Intermediate Pyrolysis of Bambara Groundnut Shell (BGS) in Various Inert Gases (N2, CO2, and N2/CO2)
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass Sample Collection
2.2. Feed Characterization
2.3. Intermediate Pyrolysis
2.4. Products Characterization
2.4.1. Bio-Oil Characterization
2.4.2. Gas Chromatography and Mass Spectrometry (GC-MS) Analysis
2.4.3. The Fourier Transform Infra-Red (FTIR) Spectroscopy
2.4.4. Biochar Characterization
3. Results
3.1. Feedstock Properties
3.2. The Product Yields
3.3. Bio-Oil Energy and pH of Bio-Oil
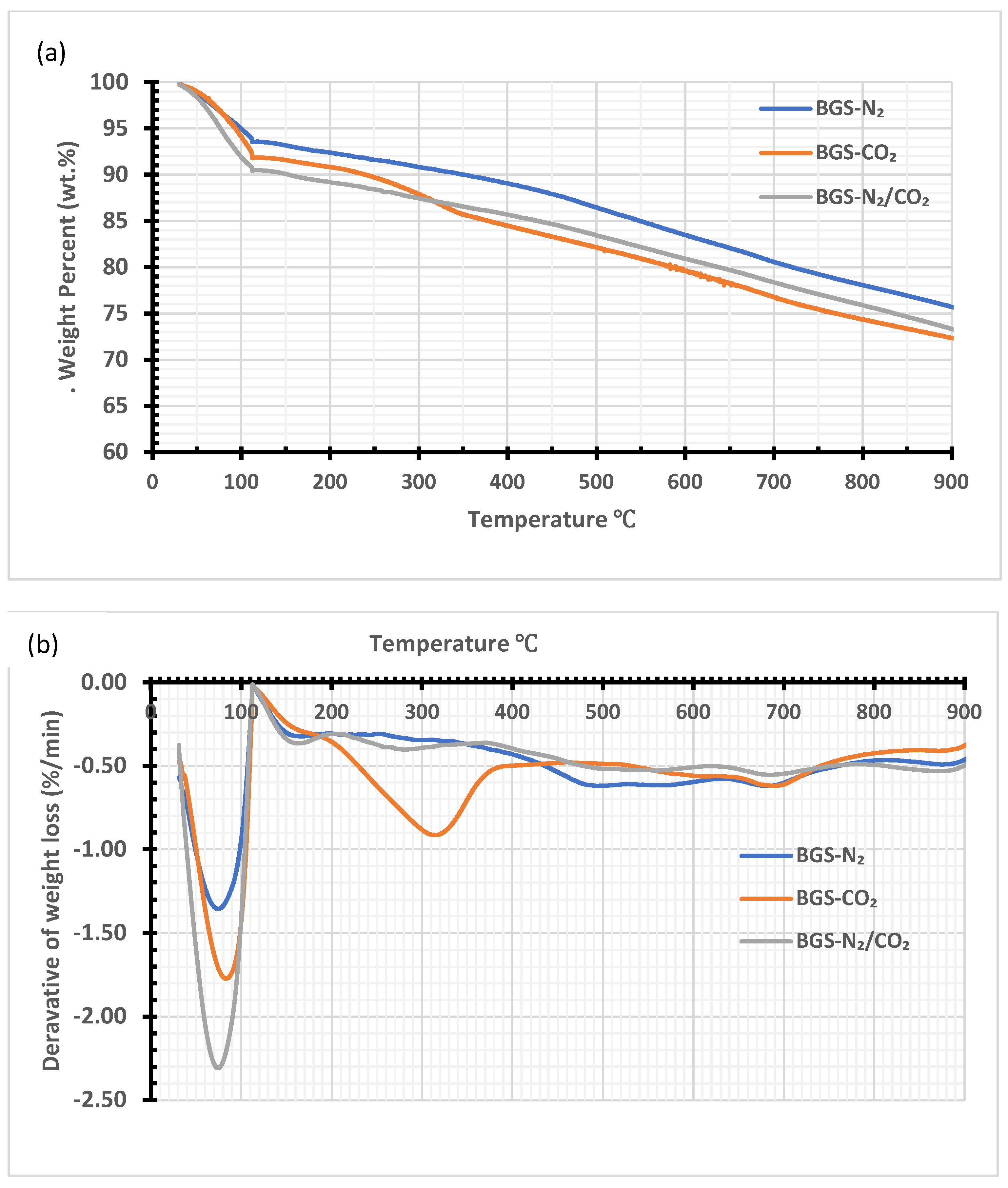
3.4. Thermogravimetric Analysis
3.5. FT-IR Analysis
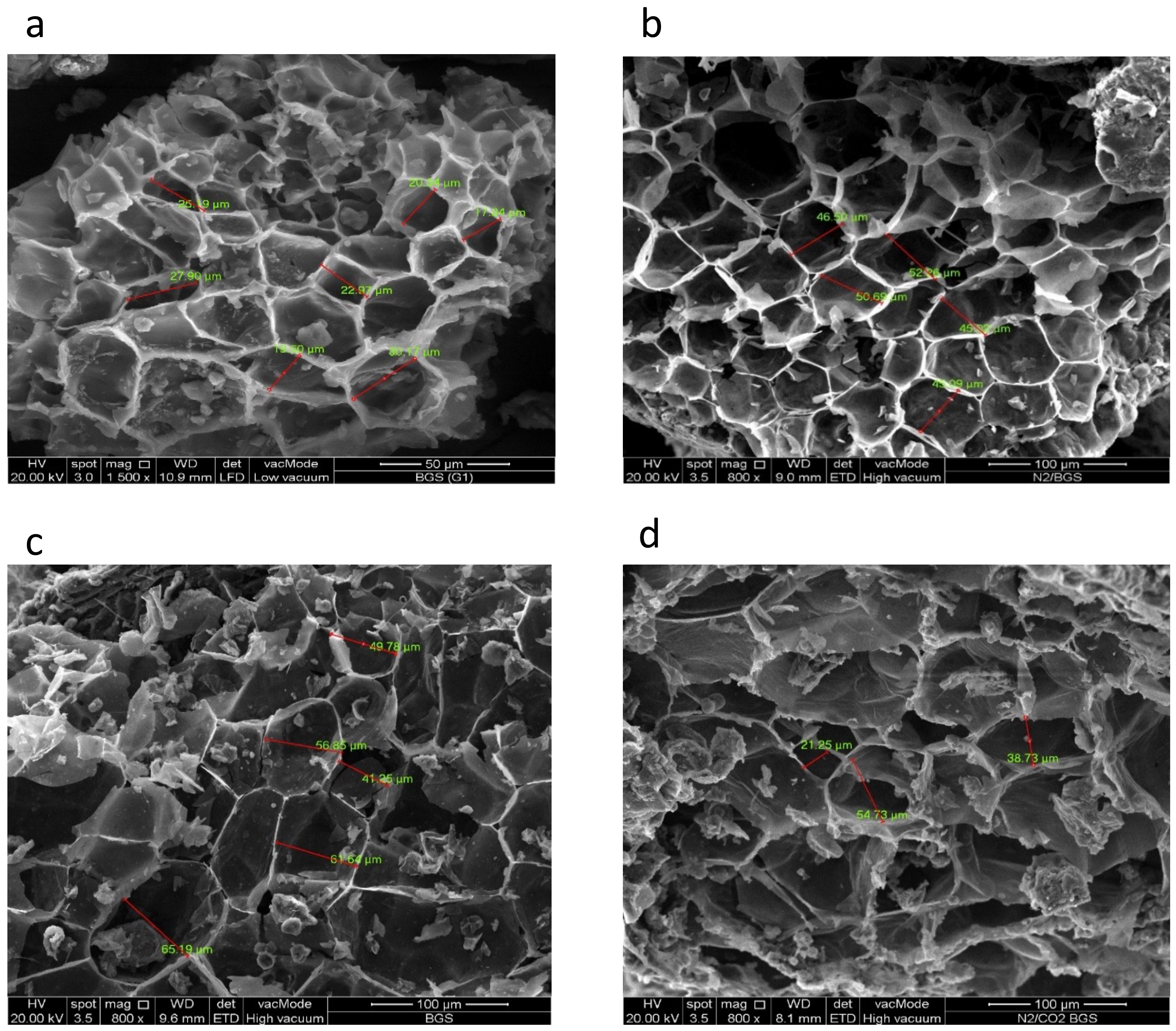
3.6. FESEM/EDX Analysis
3.7. Bio-Oil Chemical Composition through GC-MS
| # | ALKANE | BGS-N2 | BGS-CO2 | BGS-N2/CO2 |
|---|---|---|---|---|
| 1 | (+)-2-Aminoheptane | 1.737 | ||
| 2 | Tridecane | 0.274 | 0.502 | |
| 3 | Heptadecane, 2,6,10,15-tetramethyl- | 0.578 | ||
| 4 | Stigmastan-6,22-dien, 3,5-dedihydro- | 0.527 | ||
| Sub-Total | 0.274 | 1.607 | 1.737 | |
| ALKENE | ||||
| 5 | Cyclobutene, 2-propenylidene- | 1.528 | ||
| 6 | Naphthalene, 2,2-dimethyl-1-oxa-2-sila-1,2-dihydro- | 0.273 | ||
| Sub-Total | 0.273 | 1.528 | 0 | |
| AMIDE | ||||
| 7 | Pyrimidine, 4,5-dimethyl- | 0.687 | ||
| 8 | Nonadecanamide | 0.541 | ||
| Sub-Total | 0 | 1.228 | 0 | |
| ALDEHYDE | ||||
| 9 | Pentanal, 2,3-dimethyL- | 0.969 | ||
| Sub-Total | 0 | 0.969 | 0 | |
| ALCOHOL Derivatives | ||||
| 10 | 1-Propanol, 2-amino-, (ñ)- | 1.737 | ||
| 11 | Oxiranemethanol, (R)- | 0.859 | ||
| 12 | Cyclobutanol | 0.506 | ||
| 13 | 1,6-Heptadien-4-ol | 0.383 | ||
| 14 | Ethanol, 2-(9,12-octadecadienyloxy)-, (Z,Z)- | 0.476 | ||
| Sub-Total | 0 | 0.476 | 3.485 | |
| ESTER | ||||
| 15 | Nicotinic acid, 2-phenylethyl ester | 0.637 | ||
| 16 | Formic acid, tetrahydrofurfuryl ester | 0.354 | ||
| 17 | Propanoic acid, 3-chloro-, 4-formylphenyl ester | 1.004 | ||
| 18 | Oxalic acid, 2-isopropylphenyl pentyl ester | 0.887 | ||
| 19 | Hexadecanoic acid, methyl ester | 0.678 | ||
| 20 | 12,15-Octadecadienoic acid, methyl ester | 0.528 | ||
| 21 | 6-Octadecenoic acid, methyl ester, (Z)- | 2.736 | ||
| Sub-Total | 0 | 5.583 | 1.241 | |
| CARBOXYLIC ACID | ||||
| 22 | Butanoic acid, 4-hydroxy- | 1.004 | ||
| 23 | Phosphonic acid, (p-hydroxyphenyl)- | 3.125 | 0.463 | |
| 24 | n-Hexadecanoic acid | 0.357 | 3.991 | 0.463 |
| 25 | trans-13-Octadecenoic acid | 0.887 | ||
| Sub-Total | 3.482 | 6.345 | 0.463 | |
| PHENOL Derivatives | ||||
| 26 | Phenol | 3.125 | 4.696 | 9.849 |
| 27 | Phenol, 2-methyl- | 2.203 | ||
| 28 | Phenol, 3-methyl- | 3.689 | 2.936 | 13.947 |
| 29 | Phenol, 2-methoxy- | 1.618 | ||
| 30 | Phenol, 3-methyl- | 4.507 | ||
| 31 | Phenol, 2-methoxy- | 3.557 | 4.165 | |
| 32 | Phenol, 2,6-dimethyl- | 0.445 | 0.53 | 1.021 |
| 33 | Phenol, 2,5-dimethyl- | |||
| 34 | Phenol, 2-ethyl- | 0.54 | 1.095 | |
| 35 | Phenol, 2,5-dimethyl- | 1.667 | 2.359 | |
| 36 | Phenol, 4-ethyl- | 1.268 | 1.477 | 3.599 |
| 37 | Phenol, 2-ethyl-5-methyl- | 0.371 | 0.763 | |
| 38 | 2-Methoxy-5-methylphenol | 1.054 | 0.642 | |
| 39 | Phenol, 3,4-dimethyl- | 0.454 | 0.384 | |
| 40 | Phenol, 2,3,5-trimethyl- | 0.407 | ||
| 41 | Phenol, 2-ethyl-4-methyl- | 1.233 | 1.279 | |
| 42 | Phenol, 3,4,5-trimethyl- | 0.476 | ||
| 43 | Phenol, 4-ethyl-3-methyl- | 0.849 | 1.141 | |
| 44 | Phenol, 3-propyl- | 0.431 | 0.691 | 0.776 |
| 45 | Phenol, 4-ethyl-2-methoxy | 1.789 | ||
| 46 | 2,5-Diethylphenol | 0.33 | 0.831 | |
| 47 | Phenol, 2-ethyl-4,5-dimethyl- | 0.618 | ||
| 48 | 2-Methoxy-4-vinylphenol | 0.975 | ||
| 49 | Phenol, 2,6-dimethoxy | 0.321 | 0.761 | 0.748 |
| 50 | Phenol, 2-methoxy-4-propyl- | 0.736 | 0.562 | |
| 51 | Phenol, 2-methoxy-6-(2-propenyl)- | 0.448 | 0.984 | 1.116 |
| Sub-Total | 17.689 | 24.326 | 47.448 | |
| KETONE | ||||
| 52 | 3-Hexanone | 0.552 | ||
| 53 | Cyclopentanone | 0.556 | 1.005 | |
| 54 | Cyclopentanone, 2-methyl- | 0.229 | ||
| 55 | 2-Cyclopenten-1-one | 0.449 | ||
| 56 | Cyclohexanone | 0.933 | ||
| 57 | Cyclopentanone, 2-methyl- | 0.7 | ||
| 58 | 2-Cyclopenten-1-one, 2-methyl- | 0.319 | 0.753 | 0.874 |
| 59 | 2-Cyclopenten-1-one, 3,4-dimethyl- | 0.323 | 1.678 | 2.11 |
| Sub-Total | 0.871 | 3.687 | 5.923 | |
| BENZENE Derivatives | ||||
| 60 | Benzene, 1,3-dimethyl- | 0.309 | 0.619 | 0.816 |
| 61 | Benzenepropanoyl bromide | 0.236 | 0.637 | 0.452 |
| 62 | Benzene, 1,2,3-trimethy | 0.367 | ||
| 63 | Benzene, 1-ethyl-2-methyl- | 0.358 | ||
| 64 | 3-Butynylbenzene | 1.319 | ||
| 65 | Benzene, pentyl- | 1.052 | ||
| 66 | Benzene, 1-methoxy-4-methyl- | 0.499 | ||
| 67 | Benzene, 1,4-dimethoxy-2-methyl- | 0.921 | 2.138 | |
| 68 | 2,5,6-Trimethylbenzimidazole | 0.241 | 0.464 | |
| 69 | 1,2-Diethoxy-4-ethylbenzene | 0.404 | ||
| 70 | ndolizine, 1-methyl- | 0.415 | ||
| 71 | 1,4-Benzenediol, 2,5-dimethyl- | 0.278 | ||
| 72 | Benzene, 1,1’-(diazomethylene)bis- | 0.439 | ||
| 73 | Benzene, (nitromethyl)- | 6.682 | ||
| 75 | Benzonitrile, 2-(4-benzyloxybenzylidenamino)- | 1.984 | ||
| 77 | Benzonitrile, m-phenethyl- | 0.408 | ||
| Sub-Total | 11.925 | 4.446 | 4.667 | |
| Total | 34.514 | 50.195 | 64.964 |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IRENA. Renewable Capacity Statistics 2020; International Renewable Energy Agency (IRENA): Abu Dhabi, United Arab Emirates, 2020; ISBN 978-92-9260-239-0. [Google Scholar]
- Kumar, M.; Rai, D.; Bhardwaj, G.; Upadhyay, S.N.; Mishra, P.K. Pyrolysis of peanut shell: Kinetic analysis and optimization of thermal degradation process. Ind. Crops Prod. 2021, 174, 114128. [Google Scholar] [CrossRef]
- Sipra, A.T.; Gao, N.; Sarwar, H. Municipal solid waste (MSW) pyrolysis for bio-fuel production: A review of effects of MSW components and catalysts. Fuel Process. Technol. 2018, 175, 131–147. [Google Scholar] [CrossRef]
- Waluyo, J.; Makertihartha, I.G.B.N.; Susanto, H. Pyrolysis with intermediate heating rate of palm kernel shells: Effect temperature and catalyst on product distribution. AIP Conf. Proc. 2018, 1977, 020026. [Google Scholar] [CrossRef]
- Bouai, H.; Tabal, A.; Barakat, A.; el Harfi, K.; Aboulkas, A. Optimal parameters and structural composition of bio-oil and biochar from intermediate pyrolysis of red algal biomass. Comptes Rendus. Chim. 2021, 24, 85–99. [Google Scholar] [CrossRef]
- Martínez, J.D.; Campuzano, F.; Agudelo, A.F.; Cardona-Uribe, N.; Arenas, C.N. Chemical recycling of end-of-life tires by intermediate pyrolysis using a twin-auger reactor: Validation in a laboratory environment. J. Anal. Appl. Pyrolysis 2021, 159, 105298. [Google Scholar] [CrossRef]
- Hornung, A. 2013 Intermediate pyrolysis of biomass. In Biomass Combustion Science, Technology and Engineering; Woodhead Publishing Limited: Cambridge, UK, 2013. [Google Scholar] [CrossRef]
- Zadeh, Z.E.; Abdulkhani, A.; Saha, B. Characterization of Fast Pyrolysis Bio-Oil from Hardwood and Softwood lignin. Energies 2020, 13, 887. [Google Scholar] [CrossRef]
- Alvarez, J.; Lopez, G.; Amutio, M.; Artetxe, M.; Barbarias, I.; Arregi, A.; Bilbao, J.; Olazar, M. Characterization of the bio-oil obtained by fast pyrolysis of sewage sludge in a conical spouted bed reactor. Fuel Process. Technol. 2016, 149, 169–175. [Google Scholar] [CrossRef]
- Mohammed, I.Y.; Abakr, Y.A.; Hui, J.N.X.; Alaba, P.A.; Morris, K.I.; Ibrahim, M.D. Recovery of clean energy precursors from Bambara groundnut waste via pyrolysis: Kinetics, products distribution and optimisation using response surface methodology. J. Clean. Prod. 2017, 164, 1430–1445. [Google Scholar] [CrossRef]
- Cai, W.; Liu, R.; He, Y.; Chai, M.; Cai, J. Bio-oil production from fast pyrolysis of rice husk in a commercial-scale plant with a downdraft circulating fluidised bed reactor. Fuel Process. Technol. 2018, 171, 308–317. [Google Scholar] [CrossRef]
- Kabir, G.; Din, A.T.M.; Hameed, B.H. Pyrolysis of oil palm mesocarp fiber and palm frond in a slow-heating fixed-bed reactor: A comparative study. Bioresour. Technol. 2017, 241, 563–572. [Google Scholar] [CrossRef]
- Guizani, C.; Sanz, F.J.E.; Salvador, S. Effects of CO2 on biomass fast pyrolysis: Reaction rate, gas yields and char reactive properties. Fuel 2014, 116, 310–320. [Google Scholar] [CrossRef]
- Lai, Z.; Ma, X.; Tang, Y.; Lin, H. Thermogravimetric analysis of the thermal decomposition of MSW in N2, CO2 and CO2/N2 atmospheres. Fuel Process. Technol. 2012, 102, 18–23. [Google Scholar] [CrossRef]
- Hou, B.; Liu, Z.; Zhao, J.; Li, C. Study on the Pyrolysis Characterization of Rice Husk Var in CO2/N2Atmosphere. In Proceedings of the 2022 4th Asia Energy and Electrical Engineering Symposium (AEEES), Chengdu, China, 25–28 March 2022; pp. 478–483. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J.; Liu, J.; He, Y.; Evrendilek, F.; Buyukada, M.; Xie, W.; Sun, S. Co-pyrolytic mechanisms, kinetics, emissions and products of biomass and sewage sludge in N2, CO2 and mixed atmospheres. Chem. Eng. J. 2020, 397, 125372. [Google Scholar] [CrossRef]
- Kim, J.H.; Jung, S.; Lin, K.Y.A.; Rinklebe, J.; Kwon, E.E. Comparative study on carbon dioxide-cofed catalytic pyrolysis of grass and woody biomass. Bioresour. Technol. 2021, 323, 124633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xiao, R.; Wang, D.; He, G.; Shao, S.; Zhang, J.; Zhong, Z. Biomass fast pyrolysis in a fluidized bed reactor under N2, CO2, CO, CH4 and H2 atmospheres. Bioresour. Technol. 2011, 102, 4258–4264. [Google Scholar] [CrossRef] [PubMed]
- Agyeman, K.; Asante, B.O.; Berchie, J.N.; Sarkodie-Addo, J.; Marno, P.; Adabah, R. Farmers’ perceptions, constraints and preferences for improved Bambara groundnut varieties in Ghana. J. Agric. Food Res. 2021, 3, 100097. [Google Scholar] [CrossRef]
- Ajilogba, C.F.; Olanrewaju, O.S.; Babalola, O.O. Improving Bambara Groundnut Production: Insight Into the Role of Omics and Beneficial Bacteria. Front. Plant Sci. 2022, 13, 836133. [Google Scholar] [CrossRef]
- Jahanshiri, E.; Von Goh, E.; Wimalasiri, E.M.; Azam-Ali, S.; Mayes, S.; Suhairi, T.A.S.T.M.; Nizar, N.M.M.; Sinin, S.S.M. The potential of Bambara groundnut: An analysis for the People’s Republic of China. Food Energy Secur. 2022, 11, 614–628. [Google Scholar] [CrossRef]
- Majola, N.G.; Gerrano, A.S.; Shimelis, H. Utilisation and Genetic Improvement in Sub-Saharan Africa. Agronomy 2021, 11, 1–16. [Google Scholar]
- Chong, Y.Y.; Gopakumar, S.T.; Gan, S.; Ng, H.K.; Lee, L.Y. Synergic Effect and Kinetic Mechanisms for Co-Pyrolysis of Empty Fruit Bunch and Palm Oil Sludge. Inst. Eng. Malaysia 2017, 78, 1–5. [Google Scholar]
- Tsai, W.T.; Liu, S.C.; Chen, H.R.; Chang, Y.M.; Tsai, Y.L. Textural and chemical properties of swine-manure-derived biochar pertinent to its potential use as a soil amendment. Chemosphere 2012, 89, 198–203. [Google Scholar] [CrossRef]
- Bieniek, A.; Jerzak, W.; Sieradzka, M.; Mika, Ł.; Sztekler, K.; Magdziarz, A. Intermediate Pyrolysis of Brewer’s Spent Grain: Impact of Gas Atmosphere. Energies 2022, 15, 2491. [Google Scholar] [CrossRef]
- Shen, Y.; Ma, D.; Ge, X. CO2-looping in biomass pyrolysis or gasification. Sustain. Energy Fuels 2017, 1, 1700–1729. [Google Scholar] [CrossRef]
- Jaideep, R.; Lo, W.H.; Lim, G.P.; Chua, C.X.; Gan, S.; Lee, L.Y.; Thangalazhy-Gopakumar, S. Enhancement of fuel properties of yard waste through dry torrefaction. Mater. Sci. Energy Technol. 2021, 4, 156–165. [Google Scholar] [CrossRef]
- Onsree, T.; Tippayawong, N. Torrefaction of Maize Residue Pellets with Dry Flue Gas. BioEnergy Res. 2020, 13, 358–368. [Google Scholar] [CrossRef]
- Sadaka, S.; Boateng, A.A. Pyrolysis and Bio Oil. Agric. Nat. Resour. 2017, 1, 1–6. [Google Scholar]
- Aziz, S.M.A.; Wahi, R.; Ngaini, Z.; Hamdan, S.; Yahaya, S.A. Esterification of Microwave Pyrolytic Oil from Palm Oil Kernel Shell. J. Chem. 2017, 2017, 8359238. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, A.; Tekasakul, P.; Lam, S.S.; Palamanit, A. Comparative Investigation of Yield and Quality of Bio-Oil and Biochar from Pyrolysis of Woody and Non-Woody Biomasses. Energies 2021, 14, 1092. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Singh, S.V.; Dhyani, V.C.; Govindaraju, K.; Vinu, R.; Mandal, S. Characterization, bioenergy value, and thermal stability of biochars derived from diverse agriculture and forestry lignocellulosic wastes. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Chukwuneke, J.L.; Ewulonu, M.C.; Chukwujike, I.C.; Okolie, P.C. Physico-chemical analysis of pyrolyzed bio-oil from swietenia macrophylla (mahogany) wood. Heliyon 2019, 5, e01790. [Google Scholar] [CrossRef]
- Singh, S.B.; De, M. Thermally exfoliated graphene oxide for hydrogen storage. Mater. Chem. Phys. 2019, 239, 122102. [Google Scholar] [CrossRef]
- Yang, X.; Lyu, H.; Chen, K.; Zhu, X.; Zhang, S.; Chen, J. Selective Extraction of Bio-oil from Hydrothermal Liquefaction of Salix psammophila by Organic Solvents with Different Polarities through Multistep Extraction Separation. BioResources 2014, 9, 5219–5233. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, N.; Xiao, Z.; Li, Z.; Zhang, D. Characterization of biochars derived from different materials and their effects on microbial dechlorination of pentachlorophenol in a consortium. RSC Adv. 2019, 9, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yang, X.; Song, H.; Ok, Y.S.; Kwon, E.E. Effects of carbon dioxide on pyrolysis of peat. Energy 2017, 120, 929–936. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, S.; Kwon, E.E.; Lee, J. Effect of carbon dioxide on thermal treatment of food waste as a sustainable disposal method. J. CO2 Util. 2020, 36, 76–81. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, Q.; Guo, Y.; Wu, X.; Wang, H.; Han, J.; Ge, Q.; Zhu, X. Effect of postsynthesis preparation methods on catalytic performance of Ti-Beta zeolite in ketonisation of propionic acid. Microporous Mesoporous Mater. 2021, 330, 111625. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, Z.; Li, X.; Qiao, X.; Song, W.; Guo, L. Initial reaction mechanisms of cellulose pyrolysis revealed by ReaxFF molecular dynamics. Fuel 2016, 177, 130–141. [Google Scholar] [CrossRef]
- Patwardhan, P.R.; Brown, R.C.; Shanks, B.H. Product Distribution from the Fast Pyrolysis of Hemicellulose. ChemSusChem 2011, 4, 636–643. [Google Scholar] [CrossRef]
- Thangalazhy-Gopakumar, S.; Adhikari, S.; Gupta, R.B.; Fernando, S.D. Influence of pyrolysis operating conditions on bio-oil components: A microscale study in a pyroprobe. Energy Fuels 2011, 25, 1191–1199. [Google Scholar] [CrossRef]




| S/No | Biomass | Yield (wt.%) | Carbon Content | pH | Ref. |
|---|---|---|---|---|---|
| 1 | Chouka Kraft Lignin | 24.4%, | 64.80 | 4.57 | [8] |
| 2 | Sigma Kraft Lignin | 30.2% | 68.50 | 5.33 | [8] |
| 3 | Sewage sludge | 77 (*) | 45 | 8.5 | [9] |
| 4 | BGS | 36.49 | 48.10 ± 0.51 | 3.80–4.20 | [10] |
| 5 | Rice Husk | 47.7 ± 2.7 | - | 3.05 ± 0.09 | [11] |
| 6 | Palm mesocarp fibre and Palm frond | 48 and 47 | 67.77 and 60.81 | 3.0 | [12] |
| Components | C | H | N | S | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| #RAW-BGS | 39.7 | 6 | 1.2 | 0.3 | ||||||
| Bio-oil | ||||||||||
| BGS-N2 | 60.49 | 3.035 | 2.56 | 0.377 | ||||||
| BGS-CO2 | 53.57 | 2.631 | 1.66 | 0.257 | ||||||
| BGS-N2/CO2 | 58.62 | 2.734 | 2.51 | 0.347 | ||||||
| Biochar | ||||||||||
| BGS-N2 | 50.04 | 2.659 | 1.9 | 0.253 | ||||||
| BGS-CO2 | 58.13 | 2.718 | 1.38 | 0.157 | ||||||
| BGS-N2/CO2 | 55.79 | 2.525 | 1.99 | 0.264 | ||||||
| EDX | C K | O K | K K | Al K | Si K | S K | Cl K | Mg K | Fe K | P K |
| BGS-N2 | 68.7 ± 3.0 | 22.7 ± 4.6 | 10.5 ± 5.9 | 1.7 ± 1.6 | 2.2 ± 2.2 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.2 ± 0.0 | 0.9 ± 1.1 | |
| BGS-CO2 | 50.1 ± 5.0 | 37.8 ± 3.8 | 9.1 ± 5.1 | 5.5 ± 3.9 | 5.1 ± 3.2 | 0.3 ± 0.2 | 0.2 ± 0.1 | 0.3 ± 0.1 | 1.3 ± 1.0 | 1.3 |
| BGS-N2/CO2 | 57.2 ± 8.6 | 27.0 ± 3.7 | 9.2 ± 3.7 | 2.2 ± 0.8 | 2.4 ± 1.0 | 0.2 ± 0.1 | 0.3 ± 0.0 | 0.3 ± 0.2 | 0.5 ± 0.2 | 1.0 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, M.D.; Abakr, Y.A.; Gan, S.; Lee, L.Y.; Thangalazhy-Gopakumar, S. Intermediate Pyrolysis of Bambara Groundnut Shell (BGS) in Various Inert Gases (N2, CO2, and N2/CO2). Energies 2022, 15, 8421. https://doi.org/10.3390/en15228421
Ibrahim MD, Abakr YA, Gan S, Lee LY, Thangalazhy-Gopakumar S. Intermediate Pyrolysis of Bambara Groundnut Shell (BGS) in Various Inert Gases (N2, CO2, and N2/CO2). Energies. 2022; 15(22):8421. https://doi.org/10.3390/en15228421
Chicago/Turabian StyleIbrahim, Mustapha Danladi, Yousif Abdalla Abakr, Suyin Gan, Lai Yee Lee, and Suchithra Thangalazhy-Gopakumar. 2022. "Intermediate Pyrolysis of Bambara Groundnut Shell (BGS) in Various Inert Gases (N2, CO2, and N2/CO2)" Energies 15, no. 22: 8421. https://doi.org/10.3390/en15228421
APA StyleIbrahim, M. D., Abakr, Y. A., Gan, S., Lee, L. Y., & Thangalazhy-Gopakumar, S. (2022). Intermediate Pyrolysis of Bambara Groundnut Shell (BGS) in Various Inert Gases (N2, CO2, and N2/CO2). Energies, 15(22), 8421. https://doi.org/10.3390/en15228421










