Humic Substances—Common Carriers of Micropollutants in Municipal Engineering
Abstract
1. Introduction
2. Materials and Methods
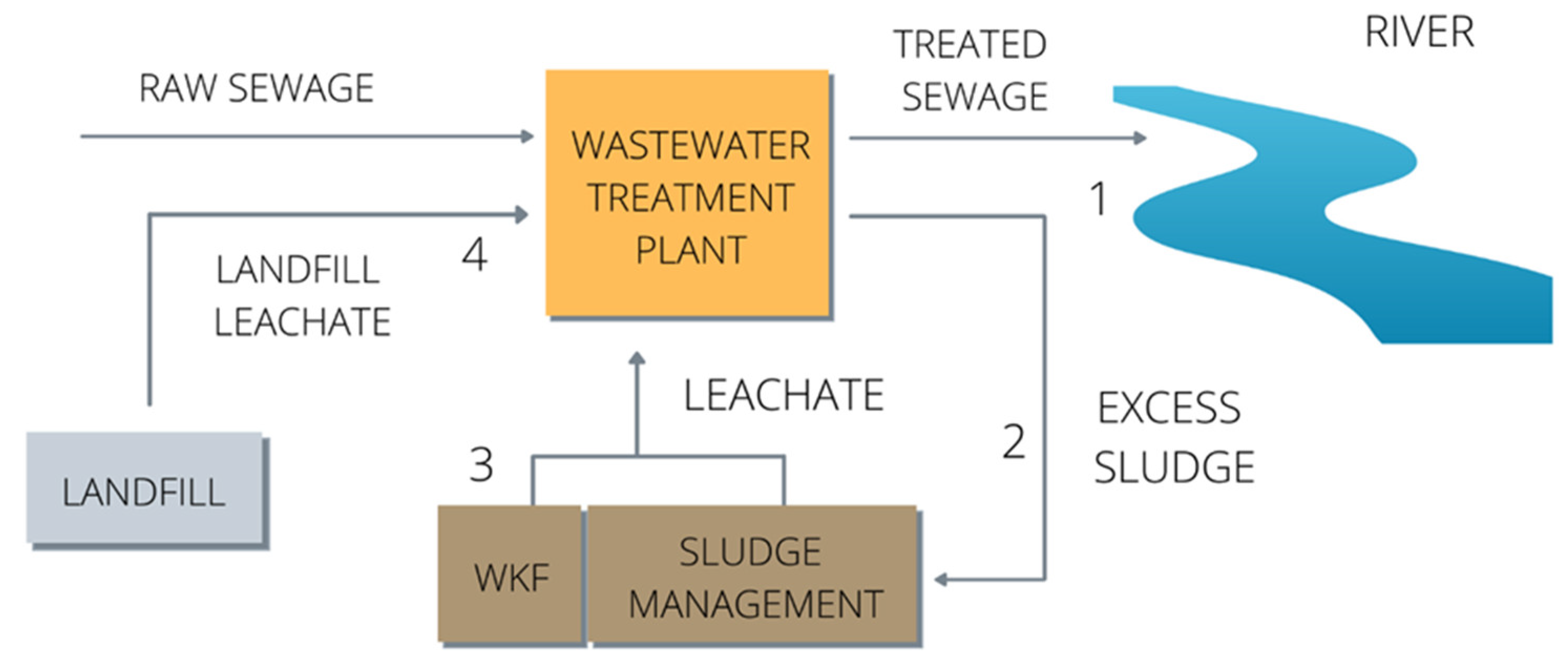
2.1. Materials and Sampling Points
2.2. Procedure of HAs Extraction and Analytic
2.3. Sorption Experiments with Pure HS and Selected Pharmaceutics
3. Results
3.1. Infrared Spectrum
3.2. Elemental Composition
3.3. Inorganic Micropollutants in FAs
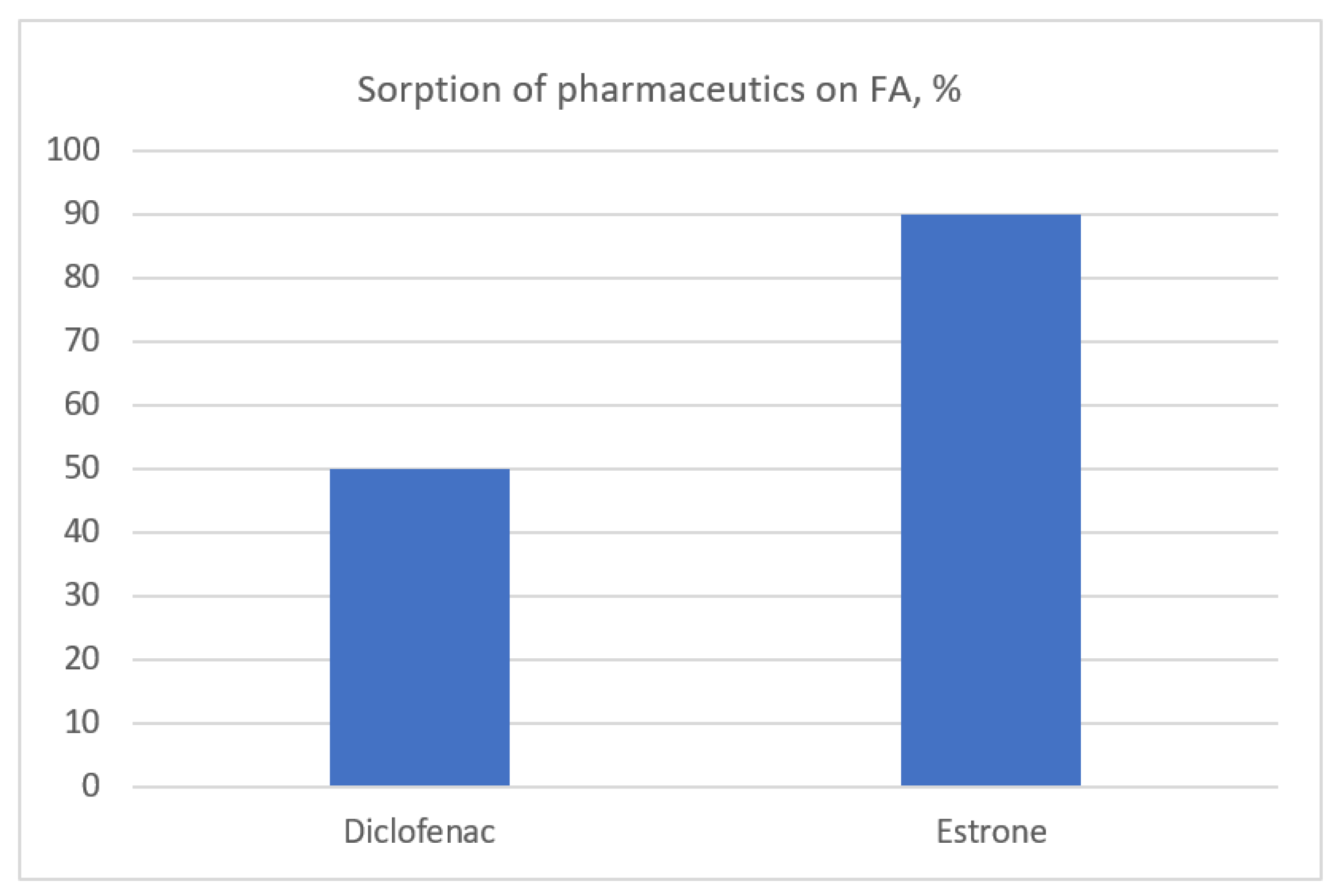
3.4. Organic Micropollutants in FAs
4. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meiczinger, M.; Varga, B.; Wolmarans, L.; Hajba, L.; Somogyi, V. Stability improvement of laccase for micropollutant removal of pharmaceutical origins from municipal wastewater. Clean Technol. Environ. Policy 2022, 24, 1–11. [Google Scholar] [CrossRef]
- Włodarczyk-Makuła, M. Mikrozanieczyszczenia w Środowisku Wodnym Wywołane Czynnikami Antropogenicznymi. In Wpływ Zmian Klimatu na Gospodarkę Wodno-ściekową w Aspekcie Bezpieczeństwa Zdrowotnego Wody, 1st ed; Kowalska, B., Ed.; Wydawnictwo Polskiej Akademii Nauk Monografie Komitetu Inżynierii Środowiska: Lublin, Poland, 2021; Volume 176, pp. 47–65. [Google Scholar]
- Mulla, S.I.; Hu, A.; Sun, Q.; Li, J.; Suanon, F.; Ashfaq, M.; Yu, C.-P. Biodegradation of sulfamethoxazole in bacteria from three different origins. J. Environ. Manag. 2018, 206, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Mulla, S.I.; Bharagava, R.N.; Belhaj, D.; Ameen, F.; Saratale, G.D.; Gupta, S.K.; Tyagi, S.; Patil, K.S.; Hu, A. A Review of Micropollutant Removal by Microalgae. In Application of Microalgae in Wastewater Treatment, 1st ed.; Gupta, S.K., Bux, F., Eds.; Springer: Cham, Switzerland, 2019; Volume 1, pp. 154–196. [Google Scholar] [CrossRef]
- Ruksrithong, C.; Phattarapattamawong, S. Removals of estrone and 17β-estradiol by microalgae cultivation: Kinetics and removal mechanisms. Environ. Technol. 2017, 40, 163–170. [Google Scholar] [CrossRef]
- Yu, C.-P.; Deeb, R.A.; Chu, K.-H. Microbial degradation of steroidal estrogens. Chemosphere 2013, 91, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Kruć, R.; Dragon, K.; Górski, J. Migration of Pharmaceuticals from the Warta River to the Aquifer at a Riverbank Filtration Site in Krajkowo (Poland). Water 2019, 11, 2238. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Z.; Zhang, J.; Wu, S.; Yang, L.; Chen, L.; Shao, Y. The challenge of micropollutants in surface water of the Yangtze River. Sci. Total Environ. 2021, 780, 146537. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Farnazo, D.M. Toxicity characteristics of sewage treatment effluents and potential contribution of micropollutant residuals. J. Ecol. Environ. 2017, 41, 39. [Google Scholar] [CrossRef]
- Anielak, A.M.; Piaskowski, K. Influence of zeolites on kinetics and effectiveness of the process of sewage biological purifi-cation in sequencing batch reactors. Environ. Prot. Eng. 2005, 31, 21–32. [Google Scholar]
- Kreuzig, R.; Haller-Jans, J.; Bischoff, C.; Leppin, J.; Germer, J.; Mohr, M.; Bliedung, A.; Dockhorn, T. Reclaimed water driven lettuce cultivation in a hydroponic system: The need of micropollutant removal by advanced wastewater treatment. Environ. Sci. Pollut. Res. 2021, 28, 50052–50062. [Google Scholar] [CrossRef]
- Wicht, A.-J.; Heye, K.; Schmidt, A.; Oehlmann, J.; Huhn, C. The wastewater micropollutant carbamazepine in insectivorous birds—An exposure estimate. Anal. Bioanal. Chem. 2022, 414, 4909–4917. [Google Scholar] [CrossRef]
- Chamberlain, E.; Adams, C. Oxidation of sulfonamides, macrolides, and carbadox with free chlorine and monochloramine. Water Res. 2006, 40, 2517–2526. [Google Scholar] [CrossRef]
- Adams, C.; Wang, Y.; Loftin, K.; Meyer, M. Removal of Antibiotics from Surface and Distilled Water in Conventional Water Treatment Processes. J. Environ. Eng. 2002, 128, 253–260. [Google Scholar] [CrossRef]
- Russo, D.; Siciliano, A.; Guida, M.; Andreozzi, R.; Reis, N.M.; Puma, G.L.; Marotta, R. Removal of antiretroviral drugs stavudine and zidovudine in water under UV254 and UV254/H2O2 processes: Quantum yields, kinetics and ecotoxicology assessment. J. Hazard. Mater. 2018, 349, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Caglak, A.; Chormey, D.S.; Bakirdere, S.; Engin, G.O. Performance evaluation of ceramic membrane bioreactor: Effect of operational parameters on micropollutant removal and membrane fouling. Environ. Sci. Pollut. Res. 2022, 29, 68306–68319. [Google Scholar] [CrossRef] [PubMed]
- Ivančev-Tumbas, I.; Lužanin, Z.; Česen, M.; Bogunović, M.; Sekulić, T.D.; Heath, D.; Heath, E. Insight into selected emerging micropollutant interactions with wastewater colloidal organic carbon: Implications for water treatment and analysis. Environ. Sci. Pollut. Res. 2020, 28, 59368–59381. [Google Scholar] [CrossRef] [PubMed]
- Moreira, W.M.; Viotti, P.V.; de Moura, A.A.; Gimenes, M.L.; Vieira, M.G.A. Synthesis of a biobased resin and its screening as an alternative adsorbent for organic and inorganic micropollutant removal. Environ. Sci. Pollut. Res. 2022, 29, 79935–79953. [Google Scholar] [CrossRef]
- Harnisch, F.; Gimkiewicz, C.; Bogunovic, B.; Kreuzig, R.; Schröder, U. On the removal of sulfonamides using microbial bioelectrochemical systems. Electrochem. Commun. 2013, 26, 77–80. [Google Scholar] [CrossRef]
- Radjenović, J.; Farré, M.J.; Mu, Y.; Gernjak, W.; Keller, J. Reductive electrochemical remediation of emerging and regulated disinfection byproducts. Water Res. 2012, 46, 1705–1714. [Google Scholar] [CrossRef]
- Kumari, R.; Sachan, S.G. Bioconversion of toxic micropollutant triclosan to 2,4-dichlorophenol using a wastewater isolate Pseudomonas aeruginosa KS2002. Int. J. Environ. Sci. Technol. 2018, 16, 7663–7672. [Google Scholar] [CrossRef]
- Tas, D.O.; Sari, S.; Aydın, E.; Topuz, E.; Pehlivanoğlu-Mantaş, E. Fate and biodegradability potential of an emerging micropollutant diclofenac in subsurface environment. Int. J. Environ. Sci. Technol. 2017, 15, 1201–1210. [Google Scholar] [CrossRef]
- Goldstein, M.; Shenker, M.; Chefetz, B. Insights into the Uptake Processes of Wastewater-Borne Pharmaceuticals by Vegetables. Environ. Sci. Technol. 2014, 48, 5593–5600. [Google Scholar] [CrossRef] [PubMed]
- Norvill, Z.N.; Shilton, A.; Guieysse, B. Emerging contaminant degradation and removal in algal wastewater treatment ponds: Identifying the research gaps. J. Hazard. Mater. 2016, 313, 291–309. [Google Scholar] [CrossRef] [PubMed]
- Łomińska-Płatek, D.; Anielak, A.M. Quantitative balance and analysis of fulvic acids changes in the process of municipal sewage treatment. Water Resour. Ind. 2021, 26, 100155. [Google Scholar] [CrossRef]
- Anielak, A.M.; Kryłów, M.; Łomińska-Płatek, D. Characterization of fulvic acids contained in municipal sewage purified with activated sludge. Arch. Environ. Prot. 2018, 44, 70–76. [Google Scholar] [CrossRef]
- Anielak, A.M.; Kłeczek, A. Humus Acids in the Digested Sludge and Their Properties. Materials 2022, 15, 1475. [Google Scholar] [CrossRef] [PubMed]
- Styszko, K.; Durak, J.; Malicka, A.; Bochnia, T.; Zaba, T. The occurrence of chemicals of emerging concern in samples of surface water and wastewater collected in Kraków, Poland. Desalination Water Treat. 2021, 232, 308–323. [Google Scholar] [CrossRef]
- Orliński, T.; Anielak, A.M. Characteristics of fulvic acids generated in communes waste landfills. Arch. Environ. Prot. 2021, 47, 41–52. [Google Scholar] [CrossRef]
- Han, Y.-S.; Lee, J.-Y.; Miller, C.J.; Franklin, L. Characterization of humic substances in landfill leachate and impact on the hydraulic conductivity of geosynthetic clay liners. Waste Manag. Res. 2009, 27, 233–241. [Google Scholar] [CrossRef]
- Huo, S.; Xi, B.; Yu, H.; He, L.; Fan, S.; Liu, H. Characteristics of dissolved organic matter (DOM) in leachate with different landfill ages. J. Environ. Sci. 2008, 20, 492–498. [Google Scholar] [CrossRef]
- Zhang, S.; Yuan, L.; Li, W.; Lin, Z.; Li, Y.; Hu, S.; Zhao, B. Characterization of pH-fractionated humic acids derived from Chinese weathered coal. Chemosphere 2017, 166, 334–342. [Google Scholar] [CrossRef]
- Anielak, A.M. Kwasy humusowe: Ekstrakcja, analiza i znaczenie w środowisku oraz metody ich usuwania. Przem. Chem. 2019, 98, 1580–1586. [Google Scholar] [CrossRef]
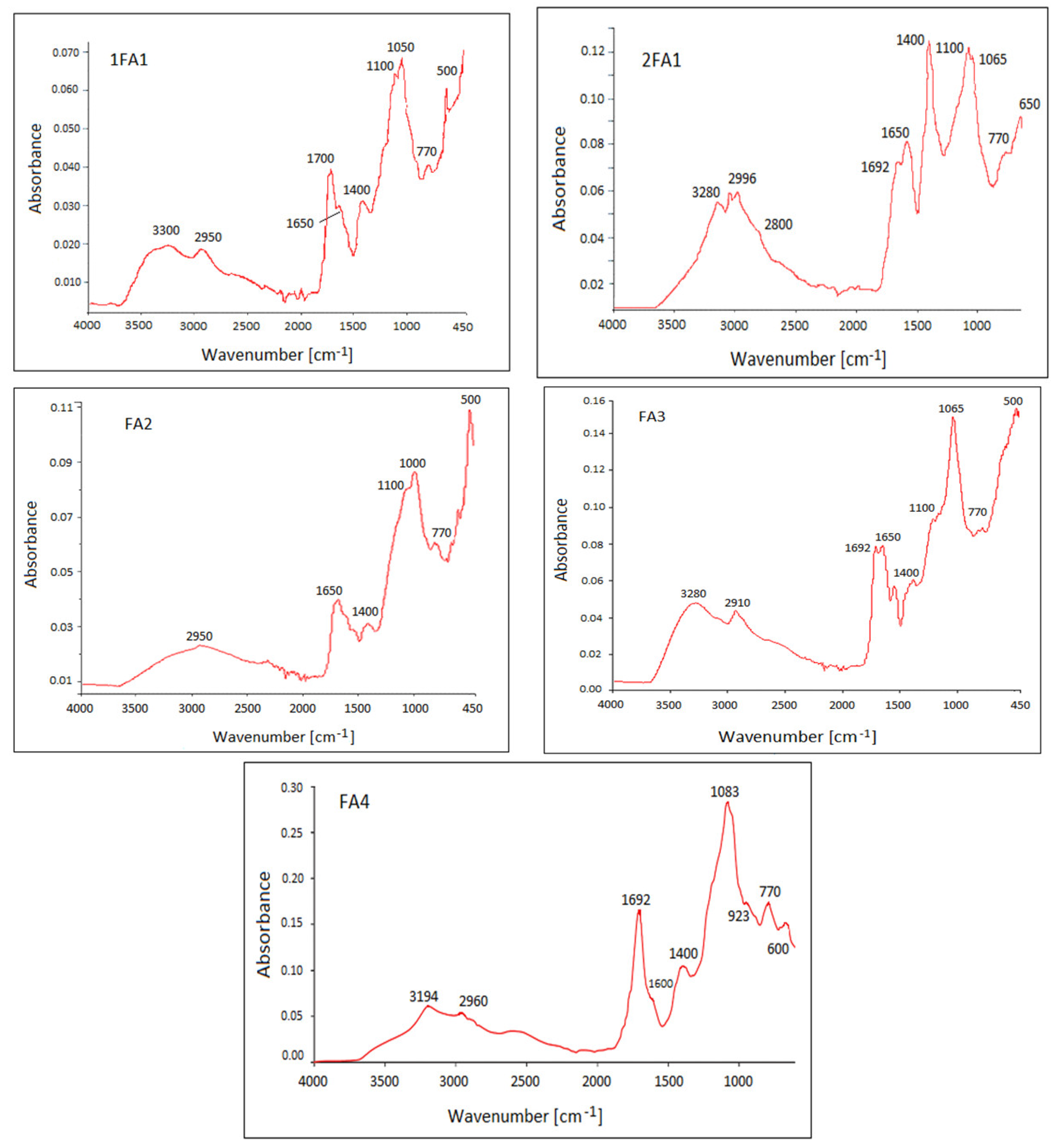
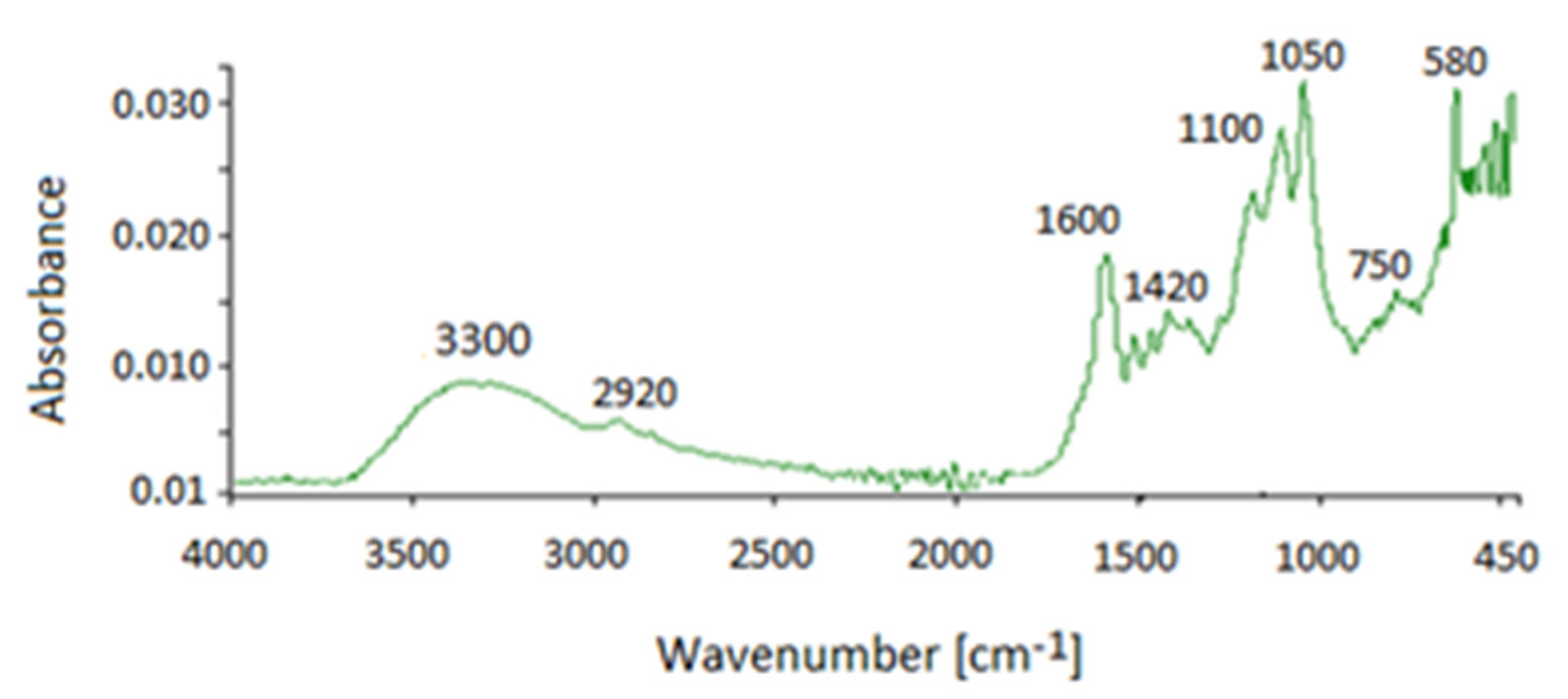

| Wave Number cm−1 | 1FA1 | 2FA1 | FA2 | FA3 | FA4 1 | |
|---|---|---|---|---|---|---|
| 3300–2500 | X | X | X | X | X | Broad absorption, stretching vibration bands in carboxylic acids (forming O-H hydrogen bonds). |
| 1700–1650 | X | X | X | X | X | Intense stretching carbonyl band, C=O bond in acids, aromatic acids |
| Fingerprint region | ||||||
| 1400 | X | X | X | X | X | Bending deformation of O-H, stretching of C-O and phenolic OH, and asymmetric stretching of COO-, acids |
| 1100–1065 | X | X | X | X | X | Stretching vibration of C-O in acids |
| 770 | X | X | X | X | X | Deformation vibration of δNO2, out-of-plane deformation of NH2 |
| 650–500 | X | X | X | X | X | Stretching vibration band of S-S in disulphides |
| FA * | Elementals Content Ash Free (%) | Atomic Ratios (-) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | H | N | O | Ash | O/C | H/C | C/N | O/H | |
| 1 FA1 | 46.02 | 5.13 | 1.57 | 47.28 | 14.77 | 0.77 | 1.34 | 34.20 | 0.58 |
| 2 FA1 | 43.51 | 5.37 | 9.44 | 41.68 | 9.74 | 0.72 | 1.47 | 5.41 | 0.49 |
| FA2 | 52.07 | 6.07 | 7.12 | 34.74 | 36.07 | 0.50 | 1.41 | 8.51 | 0.36 |
| FA3 | 44.83 | 6.47 | 5.23 | 43.47 | 14.66 | 0.97 | 1.72 | 10.08 | 0.56 |
| FA4 1 | 55.36 | 4.29 | 1.07 | 43.42 | 27.94 | 0.59 | 0.93 | 60.66 | 0.63 |
| Element | Concentration, mg/gFA | ||||
|---|---|---|---|---|---|
| 1FA1 | 2FA1 | FA2 | FA3 | FA4 1 | |
| Cl | nf | nf | 58.5 | 60.8 | 4.2 |
| Si | nf | 15.5 | 5.54 | nf | 155.8 |
| Na | nf | 14.5 | nf | 18.4 | 15.6 |
| Ca | nf | 8.80 | 25.8 | 8.0 | 6.0 |
| K | 10.83 | 2.50 | 4.71 | 0.08 | 2.8 |
| Mg | 20.31 | 1.30 | 17.2 | 0.005 | 0.04 |
| P | nf | 1.60 | 207 | 16.3 | 2.9 |
| Br | nf | 0.20 | nf | 5.0 | nf |
| Al | 13.54 | - | nf | - | - |
| Cu | 0.0074 | - | - | - | - |
| Cr | 0.008 | nf | 0.16 | 0.02 | nf |
| Zn | 0.0468 | 0.09 | 1.66 | - | nf |
| Ni | 0.0038 | - | - | - | - |
| Pb | <0.0001 | - | - | - | - |
| Cd | <0.0001 | - | - | - | - |
| Mn | 4.69 | - | - | - | - |
| Fe | 0.11 | 0.6 | 18.3 | 4.3 | 1.2 |
| Ash, % | 14.77 | 9.74 | 36.07 | 14.66 | 27.94 |
| Element | Result | Unit |
|---|---|---|
| C | 34.24 | % (m/m) |
| H | 3.21 | % (m/m) |
| N | 0.83 | % (m/m) |
| O | 53.42 | % (m/m) |
| Al | 31 | mg/kg |
| B | 202 | mg/kg |
| Ba | 2 | mg/kg |
| Co | 101 | mg/kg |
| Ca | 992 | mg/kg |
| Cu | 776 | mg/kg |
| Fe | 47 | mg/kg |
| K | 76,405 | mg/kg |
| Mg | 150 | mg/kg |
| Na | 1636 | mg/kg |
| Mn | 165 | mg/kg |
| Sr | 6 | mg/kg |
| Zn | 157 | mg/kg |
| Ash | 38.13 | % (m/m) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anielak, A.M.; Styszko, K.; Kłeczek, A.; Łomińska-Płatek, D. Humic Substances—Common Carriers of Micropollutants in Municipal Engineering. Energies 2022, 15, 8496. https://doi.org/10.3390/en15228496
Anielak AM, Styszko K, Kłeczek A, Łomińska-Płatek D. Humic Substances—Common Carriers of Micropollutants in Municipal Engineering. Energies. 2022; 15(22):8496. https://doi.org/10.3390/en15228496
Chicago/Turabian StyleAnielak, Anna M., Katarzyna Styszko, Aneta Kłeczek, and Dominika Łomińska-Płatek. 2022. "Humic Substances—Common Carriers of Micropollutants in Municipal Engineering" Energies 15, no. 22: 8496. https://doi.org/10.3390/en15228496
APA StyleAnielak, A. M., Styszko, K., Kłeczek, A., & Łomińska-Płatek, D. (2022). Humic Substances—Common Carriers of Micropollutants in Municipal Engineering. Energies, 15(22), 8496. https://doi.org/10.3390/en15228496





