| Sample(co-precipitation-calcin in air/MnFe2O4) [53] | 5.0–15.00 | 8.3359 |
| Sample(co-precipitation-calcin under argon/MnFe2O4) [54] | 20.10–80.00 | 8.2399 |
| Nearest Neighbors | JMn-Mn | JMn-Fe | JFe-Fe |
|---|---|---|---|
| Exchange couplings (meV) | 1.18 | −1.87 | −1.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aghrich, K.; Mtougui, S.; Goumrhar, F.; Abdellaoui, M.; Mamouni, N.; Fekhaoui, M.; El Moutaouakil, A.; Mounkachi, O. Experimental and Theoretical Investigation of the Synthesis, Electronic and Magnetic Properties of MnFe2O4 Spinel Ferrite. Energies 2022, 15, 8386. https://doi.org/10.3390/en15228386
Aghrich K, Mtougui S, Goumrhar F, Abdellaoui M, Mamouni N, Fekhaoui M, El Moutaouakil A, Mounkachi O. Experimental and Theoretical Investigation of the Synthesis, Electronic and Magnetic Properties of MnFe2O4 Spinel Ferrite. Energies. 2022; 15(22):8386. https://doi.org/10.3390/en15228386
Chicago/Turabian StyleAghrich, Khaoula, Sara Mtougui, Fayçal Goumrhar, Mustapha Abdellaoui, Nabila Mamouni, Mohammed Fekhaoui, Amine El Moutaouakil, and Omar Mounkachi. 2022. "Experimental and Theoretical Investigation of the Synthesis, Electronic and Magnetic Properties of MnFe2O4 Spinel Ferrite" Energies 15, no. 22: 8386. https://doi.org/10.3390/en15228386
APA StyleAghrich, K., Mtougui, S., Goumrhar, F., Abdellaoui, M., Mamouni, N., Fekhaoui, M., El Moutaouakil, A., & Mounkachi, O. (2022). Experimental and Theoretical Investigation of the Synthesis, Electronic and Magnetic Properties of MnFe2O4 Spinel Ferrite. Energies, 15(22), 8386. https://doi.org/10.3390/en15228386








