Potential Application of Canola Hull Fuel Pellets for the Production of Synthesis Gas and Hydrogen
Abstract
:1. Introduction
2. Experimental
2.1. Steam Gasification
2.2. Supercritical Water Gasification
2.3. Characterization Techniques
3. Results and Discussion
3.1. Steam Gasification
3.2. Supercritical Water Gasification
3.3. Physicochemical Characterization of Biomass and Biochar
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Bioenergy Association. Available online: https://www.worldbioenergy.org/uploads/201210%20WBA%20GBS%202020.pdf (accessed on 13 October 2022).
- Canola Council of Canada. Available online: https://www.canolacouncil.org/markets-stats/production/ (accessed on 23 February 2022).
- Azargohar, R.; Nanda, S.; Kang, K.; Bond, T.; Karunakaran, C.; Dalai, A.K.; Kozinski, J.A. Effects of bio-additives on the physicochemical properties and mechanical behavior of canola hull fuel pellets. Renew. Energy 2019, 132, 296–307. [Google Scholar] [CrossRef]
- Song, K.; Zhang, H.; Wu, Q.; Zhang, Z.; Zhou, C.; Zhang, Q.; Lei, T. Structure and thermal properties of tar from gasification of agricultural crop residue. J. Therm. Anal. Calorim. 2015, 119, 27–35. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification. In Pyrolysis and Torrefaction: Practical Design and Theory, 3rd ed.; Academic Press: London, UK, 2010; pp. 199–248. [Google Scholar]
- Molinoa, A.; Chianese, S.; Musmarra, D. Biomass gasification technology: The state of the art overview. J. Energy Chem. 2016, 25, 10–25. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Sheeba Narayanan, K. Hydrogen production from steam gasification of biomass: Influence of process parameters on hydrogen yield: A review. Renew. Energy 2014, 66, 570–579. [Google Scholar] [CrossRef]
- Larssona, A.; Kuba, M.; Vilches, T.B.; Seemann, M.; Hofbauer, H.; Thunman, H. Steam gasification of biomass–Typical gas quality and operational strategies derived from industrial-scale plants. Fuel Process. Technol. 2021, 212, 106609. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, Q.; Wang, Y.; Guo, P.; Wang, Z.; Liu, H.; Akbari, A. Investigation of biomass gasification potential in syngas production: Characteristics of dried biomass gasification using steam as the gasification agent. Energy Fuels 2020, 34, 1033–1040. [Google Scholar] [CrossRef]
- Wang, H.; Miao, R.; Yang, Y.; Qiao, Y.; Zhang, Q.; Li, C.; Huang, J. Study on the catalytic gasification of alkali lignin over Ru/C nanotubes in supercritical water. J. Fuel Chem. Technol. 2015, 43, 1195–1201. [Google Scholar] [CrossRef]
- Cao, W.; Cao, C.; Guo, L.; Jin, H.; Dargusch, M.; Bernhardt, D.; Yao, X. Hydrogen production from supercritical water gasification of chicken manure. Int. J. Hydrogen Energy 2016, 41, 22722–22731. [Google Scholar] [CrossRef]
- Secer, A.; Fak, E.; Uzden, S.T.; Hasanoglu, A. Hydrothermal co-gasification of sorghum biomass and can lignite in mild conditions: An optimization study for high yield hydrogen production. Int. J. Hydrogen Energy 2020, 45, 2668–2680. [Google Scholar] [CrossRef]
- Yang, C.; Wang, S.; Yang, J.; Xu, D.; Li, Y. Hydrothermal liquefaction and gasification of biomass and model compounds: A review. Green Chem. 2020, 22, 8210–8232. [Google Scholar] [CrossRef]
- Pinto, F.; Gominho, J.; Neto, A.R.; Gonçalves, D.; Miranda, M.; Varela, F. Improvement of gasification performance of Eucalyptus globulus stumps with torrefaction and densification pre-treatments. Fuel 2017, 206, 289–299. [Google Scholar] [CrossRef]
- Bartocci, P.; Zampilli, M.; Bidini, G.; Fantozzi, F. Hydrogen-rich gas production through steam gasification of charcoal pellet. Appl. Eng. 2018, 132, 817–823. [Google Scholar] [CrossRef]
- Abedi, A.; Cheng, H.; Dalai, A.K. Effects of natural additives on the properties of sawdust fuel pellets. Energy Fuels 2018, 32, 1863–1873. [Google Scholar] [CrossRef]
- Chojnacki, J.; Najser, J.; Rokosz, K.; Peer, V.; Kielar, J.; Berner, B. Syngas composition: Gasification of wood pellet with water steam through a reactor with continuous biomass feed system. Energies 2020, 13, 4376. [Google Scholar] [CrossRef]
- Macrì, D.; Catizzone, E.; Molino, A.; Migliori, M. Supercritical water gasification of biomass and agro-food residues: Energy assessment from modelling approach. Renew. Energy 2020, 150, 624–636. [Google Scholar] [CrossRef]
- Bakari, R.; Kivevele, T.; Huang, X.; Jande, Y.A.C. Catalytic supercritical water gasification of biomass waste using iron-doped alkaline earth catalysts. Biomass Conv. Bioref. 2022. [Google Scholar] [CrossRef]
- Tilay, A.; Azargohar, R.; Gerspacher, R.; Dalai, A.K.; Kozinski, J.A. Gasification of canola meal and factors affecting gasification process. Bioenergy Res. J. 2014, 7, 1131–1143. [Google Scholar] [CrossRef]
- Molino, A.; Larocca, V.; Chianese, S.; Musmarra, D. Biofuels Production by Biomass Gasification: A Review. Energies 2018, 11, 811. [Google Scholar] [CrossRef] [Green Version]
- Nanda, S.; Dalai, A.K.; Gökalp, I.; Kozinski, J.A. Valorization of horse manure through catalytic supercritical water gasification. Waste Manag. 2016, 52, 147–158. [Google Scholar] [CrossRef]
- Yakaboylu, O.; Harinck, J.; Smit, K.G.; Jong, W.D. Supercritical water gasification of biomass: A literature and technology overview. Energies 2015, 8, 859–894. [Google Scholar] [CrossRef]
- Hu, Y.; Gong, M.; Xing, X.; Wang, H.; Zeng, Y.; Xu, C.C. Supercritical water gasification of biomass model compounds: A review. Renew. Sustain. Energy Rev. 2020, 118, 109529. [Google Scholar] [CrossRef]
- PM, L.V.; Xiong, Z.H.; Chang, J.; Wu, C.Z.; Chen, Y.; Zhu, J.X. An experimental study on biomass air–steam gasification in a fluidized bed. Bioresour. Technol. 2004, 95, 95–101. [Google Scholar] [CrossRef]
- Arena, U.; Di Gregorio, F. Gasification of a solid recovered fuel in a pilot scale fluidized bed reactor. Fuel 2014, 117, 528–536. [Google Scholar] [CrossRef]
- Xue, G.; Kwapinska, M.; Horvat, A.; Kwapinski, W.; Rabou, L.P.L.M.; Dooley, S.; Czajka, K.M.; Leahy, J.J. Gasification of torrefied Miscanthusgiganteus in an air-blown bubbling fluidized bed gasifier. Bioresour. Technol. 2014, 159, 397–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nobre, C.; Longo, A.; Vilarinho, C.; Gonçalves, M. Gasification of pellets produced from blends of biomass wastes and refuse derived fuel chars. Renew. Energy 2020, 154, 1294–1303. [Google Scholar] [CrossRef]
- de Lasa, H.; Salaices, E.; Mazumder, J.; Lucky, R. Catalytic Steam Gasification of Biomass: Catalysts, Thermodynamics and Kinetics. Chem. Rev. 2011, 111, 5404–5433. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Zhao, C.; Pang, K. Experimental investigation of natural coke steam gasification in a bench-scale fluidized bed: Influences of temperature and oxygen flow rate. Energy Fuel 2009, 23, 805–810. [Google Scholar] [CrossRef]
- Schweitzer, D.; Gredinger, A.; Schmid, M.; Waizmann, G.; Beirow, M.; Sporl, R. Steam gasification of wood pellets, sewage sludge and manure: Gasification performance and concentration of impurities. Biomass Bioenergy 2018, 111, 308–319. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Nguyen, P.L.T.; Tran, V.B. Zero-waste biomass gasification: Use of residues after gasification of bagasse pellets as CO2 adsorbents. Therm. Sci. Eng. Prog. 2021, 26, 101080. [Google Scholar] [CrossRef]
- Azargohar, R.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Physico-chemistry of biochars produced through steam gasification and hydro-thermal gasification of canola hull and canola meal pellets. Biomass Bioenergy 2019, 120, 458–470. [Google Scholar] [CrossRef]
- Traa, Y. Is a renaissance of coal imminent? Challenges for catalysis? Chem. Commun. 2010, 46, 2175–2187. [Google Scholar] [CrossRef] [PubMed]
- de Klerk, A. Fischer–Tropsch Refining, 1st ed.; Wiley-VCH Verlag & Co.: Weinheim, Germany, 2011; pp. 1–20. [Google Scholar]
- Reddy, S.N.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Supercritical water gasification of biomass for hydrogen production. Int. J. Hydrogen Energy 2014, 39, 6912–6926. [Google Scholar] [CrossRef]
- Guo, L.J.; Lu, Y.J.; Zhang, X.M.; Ji, C.M.; Guan, Y.; Pei, A.X. Hydrogen production by biomass gasification in supercritical water: A systematic experimental and analytical study. Catal. Today 2007, 129, 275–286. [Google Scholar] [CrossRef]
- Kruse, A. Supercritical water gasification. Biofuel. Bioprod. Bioref. 2008, 2, 415–437. [Google Scholar] [CrossRef]
- Susanti, R.F.; Dianningrum, L.W.; Yuma, T.; Kim, Y.; Lee, B.G.; Kim, J. High-yield hydrogen production from glucose by supercritical water gasification without added catalyst. Int. J. Hydrogen Energy 2012, 37, 11677–11690. [Google Scholar] [CrossRef]
- Nanda, S.; Dalai, A.K.; Kozinski, J.A. Supercritical water gasification of timothy grass as an energy crop in the presence of alkali carbonate and hydroxide catalysts. Biomass Bioenergy 2016, 95, 378–387. [Google Scholar] [CrossRef]
- Nanda, S.; Isen, J.; Dalai, A.K.; Kozinski, J.A. Gasification of fruit wastes and agro-food residues in supercritical water. Energy Convers. Manag. 2016, 110, 296–306. [Google Scholar] [CrossRef]
- Li, X.-G.; Ma, B.-G.; Xu, L.; Hu, Z.-W.; Wang, X.-G. Thermogravimetric analysis of the co-combustion of the blends with high ash coal and waste tyres. Thermochim. Acta 2006, 441, 79–83. [Google Scholar] [CrossRef]
- Xia, W.; Niu, C.; Ren, C. Enhancement in floatability of sub-bituminous coal by low-temperature pyrolysis and its potential application in coal cleaning. J. Clean. Prod. 2017, 168, 1032–1038. [Google Scholar] [CrossRef]
- Kim, P.; Johnson, A.; Edmunds, C.W.; Radosevich, M.; Vogt, F.; Rials, T.G.; Labbe, N. Surface functionality and carbon structures in lignocellulosic-derived biochars produced by fast pyrolysis. Energy Fuels 2011, 25, 4693–4703. [Google Scholar] [CrossRef]
- Adriano, D.C. Trace Elements in Terrestial Environments: Biogeochemistry, Bioavailability and Risks of Metals, 2nd ed.; Springer: New York, NY, USA, 2001; pp. 167–218. [Google Scholar]
- Bolan, N.S.; Duraisamy, V.P. Role of inorganic and organic soil amendments on immobilisation and phytoavailability of heavy metals: A review involving specific case studies. Aust. J. Soil Res. 2003, 41, 533–555. [Google Scholar] [CrossRef]
- Krysanova, K.; Krylova, A.; Zaichenko, V. Properties of biochar obtained by hydrothermal carbonization and torrefaction of peat. Fuel 2019, 256, 115929. [Google Scholar] [CrossRef]
- Smith, A.; Singh, S.; Ross, A. Fate of inorganic material during hydrothermal carbonisation of biomass: Influence of feedstock on combustion behaviour of hydrochar. Fuel 2016, 169, 135–145. [Google Scholar] [CrossRef]
- Kambo, H.; Dutta, A. Comparative evaluation of torrefaction and hydrothermal carbonization of lignocellulosic biomass for the production of solid biofuel. Energy Convers. Manag. 2015, 105, 746–755. [Google Scholar] [CrossRef]
- Nanda, S.; Dalai, A.K.; Berruti, F.; Kozinski, J.A. Biochar as an exceptional bioresource for energy, agronomy, carbon sequestration, activated carbon and specialty materials. Waste Biomass Valor. 2016, 7, 201–235. [Google Scholar] [CrossRef]
- Kwona, G.; Bhatnagarb, A.; Wang, H.; Kwona, E.E.; Song, H. A review of recent advancements in utilization of biomass and industrial wastes into engineered biochar. J. Hazard. Mater. 2020, 400, 123242. [Google Scholar] [CrossRef]
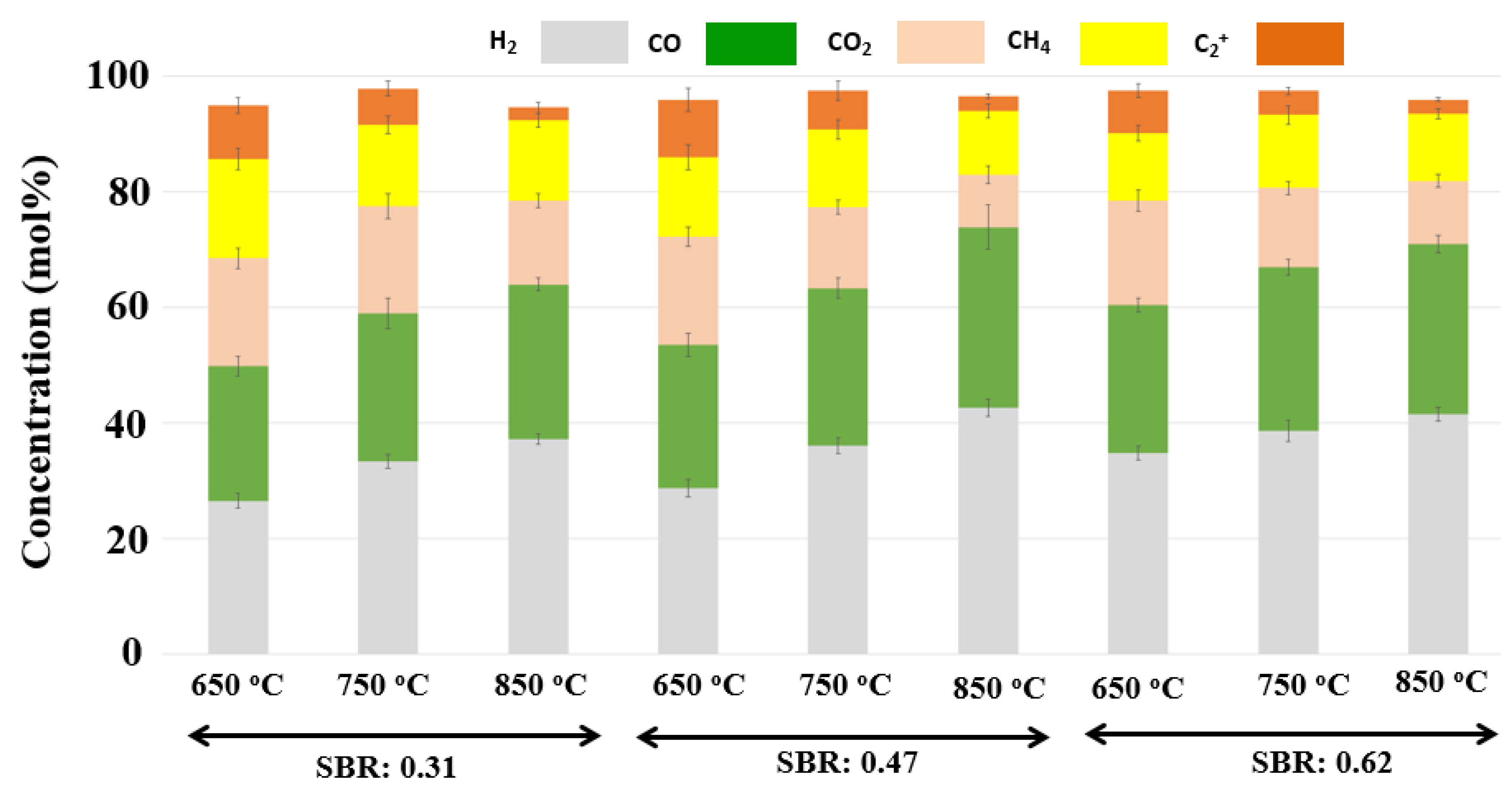

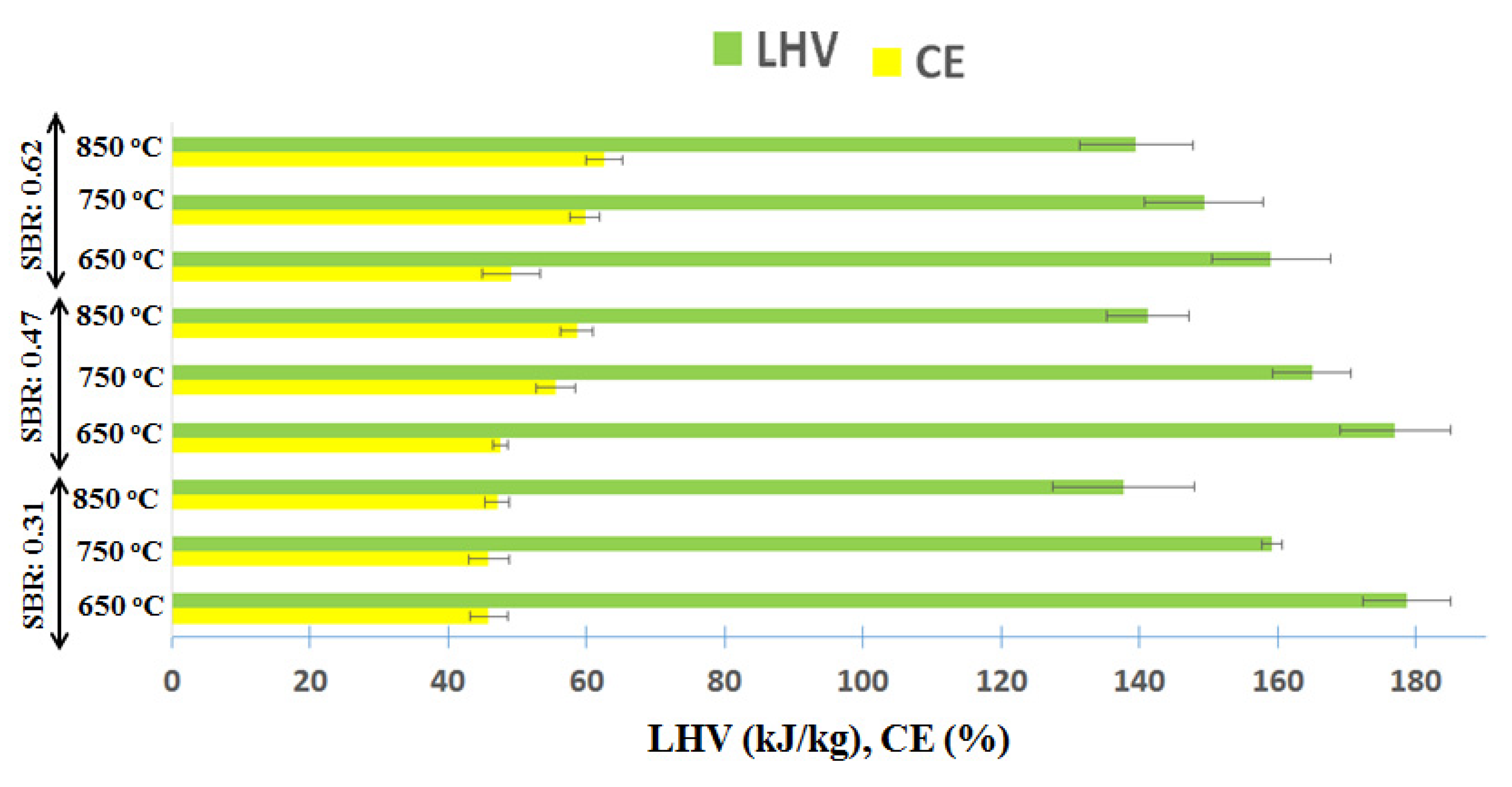
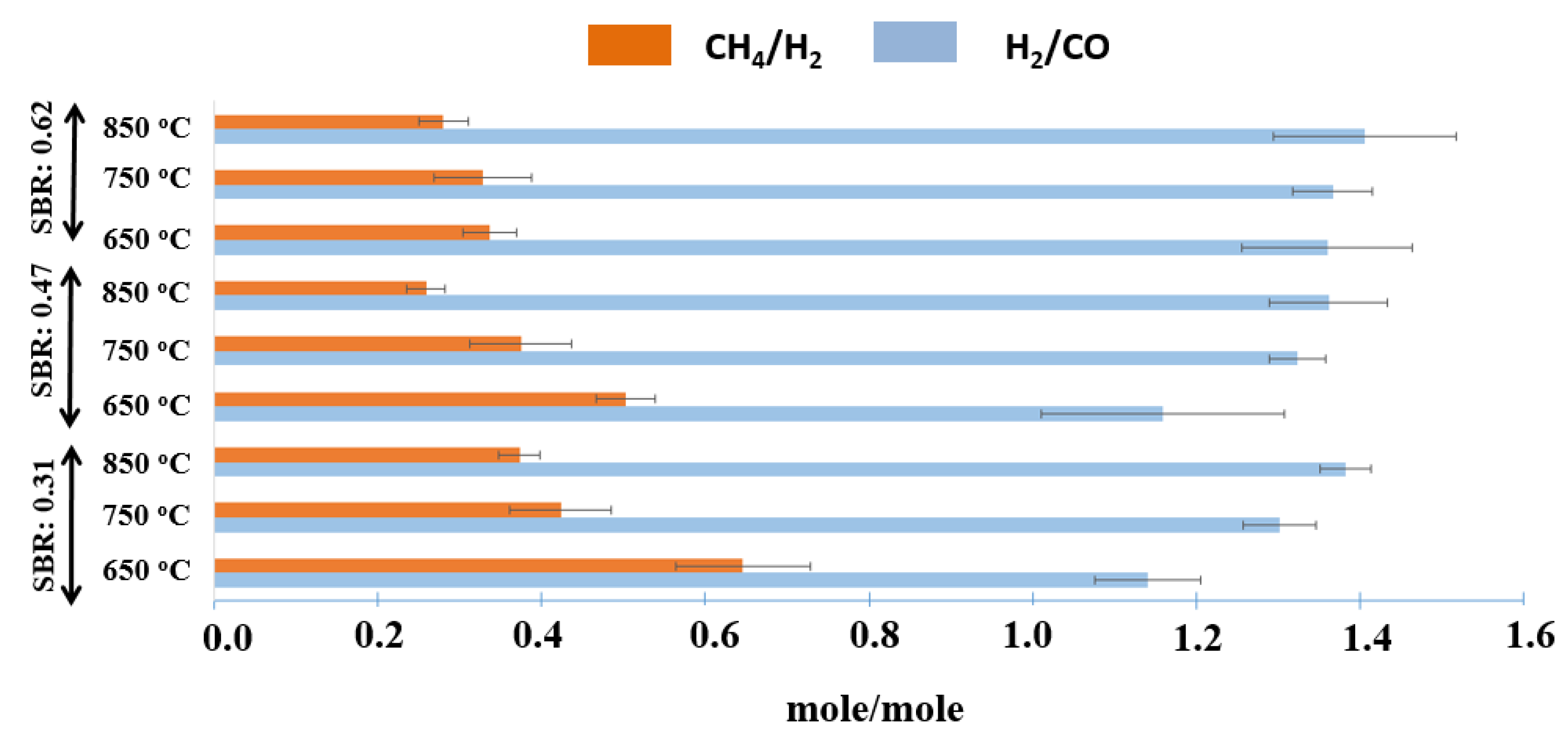


| H2/CO (mol/mol) | CH4/H2 (mol/mol) | TGY (mol/kg of Feed) | SGY (mol/kg of Feed) | LHV (kJ/m3) | |
|---|---|---|---|---|---|
| Effect of temperature (T) on 10 wt% biomass for 30 min | |||||
| T = 350 °C | 0.74 ± 0.02 | 1.05 ± 0.02 | 2.23 ± 0.06 | 0.94 ± 0.02 | 151.6 ± 4.51 |
| T = 450 °C | 1.57 ± 0.06 | 0.82 ± 0.02 | 2.96 ± 0.08 | 1.18 ± 0.03 | 173.1 ± 5.18 |
| T = 550 °C | 5.54 ± 0.15 | 0.40 ± 0.01 | 4.80 ± 0.14 | 2.42 ± 0.07 | 159.4 ± 6.75 |
| T = 650 °C | 14.41 ± 0.34 | 0.31 ± 0.01 | 6.35 ± 0.19 | 3.39 ± 0.15 | 152.6 ± 3.5 |
| Effect of feed concentration (FC) at 650 °C for 30 min | |||||
| FC = 10 wt% | 14.41 ± 0.34 | 0.31 ± 0.01 | 6.35 ± 0.19 | 3.39 ± 0.15 | 152.6 ± 4.51 |
| FC = 15 wt% | 7.69 ± 0.26 | 0.47 ± 0.02 | 6.17 ± 0.24 | 3.04 ± 0.02 | 170.7 ± 5.45 |
| FC = 20 wt% | 4.33 ± 0.12 | 0.88 ± 0.04 | 6.11 ± 0.54 | 2.56 ± 0.07 | 204.4 ± 6.15 |
| FC = 25 wt% | 2.64 ± 0.1 | 1.25 ± 0.04 | 5.59 ± 0.12 | 2.15 ± 0.02 | 226.5 ± 4.58 |
| Effect of residence time (t) on 10 wt% biomass at 650 °C | |||||
| T = 15 min | 4.17 ± 0.08 | 0.33 ± 0.04 | 4.00 ± 0.02 | 2.17 ± 0.05 | 156.0 ± 4.35 |
| T = 30 min | 14.41 ± 0.34 | 0.31 ± 0.01 | 6.35 ± 0.09 | 3.39 ± 0.15 | 152.6 ± 4.51 |
| T = 45 min | 20.37 ± 0.49 | 0.32 ± 0.05 | 8.06 ± 0.05 | 4.06 ± 0.09 | 150.6 ± 5.36 |
| T = 60 min | 40.10 ± 0.64 | 0.41 ± 0.01 | 9.87 ± 0.08 | 4.11 ± 0.05 | 146.7 ± 2.39 |
| Elements | Biochar (T = 650 °C/SBR = 0.31) 1 | Biochar (T = 750 °C/SBR = 0.47) | Biochar (T = 850 °C/SBR = 0.62) | Biochar (SCW-650 °C, 10 wt%, 60 min) |
|---|---|---|---|---|
| Essential elements (mg/g of biochar) | ||||
| Na | 3 | 5 | 8 | 4 |
| K | 12 | 17 | 22 | 10 |
| Mg | 9 | 12 | 17 | 7 |
| Ca | 7 | 11 | 17 | 4 |
| P | 13 | 13 | 27 | 13 |
| Fe | 3 | 3 | 5 | 3 |
| Total (essential elements) | 47 | 61 | 96 | 41 |
| Heavy/toxic elements (mg/g of biochar) | ||||
| Al | <1 | <1 | <1 | <1 |
| Cr | <1 | <1 | 1 | <1 |
| Cu | 1 | <1 | <1 | <1 |
| Mn | <1 | <1 | <1 | <1 |
| Mo | <1 | <1 | <1 | <1 |
| Ni | <1 | <1 | <1 | <1 |
| Zn | <1 | <1 | <1 | <1 |
| Total (heavy elements) | 2.2 | 2.1 | 2.6 | 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azargohar, R.; Nanda, S.; Cheng, H.; Dalai, A.K. Potential Application of Canola Hull Fuel Pellets for the Production of Synthesis Gas and Hydrogen. Energies 2022, 15, 8613. https://doi.org/10.3390/en15228613
Azargohar R, Nanda S, Cheng H, Dalai AK. Potential Application of Canola Hull Fuel Pellets for the Production of Synthesis Gas and Hydrogen. Energies. 2022; 15(22):8613. https://doi.org/10.3390/en15228613
Chicago/Turabian StyleAzargohar, Ramin, Sonil Nanda, He Cheng, and Ajay K. Dalai. 2022. "Potential Application of Canola Hull Fuel Pellets for the Production of Synthesis Gas and Hydrogen" Energies 15, no. 22: 8613. https://doi.org/10.3390/en15228613





