The Self-Actuating Droplet That Can Turn: A Molecular Dynamics Simulation
Abstract
1. Introduction
2. Simulation Method
2.1. Molecular Dynamics Simulation System
Simulation System
2.2. Simulation Method and Procedure
2.2.1. Simulation Method
2.2.2. Simulation Procedure
2.3. Surface Wettability Simulations
3. Results and Discussion
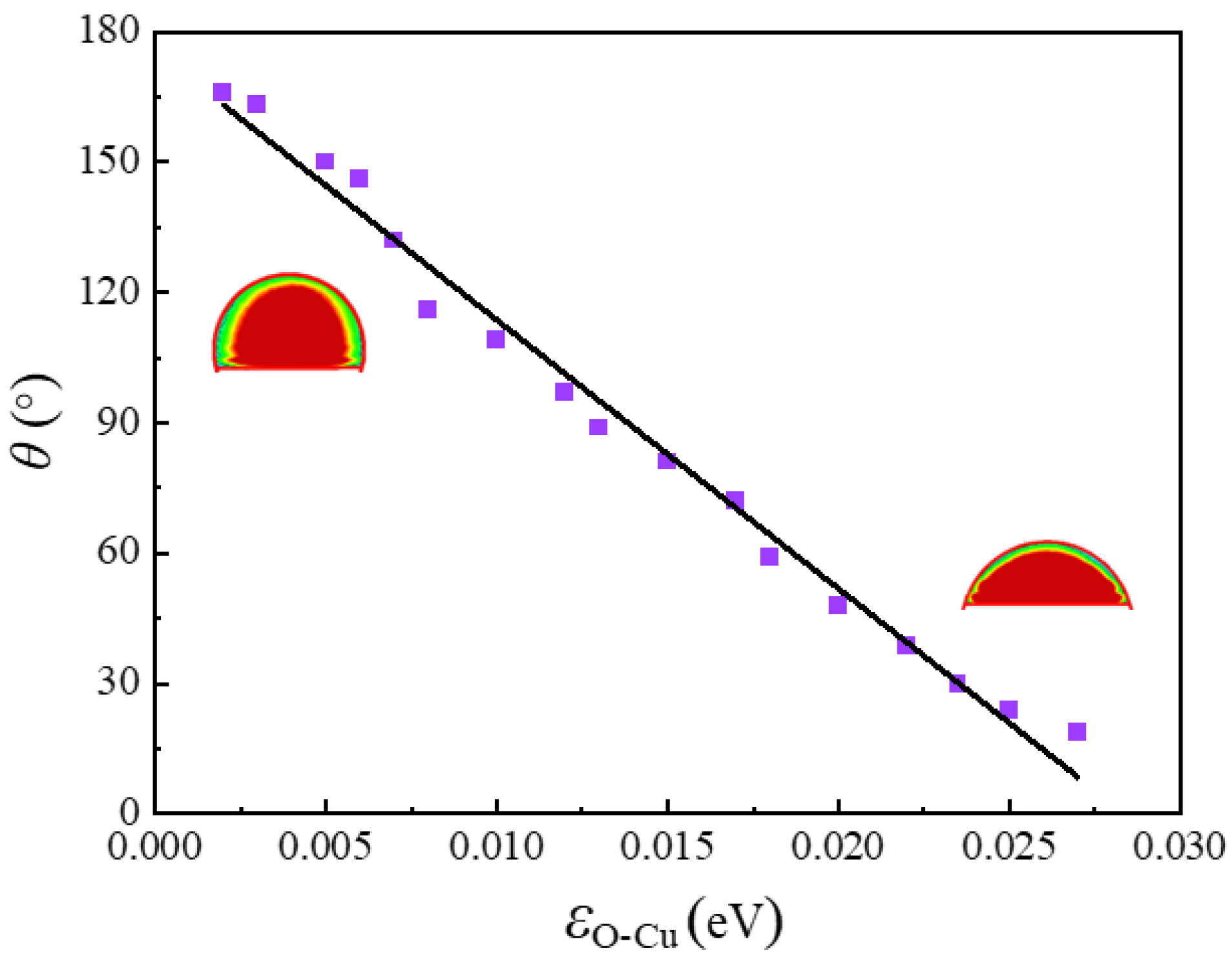
3.1. Effects of Liquid-Solid Interactions on the Contact Angle
3.2. Simulation Validation
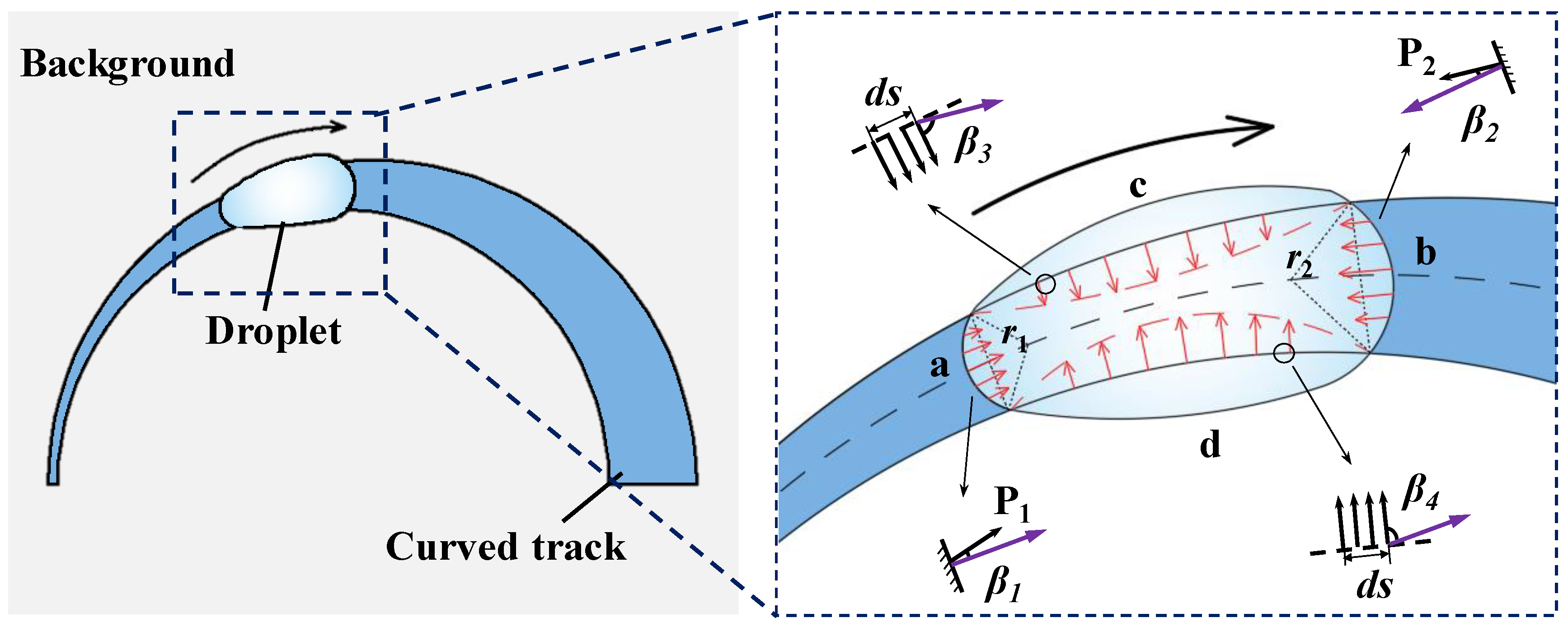
3.3. Theoretical Analysis
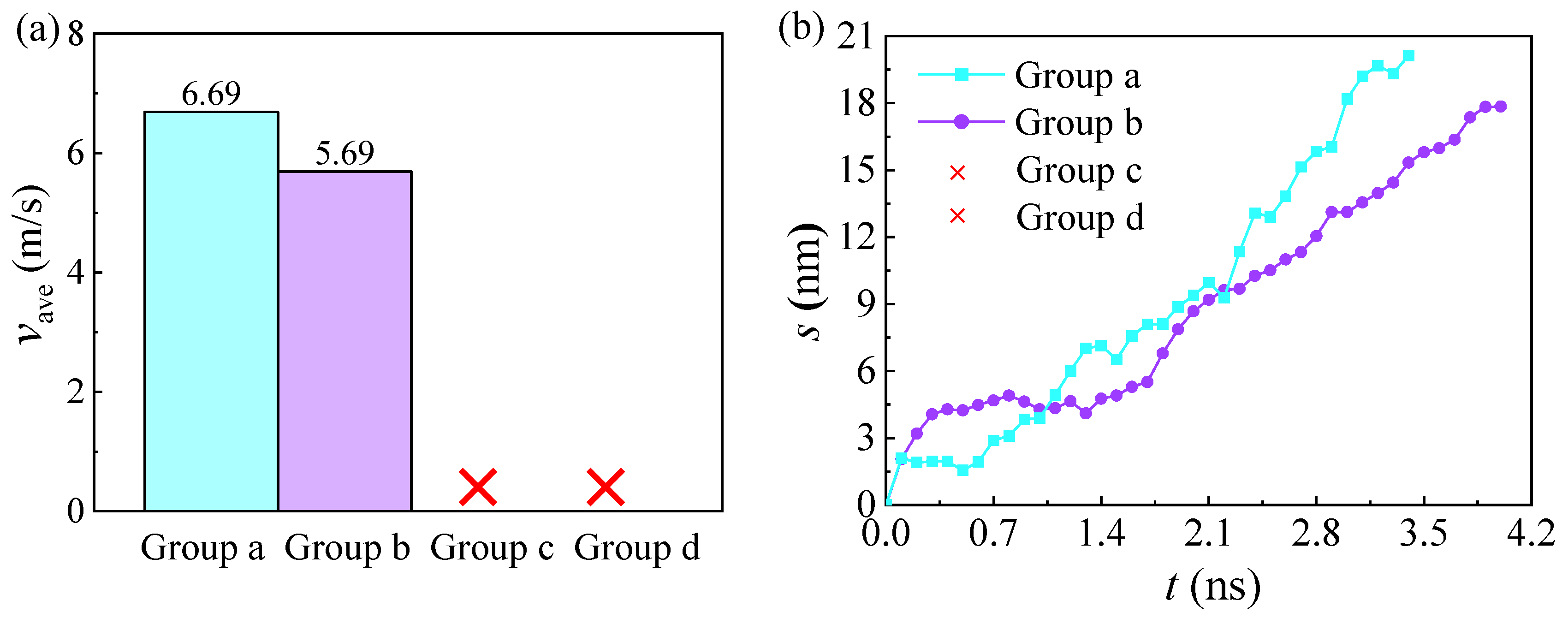
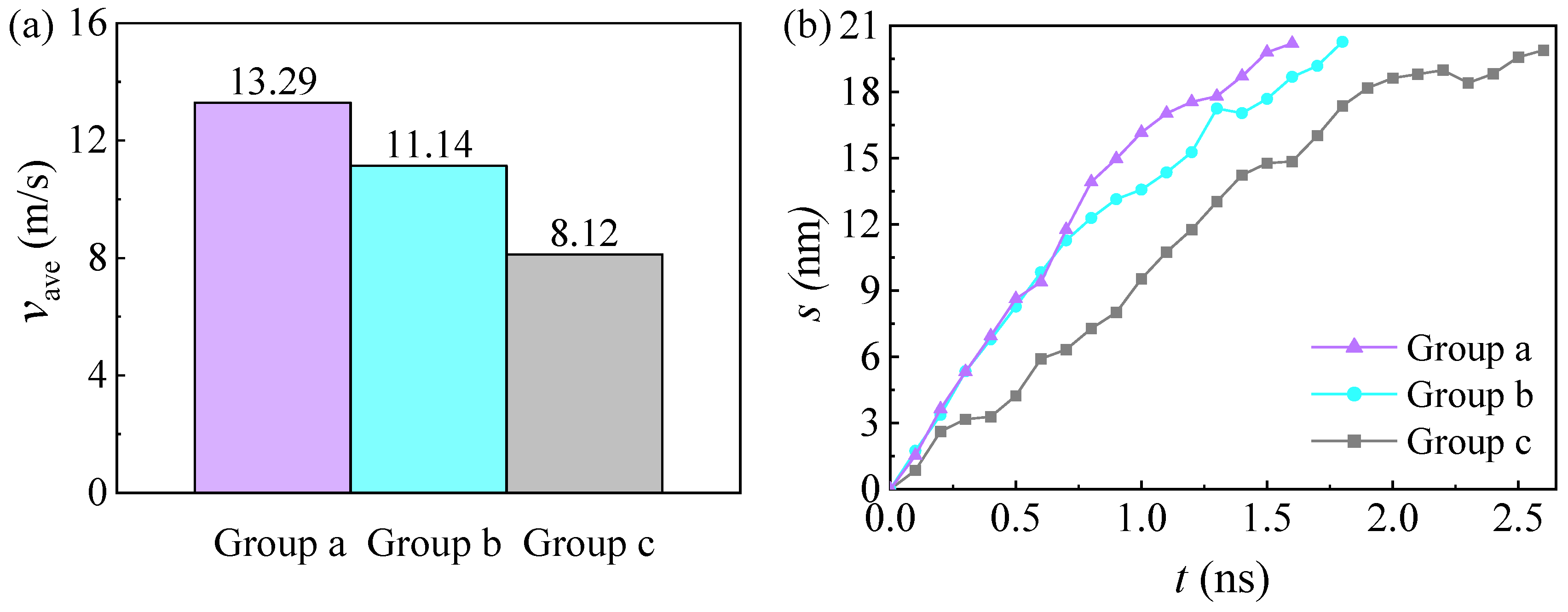
3.4. Influences of the Wettability Difference
3.5. Influences of the Track Width
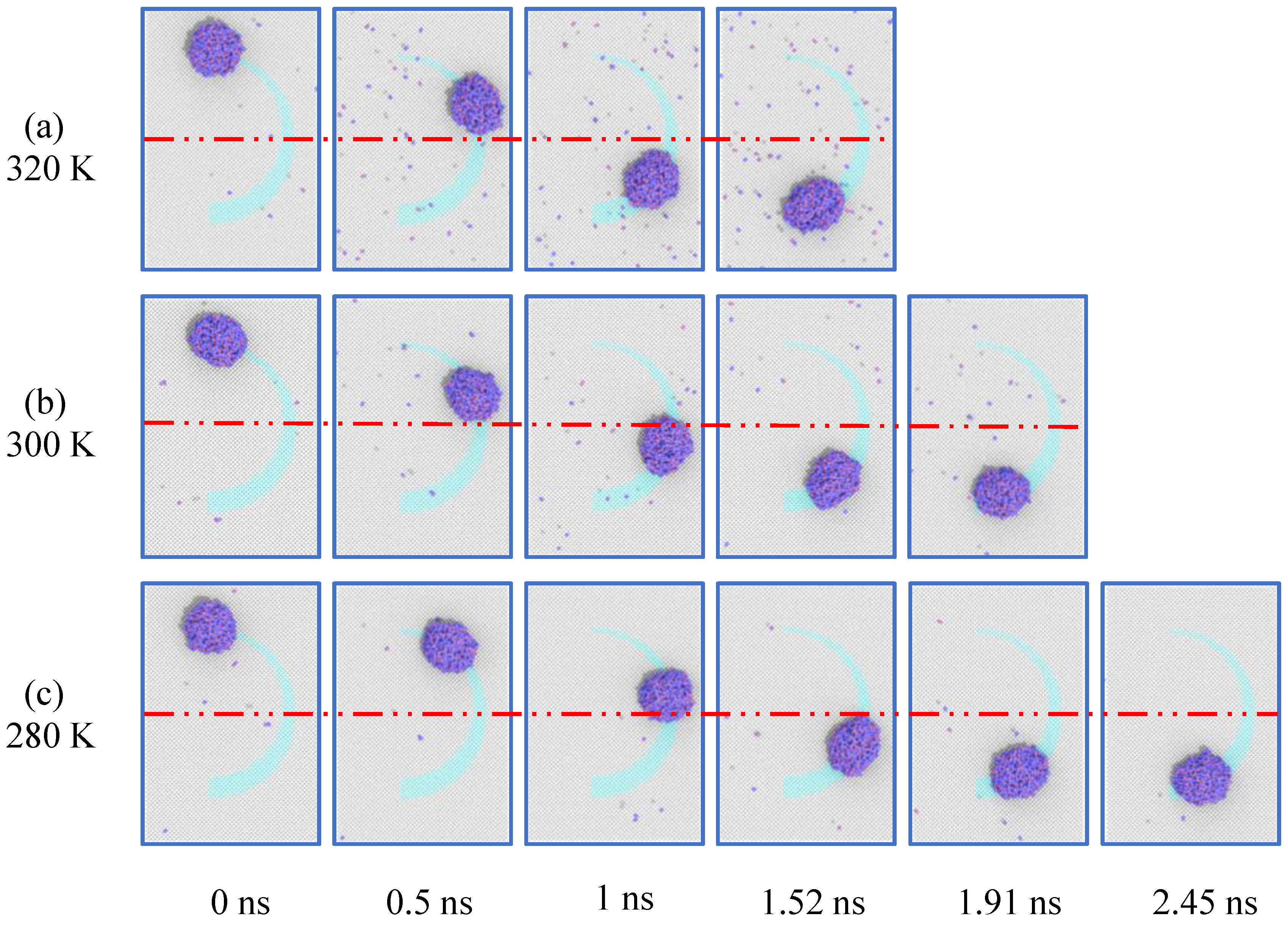
3.6. Influences of the Droplet Temperature
4. Conclusions
- (1)
- Droplet motion is achieved according to the joint action of the Laplace pressure and the wettability gradient force. The component force in the direction of motion promotes forward motion of the droplet, while the component force vertical to the direction of motion induces the droplet to turn in the direction of dynamic equilibrium. This realises droplet motion along the curved track.
- (2)
- Under the same wettability difference, the hydrophobic-hydrophobic combination of the curved track and the background provides a larger driving force on the droplet than the hydrophilic-hydrophobic surface and the hydrophilic-hydrophilic surface.
- (3)
- Increasing the width of the curved track leads to an increasingly fast motion of the droplet. However, a too large track width shrinks the contact area between the droplet and the boundary, thus decelerating the motion of the droplet and even causing the motion to stagnate. On the premise of keeping the droplet in a liquid state, the temperature rise accelerates the motion velocity of the droplet along the curved track. This is because the temperature rise increases the internal energy of molecules, leading to more violent motion, and improving the contact frequency between the droplet molecules and the surface.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| E | The lj/cut styles compute the standard 12/6 Lennard-Jones potential |
| ε | Energy units |
| σ | Distance units |
| r | Interatomic spacing |
| rc | Cutoff |
| θ | Contact angle between droplet and copper base |
| O1 | The center of the outer circle of the curved track |
| O2 | The center of the inside circle of the curved track |
| RO1 | The radius of the outer circle of the curved track |
| RO2 | The radius of the inside circle of the curve track |
| la | Narrow end distance of curved track |
| lb | Wide end distance of curved track |
| F | The resultant force exerted by the curved track on the droplet |
| FL | Laplace pressure gradient |
| FW | Wetting gradient force |
| FH | The force of surface with different wettability hindering the movement of droplets |
| P1 | Laplace pressure generated by the curved liquid surface at the rear end of the droplet along the track direction |
| P2 | Laplace pressure generated by the curved liquid surface at the front end of the droplet along the track direction |
| F1 | The force generated by Laplace pressure in the direction of droplet movement |
| F2 | The force generated by Laplace pressure perpendicular to the direction of droplet movement |
| F3 | The force generated by the wettability gradient in the direction of droplet movement |
| F4 | The force generated by the wettability gradient perpendicular to the direction of droplet motion |
| Fn | The resultant force of the droplet in the direction of the curve tracks |
| Fz | The resultant force of the droplet perpendicular to the direction of motion |
| γ | Surface tension of water |
| r1 | Local radius of the three-phase contact lines behind the droplet along the direction of the track |
| r2 | Local radius of the three-phase contact line in front of the droplet along the track direction |
| β = β1 = β2 | |
| β1 | The angle formed by force P1 and the direction of droplet movement |
| β2 | The angle formed by force P2 and the direction of droplet movement |
| β3 | The angle between the c side wetting gradient force and the direction of droplet movement. |
| β4 | The angle between the d side wetting gradient force and the direction of droplet movement. |
| S | Contact area of droplets on both sides of curved track |
References
- Bixler, G.D.; Bhushan, B. Fluid drag reduction and efficient self-cleaning with rice leaf and butterfly wing bioinspired surfaces. Nanoscale 2013, 5, 7685–7710. [Google Scholar] [CrossRef]
- Ju, J.; Bai, H.; Zheng, Y.M.; Zhao, T.Y.; Fang, R.C.; Lei, J. A multi-structural and multi-functional integrated fog collection system in cactus. Nat. Commun. 2012, 3, 1247. [Google Scholar] [CrossRef]
- Guo, L.; Tang, G.H. Experimental study on directional motion of a single droplet on cactus spines. Int. J. Heat Mass Transf. 2015, 84, 198–202. [Google Scholar] [CrossRef]
- Bai, H.; Ju, J.; Zheng, Y.M.; Jiang, L. Functional fibers with unique wettability inspired by spider silks. Adv. Mater. 2012, 24, 2786–2791. [Google Scholar] [CrossRef]
- Ju, J.; Zheng, Y.M.; Jiang, L. Bioinspired one-dimensional materials for directional liquid transport. Acc. Chem. Res. 2014, 47, 2342–2352. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.W.; Zhang, P.F.; Zhang, L.W.; Liu, H.L.; Jiang, Y.; Zhang, D.Y.; Han, Z.W. Continuous directional water transport on the peristome surface of Nepenthes alata. Nature 2016, 532, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.L.; Lv, X.D.; Zhang, Y.Y.; Li, C.Z.; Li, J.W.; Wu, H.; Xiao, Y.; Wu, S.Z.; Hu, Y.L.; Wu, D.; et al. Pitcher plant-bioinspired bubble slippery surface fabricated by femtosecond laser for buoyancy-driven bubble self-transport and efficient gas capture. Nanoscale 2019, 11, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, W.J.; Seely, M.K. Fog basking by the Namib Desert beetle, Onymacris Unguicu-laris. Nature 1976, 262, 284–285. [Google Scholar] [CrossRef]
- Parker, A.R.; Lawrence, C.R. Water capture by a desert beetle. Nature 2001, 414, 33–34. [Google Scholar] [CrossRef]
- Cieplak, M.; Koplik, J.; Banavar, J.R. Nanoscale fluid flows in the vicinity of patterned surfaces. Phys. Rev. Lett. 2006, 96, 114502. [Google Scholar] [CrossRef] [PubMed]
- Paradisanos, I.; Fotakis, C.; Anastasiadis, S.H.; Stratakis, E. Gradient induced liquid motion on laser structured black Si surfaces. Appl. Phys. Lett. 2015, 107, 111603. [Google Scholar] [CrossRef]
- Lv, C.J.; Chen, C.; Chuang, Y.C.; Tseng, F.G.; Yin, Y.J.; Grey, F.; Zheng, Q.S. Substrate curvature gradient drives rapid droplet motion. Phys. Rev. Lett. 2014, 113, 26101. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Tian, X.L.; Zheng, Y.M.; Ju, J.; Zhao, Y.; Jiang, L. Direction controlled driving of tiny water drops on bioinspired artificial spider silks. Adv. Mater. 2010, 22, 5521–5525. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Zhao, Y.; Tian, Y.; Li, K.; Jiang, L. Electrospun multiscale structured membrane for efficient water collection and directional transport. Small 2016, 12, 1000–1005. [Google Scholar] [CrossRef]
- Hou, Y.P.; Feng, S.L.; Dai, L.M.; Zheng, Y.M. Droplet manipulation on wettable gradient surfaces with micro-/nano-hierarchical structure. Chem. Mater. 2016, 28, 3625–3629. [Google Scholar] [CrossRef]
- Kou, J.L.; Mei, M.F.; Lu, H.J.; Wu, F.M.; Fan, J.T. Unidirectional motion of a water nanodroplet subjected to a surface energy gradient. Phys. Rev. E Stat. Nonlin. Soft. Matter Phys. 2010, 85, 988–1000. [Google Scholar] [CrossRef]
- Wang, Z.K.; Zheng, H.Y.; Xia, H.M. Femtosecond laser-induced modification of surface wettability of PMMA for fluid separation in microchannels. Microfluid. Nanofluid. 2011, 10, 225–229. [Google Scholar] [CrossRef]
- Gurera, D.; Bhushan, B. Multistep wettability gradient on bioinspired conical surf-aces for water collection from fog. Langmuir 2019, 35, 16944–16947. [Google Scholar] [CrossRef]
- Chen, H.W.; Tong, R.; Yang, G.; Zhou, J.J.; Zhang, Y.; Zhang, L.W.; Zhang, D.Y.; Jiang, L. Ultrafast water harvesting and transport in hierarchical microchannels. Nat. Mater. 2018, 17, 935–942. [Google Scholar] [CrossRef]
- Park, K.C.; Kim, P.; Grinthal, A.; He, N.; Fox, D.; Weaver, J.C.; Aizenberg, J. Condensation on slippery asymmetric bumps. Nature 2016, 531, 78–82. [Google Scholar] [CrossRef]
- Meng, Q.A.; Xu, B.J.; He, M.J.; Bian, R.X.; Meng, L.L.; Wang, P.W.; Jiang, L.; Liu, H. Bioinspired controllable liquid manipulation by fibrous array driven by elasticity. ACS Appl. Mater. 2018, 10, 26819–26824. [Google Scholar] [CrossRef]
- Liu, W.J.; Fan, P.X.; Cai, M.Y.; Luo, X.; Chen, C.H.; Pan, R.; Zhang, H.J.; Zhong, M.L. An integrative bioinspired venation network with ultra-contrasting wettability for large-scale strongly self-driven and efficient water collection. Nanoscale 2019, 11, 8940–8949. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Ju, J.; Xue, Z.X.; Ma, J.; Feng, L.; Gao, S.; Jiang, L. Structured cone arrays for continuous and effective collection of micron-sized oil droplets from water. Nat. Commun. 2013, 4, 2276. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.; Kim, M.; Kim, D.H. Candle-based process for creating a stable superhydrophobic surface. Carbon 2014, 68, 583–596. [Google Scholar] [CrossRef]
- Pei, Y.Y.; Song, Q.; Li, P. Research progress of biomimetic micro/nano-structured antibacterial surfaces. Surf. Technol. 2019, 48, 200–210. [Google Scholar] [CrossRef]
- Lan, F.; Haliburton, J.R.; Yuan, A.; Abate, A.R. Droplet barcoding for massively parallel single-molecule deep sequencing. Nat. Commun. 2016, 7, 11784. [Google Scholar] [CrossRef] [PubMed]
- Parashar, V.K.; Wacker, J.B.; Gijs, M.A. Spherical superstructures of oxide nanoparticles for catalytic reactions in microchemical reactors. Mater. Lett. 2015, 139, 182–186. [Google Scholar] [CrossRef]
- Tang, X.; Huang, J.X.; Guo, Z.G.; Liu, W.M. A combined structural and wettability gradient surface for directional droplet transport and efficient fog collection. J. Colloid Interface Sci. 2021, 604, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.L.; Wang, Q.Q.; Xing, Y.; Hou, Y.P.; Zheng, Y.M. Continuous directional water transport on integrating tapered surfaces. Adv. Mater. Interfaces 2020, 7, 2000081. [Google Scholar] [CrossRef]
- Lin, Y.C.; Hu, Z.Y.; Gao, C.L.; Guo, Z.Y.; Li, C.; Zheng, Y.M. Directional droplet spreading transport controlled on tilt-angle pillar arrays. Adv. Mater. Interfaces 2018, 5, 1800962. [Google Scholar] [CrossRef]
- Wang, M.; Liu, Q.; Zhang, H.R.; Wang, C.; Wang, L.; Xiang, B.X.; Fan, Y.T.; Guo, C.F.; Ruan, S.C. Laser direct writing of tree-shaped hierarchical cones on a superhydrophobic film for high-efficiency water collection. ACS Appl. Mater. 2017, 9, 29248–29254. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.S.; Tseng, F.G. Spontaneous high-speed transport of subnanoliter water droplet on gradient nanotextured surfaces. Appl. Phys. Lett. 2009, 95, 063108. [Google Scholar] [CrossRef]
- Zhang, J.L.; Han, Y.C. Shape-gradient composite surfaces: Water droplets move uphill. Langmuir 2007, 23, 6136–6141. [Google Scholar] [CrossRef]
- Huang, D.J.; Leu, T.S. Fabrication of a wettability-gradient surface on copper by screen-printing techniques. J. Micromech. Microeng. 2015, 25, 1325–1336. [Google Scholar] [CrossRef]
- Ody, T.J.; Panth, M.; Sommers, A.D.; Eid, K.F. Controlling the motion of ferrofluid droplets using surface tension gradients and magnetoviscous pinning. Langmuir 2016, 32, 6967–6976. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.Y.; Shang, W.F.; Feng, S.L.; Zhu, S.P.; Xing, Y.; Li, D.; Hou, Y.P.; Zheng, Y.M. Controlled droplet transport to target on a high adhesion surface with multi-gradients. Sci. Rep. 2017, 7, 45687. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yao, Y.; Li, J.J.; Peng, Z.L.; Chen, S.H. Directional sliding behavior of a water droplet on a wedge-shape patterned functional surface. J. Phys. Chem. B 2020, 124, 6905–6912. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.F.; Chen, J.; Zhou, C.L.; Xing, H.T.; Wen, X.F.; Pi, P.H.; Xu, S.P. Dro-plet motion on a shape gradient surface. Langmuir 2017, 33, 4172–4177. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.H.; Élfego, R.G.; Xu, B.B.; Wood, D.; Mchale, G.; Ledesma-Aguilar, R.; Wells, G.G. Drop transport and positioning on lubricant-impregnated surfaces. Soft. Matter 2017, 13, 3404–3410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.H.; Zhang, B.; Ma, H.Y.; Li, Z.; Xiao, X.; Zhang, Y.H.; Cui, X.Y.; Yu, C.M.; Cao, M.Y.; Jiang, L. Bioinspired pressure-tolerant asymmetric slippery surface for continuous self-transport of gas bubbles in aqueous environment. ACS Nano 2018, 12, 2048–2055. [Google Scholar] [CrossRef]
- Bai, H.; Wang, L.; Ju, J.; Sun, R.Z.; Zheng, Y.M.; Jiang, L. Efficient water collection on integrative bioinspired surfaces with star-shaped wettability patterns. Adv. Mater. 2014, 26, 5025–5030. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, C.; Peng, Z.L.; Chen, S.H. Moving behavior of nanodroplets on wedge-shaped functional surfaces. J. Phys. Chem. C 2019, 123, 1798–1805. [Google Scholar] [CrossRef]
- Xu, B.; Chen, Z.Q. Droplet movement on a composite wedge-shaped surface with multi-gradients and different gravitational field by molecular dynamics. Microgravity Sci. Tec. 2018, 30, 571–579. [Google Scholar] [CrossRef]
- Hao, S.Q.; Xie, Z.; Li, Z.; Kou, J.L.; Wu, F.M. Initial-position-driven opposite directional transport of water droplet on wedge-shaped groove. Nanoscale 2021, 13, 15963–15972. [Google Scholar] [CrossRef]
- Guo, L.; Kumar, S.; Yang, M.Y.; Tang, G.H.; Liu, Z.G. Role of the microridges on cactus spines. Nanoscale 2022, 14, 525–533. [Google Scholar] [CrossRef]
- Mo, J.W.; Wang, C.; Zeng, J.Y.; Sha, J.J.; Li, Z.G.; Chen, Y.F. Directional passive transport of nanodroplets on general axisymmetric surfaces. Phys. Chem. Chem. Phys. 2022, 24, 9727–9734. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.H.; Zhu, Y.Y.; Shi, T.L.; Tang, Z.R.; Liao, G.L. Patterned gradient surface for spontaneous droplet transportation and water collection: Simulation and experiment. J. Micromech. Microeng. 2016, 26, 115009. [Google Scholar] [CrossRef]
- Halverson, J.D.; Maldarelli, C.; Couzis, A.; Koplik, J. A molecular dynamics study of the motion of a nanodroplet of pure liquid on a wetting gradient. J. Chem. Phy. 2008, 129, 827. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.J.; Zhang, Z.Q.; Liu, Z.; Zhang, F.J.; Cheng, G.G.; Ding, J.N. Pinning effect in droplet self-driving and its reduction mechanism by monolayer graphene. Appl. Surf. Sci. 2021, 542, 148666. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Guo, X.F.; Tang, H.Y.; Ding, J.N.; Zheng, Y.G.; Li, S.F. Unidirectional self-driving liquid droplet transport on a monolayer graphene-covered textured substrate. ACS Appl. Mater. 2019, 11, 28562–28570. [Google Scholar] [CrossRef] [PubMed]





| Inter-Particle Interaction | Type of Potential Energy | Potential Energy Parameter | |
|---|---|---|---|
| ε/eV | σ/Å | ||
| O-O | lj/cut/tip4p/long | 0.00802 | 3.1589 |
| O-H | lj/cut/tip4p/long | 0 | 0 |
| H-H | lj/cut/tip4p/long | 0 | 0 |
| Cu-Cu | lj/cut | 0.1656 | 2.471 |
| H-Cu | lj/cut | 0 | 0 |
| O-Cu | lj/cut | Regulation variable | 2.815 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, Y.; Liu, Z.; Guo, L.; Qiu, Y. The Self-Actuating Droplet That Can Turn: A Molecular Dynamics Simulation. Energies 2022, 15, 8468. https://doi.org/10.3390/en15228468
Kong Y, Liu Z, Guo L, Qiu Y. The Self-Actuating Droplet That Can Turn: A Molecular Dynamics Simulation. Energies. 2022; 15(22):8468. https://doi.org/10.3390/en15228468
Chicago/Turabian StyleKong, Yalong, Zhigang Liu, Lin Guo, and Yu Qiu. 2022. "The Self-Actuating Droplet That Can Turn: A Molecular Dynamics Simulation" Energies 15, no. 22: 8468. https://doi.org/10.3390/en15228468
APA StyleKong, Y., Liu, Z., Guo, L., & Qiu, Y. (2022). The Self-Actuating Droplet That Can Turn: A Molecular Dynamics Simulation. Energies, 15(22), 8468. https://doi.org/10.3390/en15228468







