Geochemical Modelling of the Fracturing Fluid Transport in Shale Reservoirs
Abstract
:1. Introduction

2. Methodology
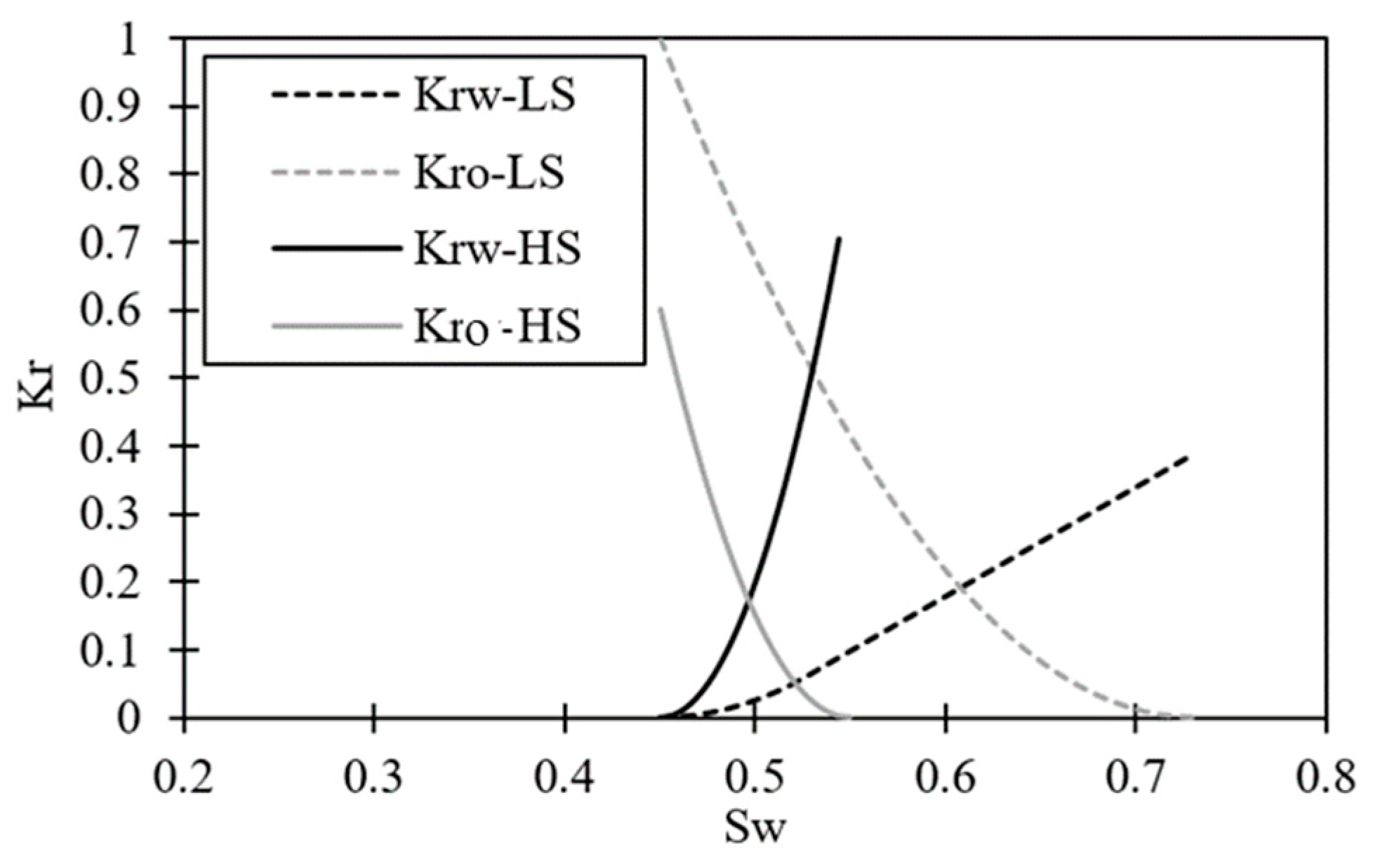
2.1. Reservoir Modeling
2.2. Geochemical Interactions
| Mineral | Area (m2/m3) | Activation Energy (J/mol) | Log Keq (mol/(m2s)) at 25 °C |
|---|---|---|---|
| Kaolinite | 17,600 | 62,760 | −13.18 |
| Illite | 26,400 | 58,620 | −14 |
| Calcite | 88 | 41,870 | −8.79 |
| Dolomite | 88 | 41,870 | −9.22 |
| Quartz | 7128 | 87,500 | −13.9 |
| K-Feldspar | 176 | 67,830 | −12 |
| Exchange Reactions | Exchange Constant (100 °C) |
|---|---|
| Ca2+ + 2NaX ⟷ 2Na+ + CaX2 | 11.31 |
| Mg2+ + 2NaX ⟷ Na+ + MgX2 | 7.25 |
| H+ + NaX ⟷ Na+ + HX | 10 |
| Ions | Slick Water | Connate Water | Sea Water | Haynesville |
|---|---|---|---|---|
| HCO3− | 49 | 354 | 12 | - |
| Ca++ | 29 | 19,040 | 650 | 26,040 |
| SO4−− | 5 | 350 | 2290 | - |
| Mg++ | 3 | 2439 | 1110 | 1460 |
| Na+ | 80 | 59,491 | 10,352 | 18,400 |
| Cl− | 30 | 102,060 | 18,379 | 71,102 |
| K+ | 984 | - | 600 | 310 |
| CO3−− | 640 | - | - | - |
| Ba+2 | 1 | - | - | - |
| Fe+2 | 1 | - | - | - |
| B+3 | 120 | - | - | - |
| Si−4 | 2 | - | - | - |
| Total | 1944 | 183,734 | 33,393 | 117,312 |
2.3. Sensitivity Analysis
3. Results
3.1. Mineral Dissolution and Precipitation
3.2. The Impact of Connate Water Composition
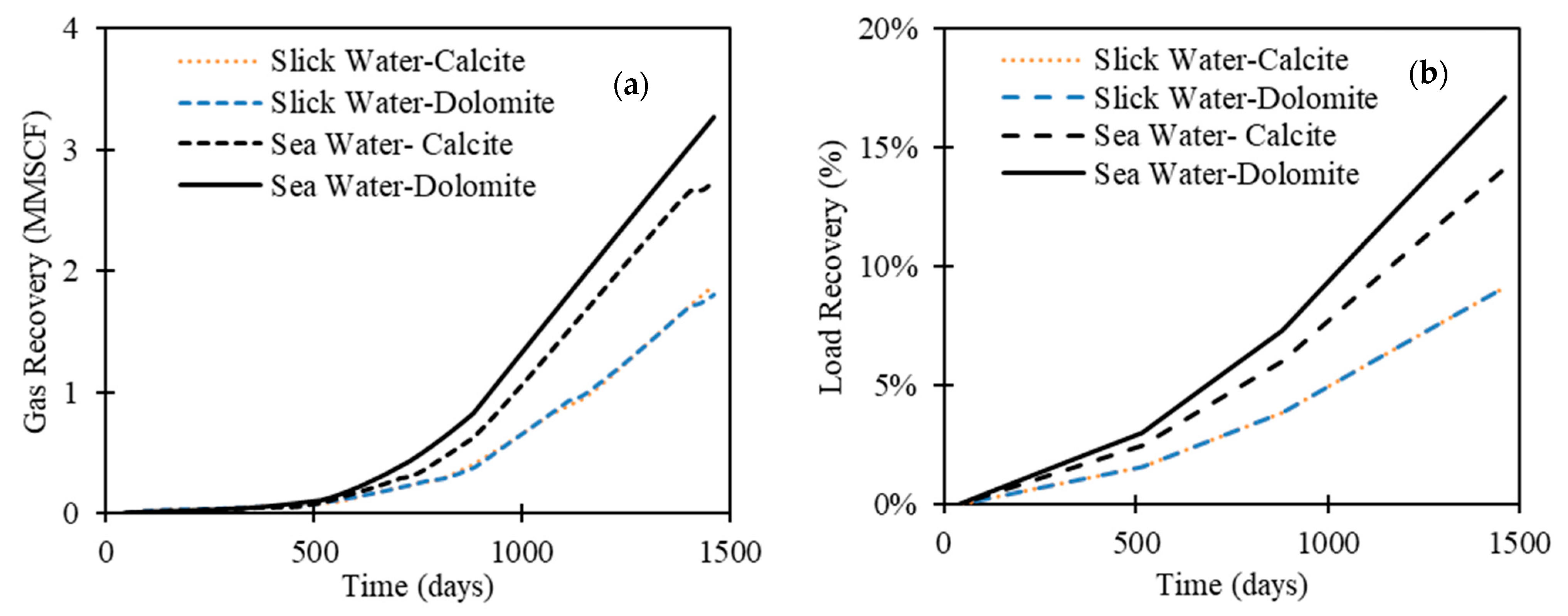
3.3. The Impact of Carbonate Mineral Type
Sensitivity Analysis Results
4. Conclusions
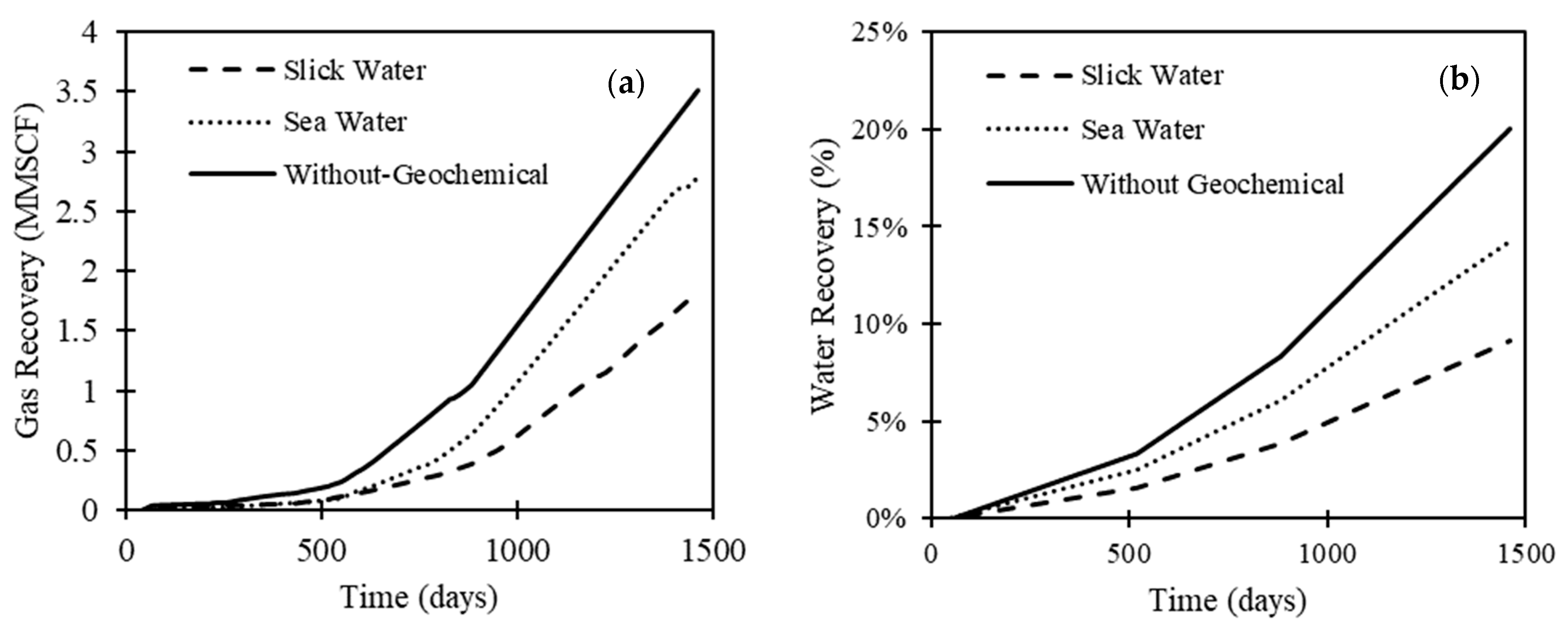
- Neglecting geochemical coupling results in an overestimation of both load and gas recovery. We observed that geochemical interactions could reduce gas recovery and load recovery by more than 50%.
- Sea water, as a fracturing fluid, consistently results in higher load and gas recovery compared to slick water (almost double). In fact, the salinity contrast between the injected fluid and the formation brine correlates negatively with well performance. We observed that sea water promotes calcite dissolution, while slick water promotes dolomite dissolution.
- In most cases studied, clay mineral interactions are minimal compared to carbonate mineral interactions. The highest amount of clay interactions are observed in the case of slick water injection into the lower-salinity connate water case of Haynesville.
- Sensitivity analysis suggests that the concentration of , K+ and Na+ ions in the fracturing fluid and illite and calcite mineral content of the rock, along with the reservoir temperature, are the main key factors affecting well performance.
Author Contributions
Funding
Conflicts of Interest
References
- Hyman, J.; Jiménez-Martínez, J.; Viswanathan, H.S.; Carey, J.W.; Porter, M.L.; Rougier, E.; Karra, S.; Kang, Q.; Frash, L.; Chen, L.; et al. Understanding hydraulic fracturing: A multi-Scale problem. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middleton, R.S.; Gupta, R.; Hyman, J.D.; Viswanathan, H.S. The shale gas revolution: Barriers, sustainability, and emerging opportunities. Appl. Energy 2017, 199, 88–95. [Google Scholar] [CrossRef]
- Middleton, R.S.; Carey, J.W.; Currier, R.P.; Hyman, J.D.; Kang, Q.; Karra, S.; Jiménez-Martínez, J.; Porter, M.L.; Viswanathan, H.S. Shale gas and non-aqueous fracturing fluids: Opportunities and challenges for supercritical CO2. Appl. Energy 2015, 147, 500–509. [Google Scholar] [CrossRef] [Green Version]
- King, G.E. Thirty years of gas shale fracturing: What have we learned? In Proceedings of the SPE Annual Technical Conference and Exhibition, Florence, Italy, 20–22 September 2010. [Google Scholar]
- Singh, H. A critical review of water uptake by shales. J. Nat. Gas Sci. Eng. 2016, 34, 751–766. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Zhao, C.; Zhao, J.; Wang, Q.; Zhao, J.; Gao, D.; Fu, C. Mechanisms of fracturing fluid spontaneous imbibition behavior in shale reservoir: A review. J. Nat. Gas Sci. Eng. 2020, 82, 103498. [Google Scholar] [CrossRef]
- Hun, L.; Bing, Y.; Xixiang, S.; Xinyi, S.; Lifei, D. Fracturing fluid retention in shale gas reservoir from the perspective of pore size based on nuclear magnetic resonance. J. Hydrol. 2021, 601, 126590. [Google Scholar] [CrossRef]
- Bai, J.; Wang, G.; Zhu, Q.; Tao, L.; Shi, W. Investigation on Flowback Behavior of Imbibition Fracturing Fluid in Gas–Shale Multiscale Pore Structure. Energies 2022, 15, 7802. [Google Scholar] [CrossRef]
- O’Malley, D.; Karra, S.; Currier, R.P.; Makedonska, N.; Hyman, J.D.; Viswanathan, H.S. Where does water go during hydraulic fracturing? Groundwater 2016, 54, 488–497. [Google Scholar] [CrossRef]
- Mehana, M.M.; Al, S.; Fahes, M. Impact of salinity and mineralogy on slick water spontaneous imbibition and formation strength in shale. Energy Fuels 2018, 32, 5725–5735. [Google Scholar] [CrossRef]
- Mehana, M.M.; Al, S.; Fahes, M. The impact of salinity on water dynamics, hydrocarbon recovery and formation softening in Shale: Experimental study. In Proceedings of the SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition, Dammam, Saudi Arabia, 24–27 April 2017. [Google Scholar]
- Mehana, M.; El-Monier, I. Numerical investigation of the osmatic flow impact on the load recovery and early well performance. J. Pet. Eng. Technol. 2015, 5, 52–64. [Google Scholar]
- Fakcharoenphol, P.; Torcuk, M.A.; Bertoncello, A.; Kazemi, H.; Wu, Y.-S.; Wallace, J.; Honarpour, M. Managing Shut-in Time to Enhance Gas Flow Rate in Hydraulic Fractured Shale Reservoirs: A Simulation Study. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 30 September–2 October 2013. [Google Scholar]
- Le, D.H.; Hoang, H.N.; Mahadevan, J. Gas recovery from tight sands: Impact of capillarity. Spe J. 2012, 17, 981–991. [Google Scholar] [CrossRef]
- Agrawal, S.; Sharma, M.M. Impact of liquid loading in hydraulic fractures on well productivity. In Proceedings of the SPE Hydraulic Fracturing Technology Conference, The Woodlands, TX, USA, 4–6 February 2013. [Google Scholar]
- Gdanski, R.; Walters, H. Impact of fracture conductivity and matrix relative permeability on load recovery. In Proceedings of the SPE Annual Technical Conference and Exhibition, Florence, Italy, 20–22 September 2010. [Google Scholar]
- Mahadevan, J.; Sharma, M.M.; Yortsos, Y.C. Evaporative cleanup of water blocks in gas wells. SPE J. 2007, 12, 209–216. [Google Scholar] [CrossRef]
- Hajirezaie, S.; Wu, X.; Soltanian, M.R.; Sakha, S. Numerical simulation of mineral precipitation in hydrocarbon reservoirs and wellbores. Fuel 2019, 238, 462–472. [Google Scholar] [CrossRef]
- Hajirezaie, S.; Wu, X.; Peters, C.A. Scale formation in porous media and its impact on reservoir performance during water flooding. J. Nat. Gas Sci. Eng. 2017, 39, 188–202. [Google Scholar] [CrossRef]
- Akrad, O.; Miskimins, J.; Prasad, M. The effects of fracturing fluids on shale rock mechanical properties and proppant embedment. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 30 October–2 November 2011. [Google Scholar]
- Das, P.; Achalpurkar, M.; Pal, O. Impact of formation softening and rock mechanical properties on selection of shale stimulation fluid: Laboratory evaluation. In Proceedings of the SPE/EAGE European Unconventional Resources Conference and Exhibition, Vienna, Austria, 25–27 February 2014. [Google Scholar]
- King, G. Fracture Fluid Additive and Formation Degradations. EPA Workshop on Hydraulic Fracturing, Workshop No. 1, Chemicals; 2015. Available online: https://19january2017snapshot.epa.gov/sites/production/files/documents/fracturefluidadditivesandformationdegradations.pdf (accessed on 18 October 2022).
- Chermak, J.A.; Schreiber, M.E. Mineralogy and trace element geochemistry of gas shales in the United States: Environmental implications. Int. J. Coal Geol. 2014, 126, 32–44. [Google Scholar] [CrossRef]
- Martin, R. Unconventional Completions–A Paradigm Shift–Evening Session; SPE: Dallas, TX, USA, 2015. [Google Scholar]
- Cipolla, C.L.; Lolon, E.P.; Erdle, J.C.; Rubin, B. Reservoir Modeling in Shale-Gas Reservoirs. SPE Reserv. Eval. Eng. 2010, 13, 638–653. [Google Scholar] [CrossRef]
- Webb, K.; Lager, A.; Black, C. Comparison of high/low salinity water/oil relative permeability. In Proceedings of the International symposium of the society of core analysts, Abu Dhabi, United Arab Emirates, 29 October–2 November 2008. [Google Scholar]
- Nghiem, L.; Sammon, P.; Grabenstetter, J.; Ohkuma, H. Modeling CO2 storage in aquifers with a fully-coupled geochemical EOS compositional simulator. In Proceedings of the SPE/DOE symposium on improved oil recovery, Tulsa, OK, USA, 17–21 April 2004; OnePetro: Richardson, TX, USA, 2004. [Google Scholar]
- Hakala, J.A.; Vankeuren, A.N.P.; Scheuermann, P.P.; Lopano, C.; Guthrie, G.D. Predicting the potential for mineral scale precipitation in unconventional reservoirs due to fluid-rock and fluid mixing geochemical reactions. Fuel 2021, 284, 118883. [Google Scholar] [CrossRef]
- Palisch, T.T.; Vincent, M.C.; Handren, P.J. Slickwater fracturing: Food for thought. In Proceedings of the SPE annual technical conference and exhibition, Denver, CO, USA, 21–24 September 2008. [Google Scholar]
- Computer Modelling Group. CMG-GEM Technical Manual; Computer Modelling Group: Calgary, AB, Canada, 2017. [Google Scholar]
- Luo, H.; Al-Shalabi, E.W.; Delshad, M.; Panthi, K.; Sepehrnoori, K. A Robust Geochemical Simulator To Model Improved-Oil-Recovery Methods. SPE J. 2016, 21, 55–73. [Google Scholar] [CrossRef]
- Fjelde, I.; Asen, S.M.; Omekeh, A. Low salinity water flooding experiments and interpretation by simulations. In Proceedings of the SPE improved oil recovery symposium, Tulsa, OK, USA, 14–18 April 2012. [Google Scholar]
- Schepdael, A.V.; Carlier, A.; Geris, L. Sensitivity analysis by design of experiments. In Uncertainty in Biology; Springer: Berlin/Heidelberg, Germany, 2016; pp. 327–366. [Google Scholar]
- Shehata, A.M.; Nasr-El-Din, H.A. Reservoir connate water chemical composition variations effect on low-salinity waterflooding. In Proceedings of the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, United Arab Emirates, 10–13 November 2014. [Google Scholar]
- Tang, H.; Li, S.; Zhang, D. The effect of heterogeneity on hydraulic fracturing in shale. J. Pet. Sci. Eng. 2018, 162, 292–308. [Google Scholar] [CrossRef]
- Yucel, A.I.; Fathi, E. Multiscale gas transport in shales with local kerogen heterogeneities. SPE J. 2012, 17, 1002–1011. [Google Scholar] [CrossRef] [Green Version]
- Fathi, E.; Akkutlu, I.Y. Matrix heterogeneity effects on gas transport and adsorption in coalbed and shale gas reservoirs. Transp. Porous Media 2009, 80, 281–304. [Google Scholar] [CrossRef]
- Borrok, D.M.; Yang, W.; Wei, M.; Mokhtari, M. Heterogeneity of the mineralogy and organic content of the Tuscaloosa Marine Shale. Mar. Pet. Geol. 2019, 109, 717–731. [Google Scholar] [CrossRef]
- Chen, Y.; Mastalerz, M.; Schimmelmann, A. Heterogeneity of shale documented by micro-FTIR and image analysis. J. Microsc. 2014, 256, 177–189. [Google Scholar] [CrossRef] [PubMed]










| Model Parameters | Value |
|---|---|
| Model Dimensions | 420 × 420 × 100 ft. |
| Reservoir Pressure | 5000 psi |
| Matrix Porosity | 7% |
| Natural Fracture Porosity | 1% |
| Matrix Permeability | 1.5 × 10−4 md |
| Fracture Conductivity | 4.13 md-ft. |
| Reservoir Temperature | 250 F |
| Natural Fracture Spacing | 10 ft. |
| Shut-in time | 1 month |
| Parameter | Slick Water | Sea Water | ||||
|---|---|---|---|---|---|---|
| Base | Lower | Upper | Base | Lower | Upper | |
| Ca+2 (Mole/L) | 0.000724 | 0.000543 | 0.000904 | 0.000299 | 0.000225 | 0.000374 |
| Cl− (Mole/L) | 0.000846 | 0.000635 | 0.001058 | 0.5184 | 0.3888 | 0.648 |
| H+ (Mole/L) | 9.9216 × 10−7 | 7.44 × 10−7 | 1.24 × 10−6 | 9.92 × 10−11 | 7.44 × 10−11 | 1.24 × 10−10 |
| HCO−3 (Mole/L) | 0.01638 | 0.01229 | 0.0205 | 0.01065 | 0.007989 | 0.01332 |
| K+ (Mole/L) | 0.02517 | 0.01888 | 0.03146 | 0.04566 | 0.03425 | 0.05708 |
| Mg+2 (Mole/L) | 0.000123 | 9.26 × 10−5 | 0.000154 | 0.4567 | 0.3377 | 0.5629 |
| Na+ (Mole/L) | 0.00348 | 0.002609 | 0.004349 | 0.02384 | 0.01788 | 0.0298 |
| (Mole/L) | 5.21 × 10−5 | 3.90 × 10−5 | 6.51 × 10−5 | 0.011534 | 1.15 × 10−2 | 1.92 × 10−2 |
| T (F) | 250 | 187.5 | 312.5 | 250 | 187.5 | 312.5 |
| Calcite | 0.15 | 0.1125 | 0.1875 | 0.15 | 0.1125 | 0.1875 |
| Dolomite | 0.15 | 0.1125 | 0.1875 | 0.15 | 0.1125 | 0.1875 |
| Illite | 0.15 | 0.1125 | 0.1875 | 0.15 | 0.1125 | 0.1875 |
| Kaolinite | 0.15 | 0.1125 | 0.1875 | 0.15 | 0.1125 | 0.1875 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehana, M.; Chen, F.; Fahes, M.; Kang, Q.; Viswanathan, H. Geochemical Modelling of the Fracturing Fluid Transport in Shale Reservoirs. Energies 2022, 15, 8557. https://doi.org/10.3390/en15228557
Mehana M, Chen F, Fahes M, Kang Q, Viswanathan H. Geochemical Modelling of the Fracturing Fluid Transport in Shale Reservoirs. Energies. 2022; 15(22):8557. https://doi.org/10.3390/en15228557
Chicago/Turabian StyleMehana, Mohamed, Fangxuan Chen, Mashhad Fahes, Qinjun Kang, and Hari Viswanathan. 2022. "Geochemical Modelling of the Fracturing Fluid Transport in Shale Reservoirs" Energies 15, no. 22: 8557. https://doi.org/10.3390/en15228557
APA StyleMehana, M., Chen, F., Fahes, M., Kang, Q., & Viswanathan, H. (2022). Geochemical Modelling of the Fracturing Fluid Transport in Shale Reservoirs. Energies, 15(22), 8557. https://doi.org/10.3390/en15228557






