Abstract
The objective of this review is to provide a deep overview of liquid biofuels produced from sugarcane bagasse and to address the economic challenges of an ethanol and acetone-butanol-ethanol blend in commercial processes. The chemistry of sugarcane bagasse is presented. Pretreatment technologies such as physical, chemical pretreatment, biological, and combination pretreatments used in the fermentation process are also provided and summarised. Different types of anaerobic bacteria Clostridia (yeast) are discussed to identify the ingredient best suited for sugarcane bagasse, which can assist the industry in commercializing ethanol and acetone-butanol-ethanol biofuel from biomass sugarcane. The use of an acetone-butanol-ethanol mixture and ethanol blend in internal combustion engines is also discussed. The literature then supports the proposal of the best operating conditions for fermentation to enhance ethanol and acetone-butanol-ethanol plant efficiency in the sugar waste industry and its application in internal combustion engines.
1. Introduction
Sugarcane is one of the main agricultural crops in the world. For example, in Australia, more than 35 million tons of sugarcane are produced annually. Four and a half million tons of raw sugar, one million tons of molasses and 10 million tons of bagasse (a fibrous cane residue) can be produced each year from the sugarcane crops. Modern sugarcane varieties can produce more than 55 tons/hectare of biomass (dry weight).
Biofuel (ethanol, butanol, and acetone-butanol-ethanol blend (ABE)) are produced from edible and non-edible sources in a variety of ways. Ethanol-biofuel is already used as an additive at all Australian fuel stations: 5% ethanol blended with petrol and produced from crop sources.
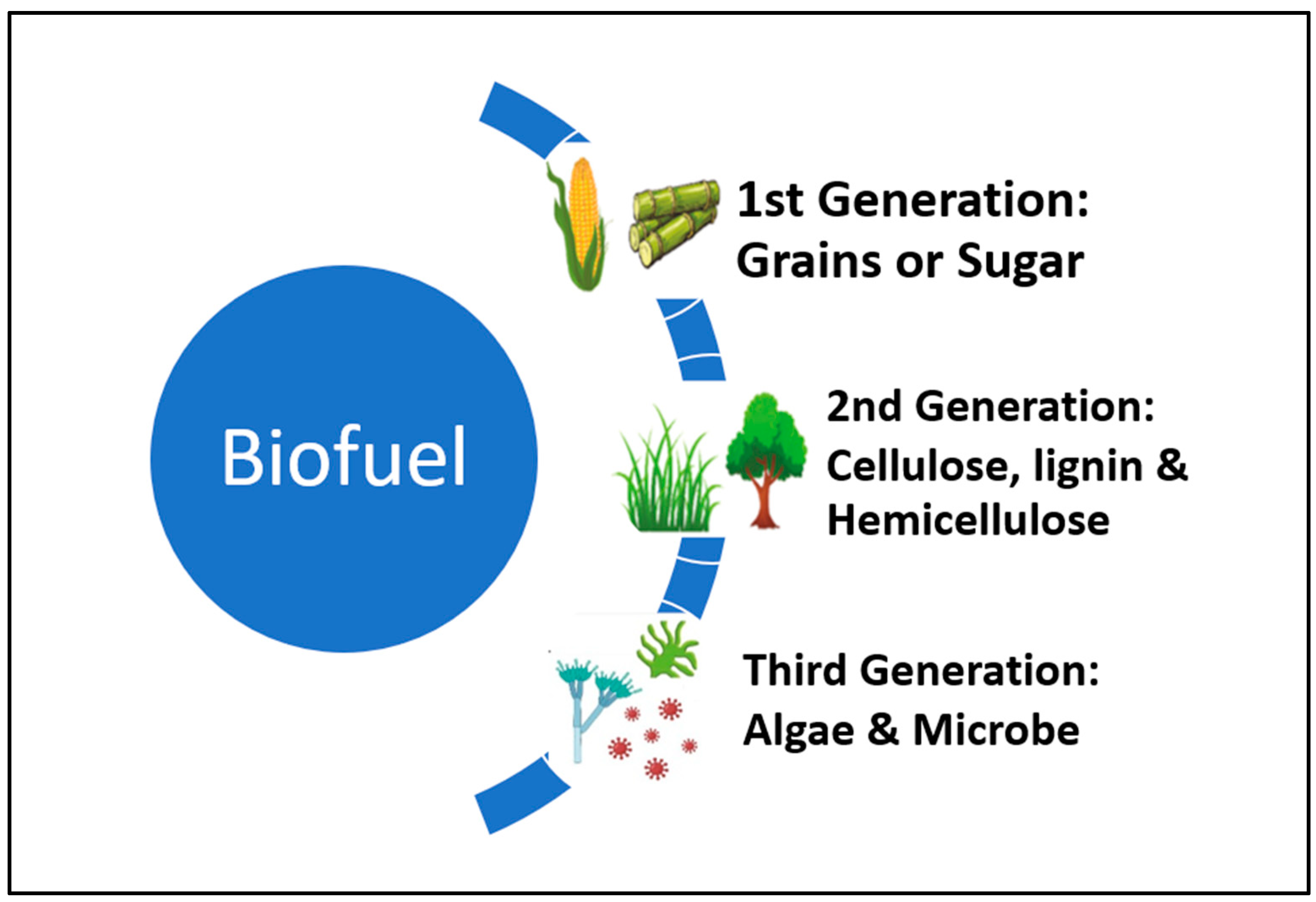
The term “first-generation biofuels” refers to a category of liquid fuels, the most common of which is ethanol, that are typically made from sugars and call for a relatively straightforward production process [1,2]. Because starch is much easier to ferment than cellulose, its six-carbon sugars (primarily glucose) are easily converted to ethanol using Clostridia (yeast). Classification of biofuel according to its generation is presented in Figure 1.
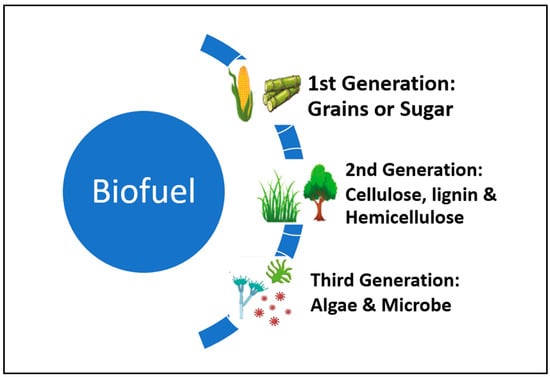
Figure 1.
Classification of biofuels according to their generation.
However, using edible sources is expensive and competes with human food. Lignocellulosic biomass as a feedstock is used to produce biofuels [3]. This industry has recently been extended due to increased demand for energy resources; a decline in fossil fuel reserves; high pollution produced by emissions from fossil fuels; and the need for alternative renewable energy resources to reduce dependence on conventional fuel.
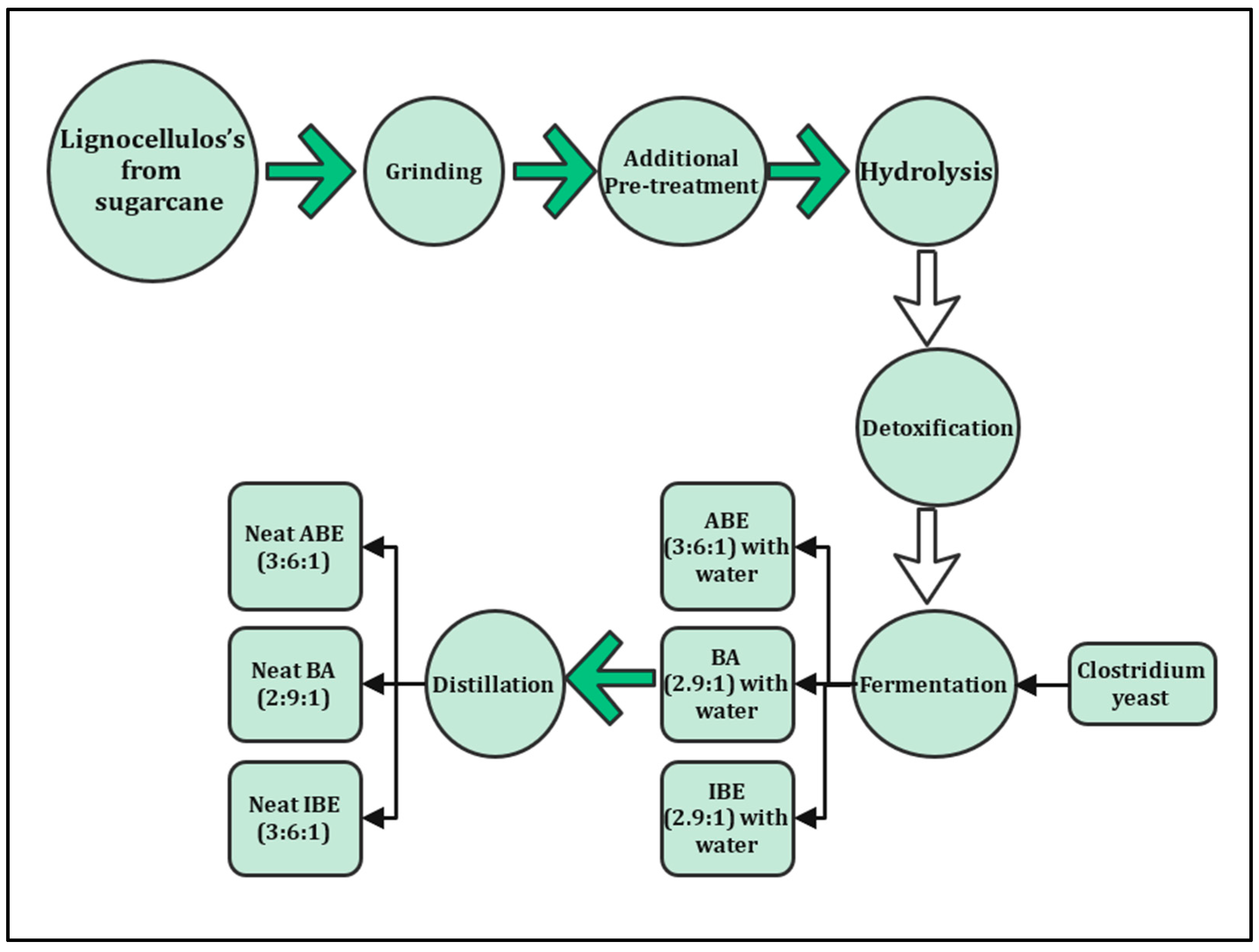
Lignocellulosic materials are mostly concentrated in sugarcane bagasse and straw. These materials mainly contain cellulose, hemicelluloses, and lignin, with lower amounts of extractives and ash. Sugarcane bagasse and straw are desirable feedstocks to produce second-generation bioethanol. They have high ratios of carbohydrate content which make them a source for biofuel production, which can help to reduce dependence on human food [4,5,6]. Figure 2 shows the processes of biofuel produced from a non-edible source (lignocellulosic) [7]. Lignocellulosic materials are a complex mixture of cellulose, hemicellulose, and lignin with minor amounts of ash, proteins, lipids, and extractive [8]. According to a bagasse fiber composition report [9], sugarcane bagasse contains cellulose typically 32–47%, hemicellulose 19–35%, lignin 18–32% on a dry basis, and 2–6% ash [10,11].
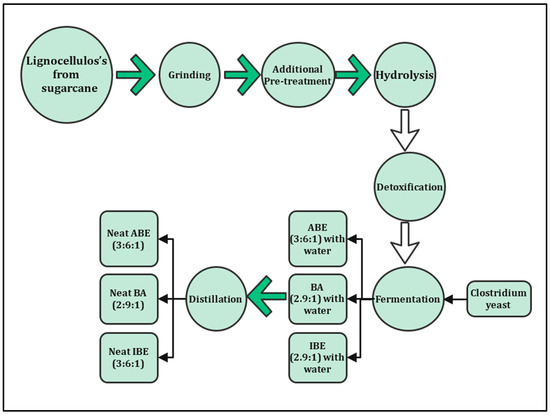
Figure 2.
Production process of biofuel from sugarcane.
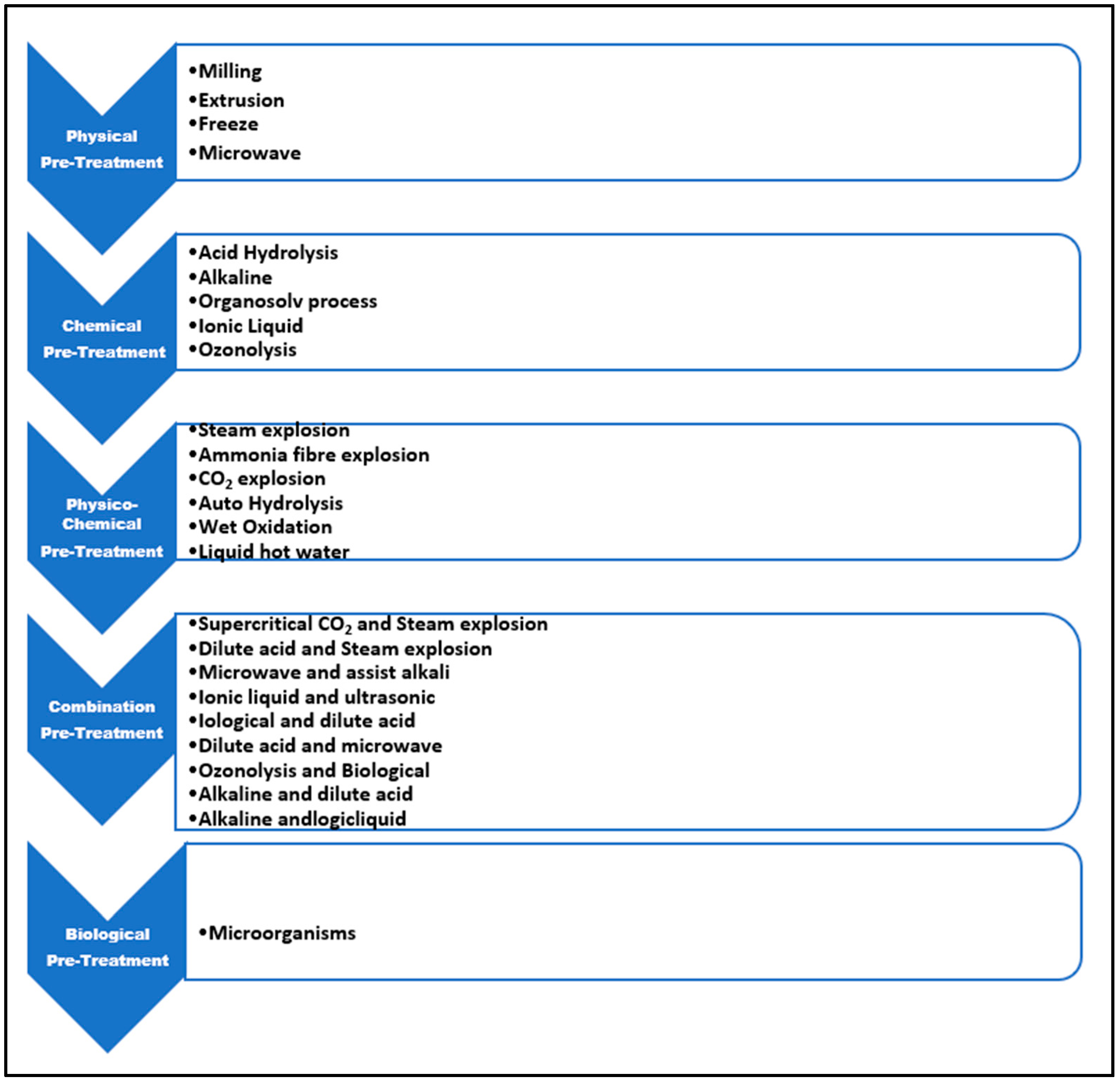
Native lignocellulosic materials are extremely resistant to enzymatic hydrolysis, so they require an efficient pretreatment process before hydrolysis can take place [12,13,14]. Bagasse pretreatment technologies can be broken down into three categories: chemical treatments, physical treatments, and biological treatments. These treatments have been used either singularly or in combination with one another (Figure 3).
Lignocellulose’s biomass can be converted to glucose through various processes. Pretreatment is a procedure that is carried out before the splitting of complex sugars like cellulose, hemicellulose, and lignin into their component simple sugars. Additionally, the advancement of genetic engineering can increase the total amount of biofuel produced via fermentation. These processes make use of a variety of anaerobic bacteria, such as the organic solvent Clostridia, and can convert a wide variety of carbon sources (such as glucose, galactose, cellobiose, mannose, and xylose) into liquid biofuel such as ABE and ethanol. Therefore, the use of lignocellulosic biomass through a fermentation process to produce ethanol, butanol, or acetone butanol ester (ABE) is a good way to meet the world’s needs for ethanol and butanol [15,16,17] or ABE or BA [18,19]. Since almost all butanol products are obtained from petrochemical processes obtained from biomass through the direct, catalytic, or aggressive conversion of cellulose, hemicellulose, and lignin, butanol is an alternative and renewable environmental resource.
Butanol is generated by first isolating it from the product of fermentation, which, depending on the parameters of the fermentation process, may be either ABE or a mixture of BA. Clostridium acetobutylicum strains release an enzyme that catalyses the anaerobic conversion of carbohydrates into ABE. This process is necessary for the breakdown of polymeric carbohydrates into monomers and results in the production of acetobutyric acid. Because the process of separating butanol from these combinations incurs a high cost, utilising ABE or BA as a biofuel is an alternate method that can be used to bring down the overall cost of production.
The hydrolysis product is a solution mostly consisting of sugars such as xylose, glucose, and arabinose. Some other composites are also produced in the solution of the hydrolysis product. Other compounds such as oligomers, furfural, and acetic acid are also released. The bonds in hemicellulos fractions are lower than in cellulosic fractions.
Recent genetic engineering developments aim to improve microbial strains and media formulations. With product recovery technology improvement, production costs can also be minimised. All these improvements in pretreatment technology could make it possible to convert biofuel from sugarcane bagasse and sugarcane leaves efficiently, thus enabling commercial use.
The objective of this review is to provide a deep overview of liquid biofuels produced from sugarcane biomass and to address pretreatment technologies; anaerobic bacteria clostridium types; cost analysis; and the internal combustion engine application of ABE and ethanol.
2. Sugarcane Biomass Extraction Pipeline
In general, energy sources can be divided into two categories: dispatchable/continuous sources such as oil, gas, coal, hydropower, and biopower, and non-dispatchable/discontinuous sources such as solar and wind power. The energy extraction pipelines for continuous and discontinuous sources are nearly analogous, since source extraction requires capacity orders, build, and installation, which implies a construction delay before the new capacity comes onstream. The main difference is that discontinuous sources require backup power to address the inherent unpredictability issue. Besides, some continuous sources (e.g., fossil fuels) are limited, hence their reserves diminish gradually. A recent study has developed a novel model for continuous and discontinuous sources described above for all energy sources, including bagasse to produce certain behaviours over time, from 1990 to 2050 [20].
The energy extraction pipeline includes four stocks and eight flows, as depicted in Figure 3. The stocks are reserves (disregarded for bagasse since they are renewable sources), capital employed, capacity under construction, and energy production capacity. The flows are new discoveries inflow, depletion outflow, capex inflow, depreciation outflow, new capacity order inflow, new capacity start-up inflow and outflow, capacity retirement outflow, and capacity bankruptcy outflow. Reserves are the proven reserves that are economically viable. Capital employed is the capacity’s current market value, which depreciates over many years. Capacity under construction is the current capacity under construction that enters service after some delay. Energy production capacity is the capacity used presently.
Figure 3.
The energy sources extraction pipeline.
Figure 3.
The energy sources extraction pipeline.
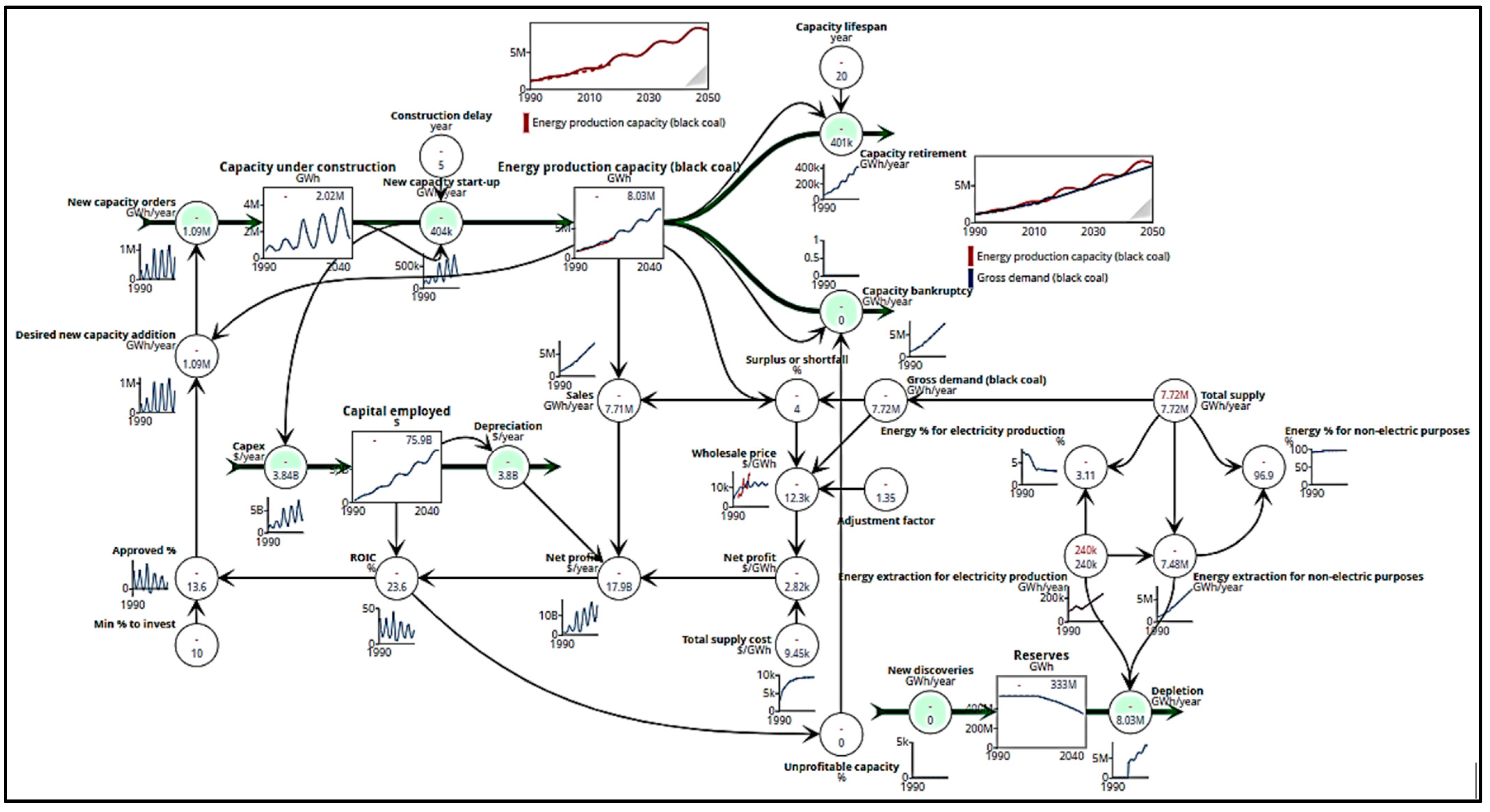
Capex refers to capital expenditure of capacity. New capacity order is the starting rate of building new capacity which is ordered when there is high confidence in future profitability; the more confidence there is, the more capacity is established. New capacity start-up is the rate at which the new capacity comes onstream, which directly adds to the total capacity. On the other hand, capacity retirement and bankruptcy reflect the total decline of capacity [20]. Capacity retirement is connected to the project’s lifetime, while capacity bankruptcy is the rate of business closing capacity that is in use. This relates to the profitability of the current capacity. The lower the profitability, the more capacity is closed.
Many variables are included in the extraction pipeline model such as gross demand, surplus or shortfall, wholesale price, adjustment factor, and total supply cost. Gross demand is subject to the desired production. Surplus is the percentage through which capacity surpasses the market’s demand. When demand surpasses supply, prices are likely to surge. Furthermore, wholesale price is subject to the supply cost and the energy demand/production rate. The adjustment factor represents the overhead expenses factor, and its value can be anywhere from 1.2 to 1.4 depending on the energy source used. This factor is important in matching demand and supply [21]. Total supply cost combines the variable and fixed costs of production.
The study established a balance of supply-demand for all energy sources including wood, wood waste, and bagasse (sugarcane pulp) for biomass, and found that the wholesale price for electricity generated from bagasse will be $71/MWh by 2030 compared to the current Australian wholesale electricity prices which is about $150/MWh for much of 2022 [22].
3. Properties and Chemistry of Sugarcane Bagasse
Bagasse is the fibre left over after the sugars have been extracted from sugarcane. Sugarcane bagasse (Saccharum officinarum) is another lignin raw material source as an agro-industrial residue. Sugarcane bagasse’s complex chemical composition limits its use as fodder for cattle and ruminants in comparison to other crops such as wheat straw, rice straw, sorghum straw, etc., making sugarcane bagasse a more appealing substrate for industry commercialization [23].
Bagasse from sugarcane has a chemical composition that is comparable to that of the cell walls of other plants. Every category of plants, including grasses, softwoods, and hardwoods, generates lignin that is primarily composed of a single variety of the phenylpropane repeat unit [24]. The lignin found in sugarcane bagasse has a higher proportion of H-type lignin, also known as hydroxyphenyl, and as a result, a lower methoxy content than the lignin found in softwood and hardwood [25]. An earlier study was able to successfully isolate seven lignin fractions by using alkali and alkaline peroxide. This study discovered that all the lignin fractions were of the SGH type, containing only a trace amount of esterified p-coumaric acid and predominantly etherified ferulic acid [26]. Sugarcane lignin (SL) and lignocellulosic biomass (LB) can only be utilised for a limited number of industrial applications due to the high lignin content.
In order to transform LB into products with added value, it is unavoidable to convert the cellulosic fraction into sugars that are ready to be fermented. Because lignin content is high in the plant cell wall, converting cell wall carbohydrate fractions is difficult. Therefore, retreatment has been employed. Retreatment can assist in producing higher chemical loadings compounds with increased temperatures and reaction times. The high cost of cellulolytic enzymes and the high number of celluloses that are required both contribute to an increase in the overall cost of the processing. The elimination of lignin results in an increase in the accessibility of cellulose and a greater amenability of cellulose to the carbohydrate framework of the plant cell wall. Sugarcane bagasse (SB) was found to have a significantly lower ash content (2–6%), which is a significant advantage when compared to other agricultural residues such as rice straw (17.5% ash) and wheat straw (11.0% ash). When one tons of sugarcane is processed, approximately 250–280 kilo grammes of bagasse are produced, which results in an annual production of approximately 54 million tons of bagasse [27]. Only a small portion of bagasse is used in the production of pulps, board materials, and composites, whereas a significant amount of it is burned as a low-grade fuel for energy recovery.
4. Pretreatment of Sugarcane Bagasse for Industrial Applications
A suitable pretreatment is required to improve the efficiency of the hydrolysis process by assisting in the removal of lignin or hemicellulose, exposing the cellulosic component. Furthermore, for pretreatment, an efficient cellulolytic enzyme cocktail; the correct enzyme loading amount; specific conditions of hydrolysing; and the right lignocellulosic material nature are essential requirements for achieving maximum hydrolysis produced from lignocellulosic material. It has frequently been reported that using pretreated substrate results in a substantial increase in the amount of lignin removal and hemicellulose depolymerisation into simpler sugars. Some traditional pretreatment methods can be used with pretreatment lignocellulosic sugarcane materials, such as alkaline hydrolysis, biological pretreatment, and acidic pretreatment. Alkaline hydrolysis happens when alkaline substances such as NaOH, Na2SO3, NH4OH, and others are added. Biological pretreatment can aid in the growth of white rot fungus or delignifying microorganisms on lignocellulosic wastes. Acidic pretreatments were carried out by introducing acidic substances (such as HCl, H2SO4, H3PO4, oxalic acid, formic acid, etc.) [28]. A pretreatment is required to make the cellulosic material more susceptible to subsequent cellulose-mediated hydrolytic processes. Figure 4 depicts the many types of pretreatments utilised in lignocellulosic fermentation.
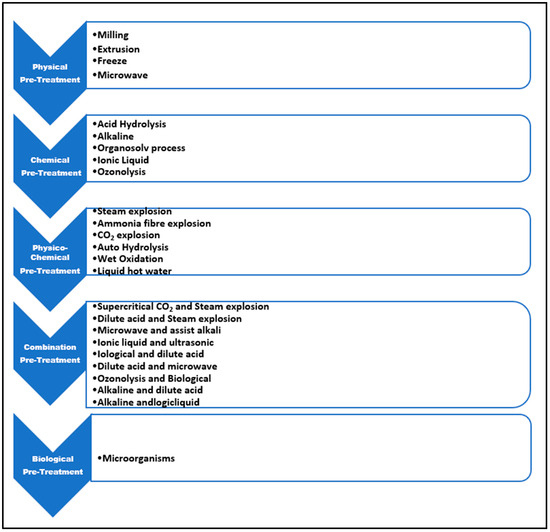
Figure 4.
Pretreatment types used for lignocellulosic fermentation.
The pre-treated SB has also been used as an inert support material for fungal biomass in the solid-state fermentation process and as an immobilisation carrier. Both applications take place in solid state. The mechanistic application of pre-treated SB that has been impregnated with suitable liquid media creates homogenous aerobic conditions throughout the bioreactor, which in turn will produce high product yield titers with relatively high purity after the cultivation cycle completion. The hemicellulose fraction is broken down into several different sugar monomers when lignocellulosic substrates are subjected to an acidic hydrolysis (xylose, arabinose, mannose, galactose, and glucose). In order to increase the yield of products that are desirable, it is necessary to remove these inhibitory substances from the hydrolysates before fermentation takes place. Lignin can be removed using pretreatments based on alkali as well as biodelignification techniques, which leave behind cellulose and hemicellulose. A mixture of cellulolytic enzymes can then be used to hydrolyze the material after it has been pre-treated [26]. This results in the formation of simpler sugars. Exoglycanase, endoglucanase, glucosidase, and other accessory enzymes required for the successful breakdown of polysaccharides found in the cell walls of lignocellulosic materials should be present in sufficient quantities in the cellulolytic cocktail [29,30].
The genus Clostridia contains a wide variety of bacteria that produce acetone, butanol, and ethanol, such as Clostridium butyricum [31], Clostridium acetobutylicum [32,33], Clostridium beijerinckii [34], and Clostridium sporogenes [35]. This process was previously referred to as ABE fermentation. The selection of raw materials that have a high fermentable sugar content and are readily available at a low cost is essential in order to ensure that the production of ethanol and butanol through a biological process is economically viable.
An increasing amount of attention is being paid to agricultural residues like barley straws, corn stoves, and sugarcane bagasse’s as sources of fermentable sugars. These agricultural residues need to be treated using pretreatment and hydrolysis processes to convert carbohydrate polymers long chains found in lignocellulosic materials into monosaccharide sugars. These processes must be carried out for the desired result to be achieved. The method of hydrolysis has a significant impact on the fermentation sugars and their contents, both of which are factors that determine the amount of butanol that can be produced from a given agricultural residue.
5. Types of Anaerobic Bacteria Clostridia (Yeast)
By using a dilute acid solution, the fermentation sugars were extracted from the sugarcane bagasse and hydrolyzed. To evaluate the use of sugarcane bagasse hydrolysate as a substrate, the butanol fermentation was carried out with a bacterial strain chosen from a variety of Clostridium species, including Clostridium butyricum (TISTR 1032), Clostridium sporogenes (TISTR 1452), Clostridium beijerinckii (TISTR 1461), and Clostridium acetobutylicum (TISTR 1462). The yield of sugar hydrolysate that was obtained in study [36] was found to be the highest when compared to that which was obtained in the works of several other researchers, as shown in Table 1.

Table 1.
Comparison of main components of biomass hydrolysates from different biomass source.
Numerous aspects, such as the type and concentration of the solvent, the temperature, the amount of time required for the reaction, and the enzyme biocatalyst, all play a role in the hydrolysis of various biomass materials. The amount of glucose that could be extracted through enzymatic hydrolysis was significantly higher than the amount that could be extracted through dilute acid hydrolysis. Additionally, a high temperature of 160 °C had a significant impact on the concentration of glucose. The diluted acid solution and low temperature of 121 °C were used [41] as the hydrolysis method to save money and energy during the production process. The amount of xylose that was obtained was comparable to the amount that was obtained in other studies using dilute acid hydrolysis and a temperature of 121 °C; however, the amount of glucose that was obtained in this study was significantly higher. This high glucose content was accomplished by using H2SO4 at a concentration of five percent by volume [42]. The chemical bonds that hold sugarcane bagasse’s sugars together can be broken down into sugars by increasing the acid concentration in the acid hydrolysis process. This could result in a powerful or comprehensive reaction.
6. Bioethanol Production from Sugarcane
Bioethanol is obtained mostly from agricultural leftovers, and it may be created by the fermentation of sucrose or simple sugars acquired through biomass treatment. It is possible to partition the processes of producing bioethanol into three distinct generations, and each generation is determined by the characteristics of the feedstock that was used initially. In every one of these processes, the lignocellulosic or cellulosic material is first transformed into simple sugars, and only after that is bioethanol produced. The substrate in the first generation is primarily composed of sucrose-containing feedstock grains and starchy materials (such as sugarcane, maise, sugar beet, sweet sorghum, corn, cassava, sweet potato, yam, wheat, barley, and oats), and bioethanol is produced through starch or sugar fermentation [43,44]. In the second generation, the substrate is primarily composed of lignocellulosic biomass (such as sugarcane bagasse, stover, stems, straw, leaves, and grass), and bioethanol is produced through enzymatic hydrolysis [45]. In the third generation, the substrates are algae biomasses, and bioethanol is produced through the fermentation of green and blue algae [46].
Sugarcane is the second most utilised raw material in bioethanol manufacturing. Sugarcane contains 12–17% total sugars by weight and 68–72% moisture (90% sucrose and 10% glucose or fructose). The average extraction efficiency for producing cane juice by crushing is approximately 95%, with cane fibre constituting the remaining solid residue (sugarcane bagasse) [47]. Cane juice is heated to 110 °C in plants that solely manufacture ethanol, decanted, occasionally concentrated by evaporation, and then fermented to reduce microbial contamination. Like maise, sugarcane has a well-established infrastructure for cultivation, harvesting, and processing. Sugarcane is also considered the most effective raw material resource for bioethanol production: the amount of fossil energy consumed during sugarcane processing is substantially lower than that of corn [48,49]. Sugarcane is an annual crop whose period of growth ranges from 9 to 24 months. This growing time could be changed depending on several factors such as variety, environmental conditions, and management [50]. After five to seven ratoon cycles, sugarcane fields are “reformed” or replanted by removing stalks (mechanically or chemically), tilling the soil, and replanting freshly cut sugarcane sprouts. Traditionally, thorough tillage is required for sugarcane soil preparation. In certain areas of Brazil and Australia, full tillage has given way to minimum tillage techniques, in which the soil is only lightly tilled in the planting row. Planting legumes during the reformation phase occasionally increases soil fertility and/or soil physical qualities [51,52,53,54].
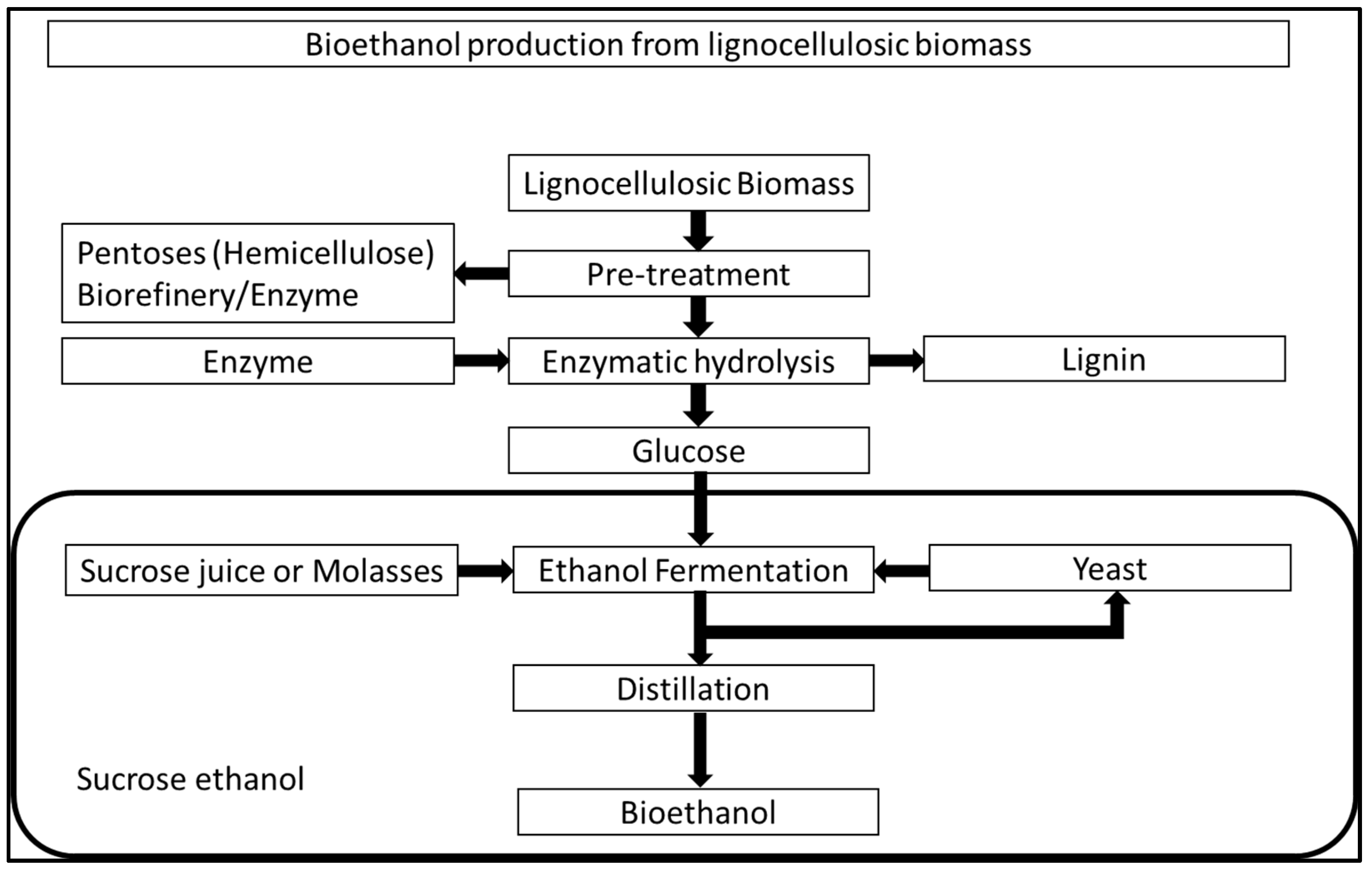
On the other hand, Sugarcane Bagasse (SCB) is primarily composed of lignin (20–30%), cellulose (40–45%), and hemicelluloses (30–35%) [55]. Because of its lower ash content (1.9%) [56], SCB offers advantages over high ash containing bagasse, such as rice straw, 14.5% [57] and wheat straw, 9.2% [58]. Currently, converting lignocellulosic biomass (such as sugarcane bagasse) into bioethanol entails three critical and interdependent steps: (i) pretreatment of lignocellulosic biomass to depolarise the lignocellulosic matrix, allowing carbohydrate polymers (e.g., cellulose, hemicellulose, and other carbohydrates) to be accessible for enzymatic hydrolysis; (ii) saccharification of pretreated material to liberate fermentable sugars through hydrolases such as cellulases and hemicellulases; and (iii) fermentation of monosaccharide to produce ethanol by using ethanogenic yeast/microorganism [59]. Figure 5 shows the pathway producing bioethanol from lignocellulosic biomass. Jugwanth et al. [60] studied the modelling and optimisation of simultaneous saccharification and fermentation (SSF) process followed by bioethanol production under the optimised SSF process conditions. They reported that the developed SSF model predicted optimum process conditions to be 39 °C (temperature), 100 U/g (enzyme loading) and one time (yeast titre) with a bioethanol concentration of 4.88 g/L. They also reported a maximum bioethanol production rate of 0.29 g/L/h with the optimised SSF process. Valladares-Diestra et al. [61] studied the bioethanol production from SCB using pretreatment with the imidazole method for enzymatic hydrolysis. They reported that this pretreatment process mostly produces the delignification of SCB without causing major changes in cellulose properties. Untreated SCB achieved maximum enzymatic conversions of 29.7% glucose and 23.6% xylose, respectively, with an enzyme incubation time of 48 h. On the other hand, in the case of the best imidazole treatment condition (160 °C, 1 h), the enzymatic conversion reached 100% for glucose and 85% for xylose after 8 h and 24 h of enzyme incubation. Thus, a higher glucose release in much less incubation time was obtained for treated SCB. Table 2 highlights studies that have focussed on bioethanol production from SCB.
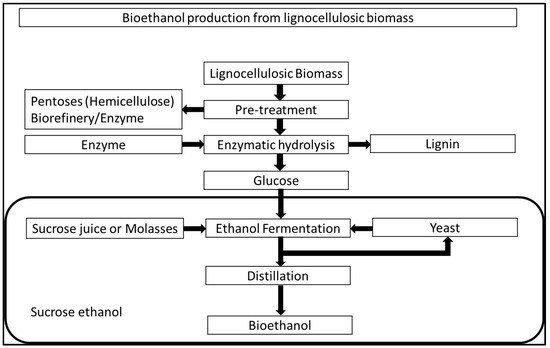
Figure 5.
Bioethanol production from lignocellulosic biomass [46].

Table 2.
Second-generation bioethanol production from SCB.
7. Cost Analysis
The United States produces 40 billion liters of bioethanol from corn/wheat annually. In comparison, Brazil produces 25 billion liters; China,3 billion liters; Canada, 2 billion liters; India, 1 billion liters; France, 1 billion liters; Germany, 750 million liters; and Australia, 500 million liters [70]. Table 3 shows the annual global fuel ethanol production (in millions of gallons) by country/region from 2016 to 2021 [71].

Table 3.
Global annual fuel ethanol production by country/region from 2016 to 2021 in millions of gallons [72].
Overall, global output is increasing, but production dropped in 2020 owing to the COVID-19 pandemic. A total of 98.64 billion liters of bioethanol was produced in 2020 [72]. The United States is the world’s greatest producer of ethanol, with over 13.9 billion gallons produced in 2020 [73]. The US and Brazil produce 82% of the world’s ethanol. Most of the ethanol produced in the United States is from maise, whereas Brazil primarily uses sugarcane.
With the rising volatility of oil prices, several nations have opted to shift their energy policies towards the usage of biofuels. Table 4 describes bioethanol output in various producing nations. The major feedstocks for different countries/regions include molasses (China), sweet sorghum (China), wheat (Belgium, Spain, Sweden, Canada), cassava (Thailand), cereal (EU, Canada), sugar beet (EU), barley (Spain), rye (Poland), corn/maise (US) and sugarcane (Brazil, Argentina, Australia).

Table 4.
Cost of bioethanol production in different countries/regions worldwide [71,72].
8. Comparison between Bioethanol from Different Sources
Concerns about food versus fuel and the severe environmental implications of large-scale production of first-generation feedstocks have brought a lot of attention to second-generation feedstocks over the past two decades [73,74,75]. This has led to an increase in the use of second-generation feedstocks. The characteristics and potential for producing bioethanol from a variety of second-generation feedstocks are summarised in Table 5.

Table 5.
Composition and bioethanol production from some of the commonly used crops.
9. Economic Challenges
Biofuel production from sugarcane waste has some challenging factors. One obstacle is the high cost of the enzymatic hydrolysis process [46] that is used for biofuel production. In addition, another challenge is the low yield produced after fermentation. Carpio and Souza [83] evaluated biofuel production from second-generation bagasse using different market prices and bagasse allocation scenarios. The results analysis showed that the bagasse allocation to second generation ethanol increases with the reduction of its production costs. They also showed that second-generation ethanol production costs 0.30 USD/L. Several researchers have evaluated and analysed the traditional processing approaches used to reach the desired reduction in second-generation biofuel production costs. For example, using enzymatic cocktails for hydrolysis is one of the most critical steps in terms of processing cost reduction [84]. Prajapati et al. [85] produced high hydrolysis efficiency with 74.9% from cellulase and hemicellulose using novel technics.
As a result of the high cost of using yeasts to convert sugarcane residue into biofuel, alternatives for co-production with other high value bioproducts have been explored. Xylitol has been identified as one of these bioproducts [86]. Unrean and Ketsub [87] produced second generation ethanol and xylitol by S. cerevisiae and Candida tropicalis, respectively, with the assistance of acid pretreatment. They produced high yield amounts of 56.1 g/L ethanol. Valladares-Diestra et al. [88] produced high yield amounts (ethanol 171.9 g) from sugarcane bagasse with a novel pretreatment. These technological improvements in pretreatment and the advancement of genetic engineering could minimise biofuel production costs associated with this second generation. Therefore, all these improvements in pretreatment technology and the enzymatic hydrolysis process could make it possible to convert biofuel from sugarcane bagasse and sugarcane leaves efficiently, thus enabling commercial use.
10. ABE and Ethanol in Internal Combustion Engines
Generally, high levels of pollution are released into the atmosphere by internal combustion engines powered by fossil fuels. There are numerous methods for reducing CO2, NOx and smoke emissions from petrol or diesel engines. One way to reduce reliance on conventional fuel and emissions is to blend fossil fuel with biofuel. As a result, researchers and scientists have widely investigated and tested biofuels such as ABE or ethanol in internal combustion engines as sole fuel or in blends with petrol or diesel under various operating conditions.
Duan et al. [89] investigated the effects of injection timing and EGR on the combustion and emission characteristics of ABE-diesel fuel blends. The results showed that the injection timing and EGR strategies significantly reduced NOx, CO, and HC emissions. Aguado-Deblas et al. [90] investigated the blending of ABE and vegetable oils. The heat release pattern and thermal efficiency of blending 20% ABE with diesel fuel were investigated by Ob Nilaphai et al. [91]. Their results showed that the 20ABE80 diesel blend (20% ABE in diesel by volume) produced a slightly lower thermal efficiency with comparable energy consumption compared to diesel fuel. A longer ignition delay was observed for the 20ABE80diesel blend, with shorter ignition in the diffusion combustion regime.
Dinesha et al. [92] conducted an experimental investigation of the SI engine using an ABE-gasoline blend. The experimental results indicated increased brake thermal efficiency and reduced CO and NOx emissions. Zhang et al. [93] researched the combustion and emission performance of a SI engine equipped with GPI and ABEDI at various engine speeds and loads. Their experimental results revealed an increase in brake power as well as a reduction in CO and NOx emissions.
Mendiburu et al. [1] examined ethanol’s use as a renewable biofuel in internal combustion engines. According to the authors, ethanol blends can significantly improve thermal efficiency and reduce NOx emissions from internal combustion engines.
Another study looked at the use of ethanol in gasoline and diesel [94]. The authors discovered that blending ethanol gasoline and diesel presented some challenges due to volatility and phase separation. Blending up to 10% ethanol in SI engines is already done commercially in Australia. Furthermore, ethanol of a lower percentage can aid in the reduction of auto-ignition time. As a result of the addition of nano additives such as TiO2 and Al2O3, BTE increased while BSFC decreased.
11. Conclusions
In the not-too-distant future, the ever-increasing cost of fossil fuel may be able to be neutralised by biofuel derived from sugarcane bagasse. For industries that produce sugar and alcohol, operational integration such as fermentation would be an efficient way to maximise the effective utilisation of waste sugarcane. This strategy would maximise the amount of sugar that could be produced from the raw substrate. At this time, bagasse from sugarcane is almost exclusively burned in boilers because it is a more cost-effective source of energy. The currently available technology for producing biofuel from biomass using sugarcane biomass cannot offer a competitive price in relation to the amount of yield produced. Therefore, developments in treatment and genetic engineering, and the use of suitable and cheaper yeast to convert sugarcane bagasse into biofuel are of commercial significance for the use of ethanol and ABE. The second step is to assist industry in the form of commercial ethanol and ABE biofuel derived from bio-mass sugarcane by optimising the operating conditions of ethanol and ABE fermentation. Wide-scale plants for the disposal of sugar waste could be set up in Australia. This would lead to the production of commercial ABE as a suitable additive for conventional diesel, which in turn would result in fewer emissions from diesel engines and less waste.
Author Contributions
S.J.M.A. wrote the original draft; I.M.R.F. wrote the ethanol section; M.L. wrote the extraction pipeline section; N.H.H. drew figures; T.Y., A.P.W. and S.M.A.R. provided editorial contributions and guidance; S.J.M.A., I.M.R.F., N.H.H. and M.L. wrote the final revision and editing draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| ABE | Acetone-butanol-ethanol |
| ABEDI | ABE direct injection |
| BA | Butanol-acetone |
| BSFC | Brake specific fuel consumption |
| BTE | Brake thermal efficiency |
| 20ABE80 | 20% ABE80% diesel |
| CO | Carbon monoxide |
| EGR | Exhaust gas recirculation |
| ICE | Internal combustion engines |
| GPI | gasoline port injection |
| HC | Hydrocarbon emission |
| SL | Sugarcane lignin |
| LB | lignocellulosic biomass |
| NOx | Nitrogen oxides emission |
| SB | Sugarcane bagasse |
| SSF | simultaneous saccharification and fermentation |
| SI | Spark ignition |
References
- Mendiburu, A.Z.; Lauermann, C.H.; Hayashi, T.C.; Mariños, D.; da Costa, R.B.R.; Coronado, C.J.; Roberts, J.J.; de Carvalho, J.A., Jr. Ethanol as a renewable biofuel: Combustion characteristics and application in engines. Energy 2022, 257, 124688. [Google Scholar] [CrossRef]
- Masum, B.M.; Masjuki, H.H.; Kalam, M.A.; Rizwanul Fattah, I.M.; Palash, S.M.; Abedin, M.J. Effect of ethanol–gasoline blend on NOx emission in SI engine. Renew. Sustain. Energy Rev. 2013, 24, 209–222. [Google Scholar] [CrossRef]
- Kandasamy, M.; Hamawand, I.; Bowtell, L.; Seneweera, S.; Chakrabarty, S.; Yusaf, T.; Shakoor, Z.; Algayyim, S.; Eberhard, F. Investigation of ethanol production potential from lignocellulosic material without enzymatic hydrolysis using the ultrasound technique. Energies 2017, 10, 62. [Google Scholar] [CrossRef]
- Zhang, W.L.; Liu, Z.Y.; Liu, Z.; Li, F.L. Butanol production from corncob residue using Clostridium beijerinckii NCIMB 8052. Lett. Appl. Microbiol. 2012, 55, 240–246. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, J.; Kuittinen, S.; Vepsäläinen, J.; Soininen, P.; Keinänen, M.; Pappinen, A. Enhanced sugar production from pretreated barley straw by additive xylanase and surfactants in enzymatic hydrolysis for acetone–butanol–ethanol fermentation. Bioresour. Technol. 2015, 189, 131–137. [Google Scholar] [CrossRef]
- Savaliya, M.L.; Dhorajiya, B.D.; Dholakiya, B.Z. Recent advancement in production of liquid biofuels from renewable resources: A review. Res. Chem. Intermed. 2013, 41, 475–509. [Google Scholar] [CrossRef]
- Algayyim, S.J.M. The Use of BA Mixture in Diesel Engines: Blend Preparation, Spray Visualisation and Engine Performance. Ph.D. Thesis, University of Southern Queensland, Toowoomba, Australia, 2019. [Google Scholar]
- Nigam, P.S.; Singh, A. Production of liquid biofuels from renewable resources. Prog. Energy Combust. Sci. 2011, 37, 52–68. [Google Scholar] [CrossRef]
- Carlucci, F.V. Proposed best operating practices to improve technical efficiency in brazilian sugar and ethanol plants. In Proceedings of the 2018 Conference of the Australian Society of Sugar Cane Technologists, Mackay, QLD, Australia, 18–20 April 2018. [Google Scholar]
- O’Hara, I. Cellulosic Ethanol from Sugar Cane Bagasse in Australia: Exploring Industry Feasibility through Systems Analysis Techno-Economic Assessment and Pilot Plant Development. Ph.D. Thesis, Queensland University of Technology, Brisbane, QLD, Australia, 2011. [Google Scholar]
- Gonçalves, A.R.; Benar, P.; Costa, S.M.; Ruzene, D.S.; Moriya, R.Y.; Luz, S.M.; Ferretti, L.P. Integrated processes for use of pulps and lignins obtained from sugarcane bagasse and straw. Appl. Biochem. Biotechnol. 2005, 123, 821–826. [Google Scholar] [CrossRef]
- Algayyim, S.J.M.; Wandel, A.P.; Yusaf, T.; Hamawand, I. Production and application of ABE as a biofuel. Renew. Sustain. Energy Rev. 2018, 82, 1195–1214. [Google Scholar] [CrossRef]
- Qureshi, N.; Bowman, M.; Saha, B.; Hector, R.; Berhow, M.; Cotta, M. Effect of cellulosic sugar degradation products (furfural and hydroxymethyl furfural) on acetone–butanol–ethanol (ABE) fermentation using Clostridium beijerinckii P260. Food Bioprod. Process. 2012, 90, 533–540. [Google Scholar] [CrossRef]
- Sun, J.-X.; Sun, X.-F.; Sun, R.-C.; Fowler, A.P.; Baird, M.S. Inhomogeneities in the Chemical Structure of Sugarcane Bagasse Lignin. J. Agric. Food Chem. 2003, 51, 6719–6725. [Google Scholar] [CrossRef] [PubMed]
- Algayyim, S.J.M.; Wandel, A.P.; Yusaf, T. Experimental and numerical investigation of spray characteristics of butanol-diesel blends. In Proceedings of the 11th Asia-Pacific Conference on Combustion, Sydney, Australia, 10–14 December 2017. [Google Scholar]
- Vandenberghe, L.P.S.; Valladares-Diestra, K.K.; Bittencourt, G.A.; Torres, L.Z.; Vieira, S.; Karp, S.G.; Sydney, E.B.; de Carvalho, J.C.; Soccol, V.T.; Soccol, C.R. Beyond sugar and ethanol: The future of sugarcane biorefineries in Brazil. Renew. Sustain. Energy Rev. 2022, 167, 112721. [Google Scholar] [CrossRef]
- Algayyim, S.J.M.; Wandel, A.P. Performance and emission levels of butanol, acetone-butanol-ethanol, butanol-acetone/diesel blends in a diesel engine. Biofuels 2022, 13, 449–459. [Google Scholar] [CrossRef]
- Veza, I.; Said, M.F.M.; Latiff, Z.A. Recent advances in butanol production by acetone-butanol-ethanol (ABE) fermentation. Biomass Bioenergy 2021, 144, 105919. [Google Scholar] [CrossRef]
- Algayyim, S.J.M.; Wandel, A.P.; Yusaf, T.F.; Hamawand, I.; Al-lwayzy, S. Experimental study of spray characteristics, engine performance and emission levels of acetone-butanol-ethanol mixture-diesel blends in a diesel engine. In Proceedings of the 11th Asia-Pacific Conference on Combustion, Sydney, Australia, 10–14 December 2017. [Google Scholar]
- Laimon, M.; Yusaf, T.; Mai, T.; Goh, S.; Alrefae, W. A systems thinking approach to address sustainability challenges to the energy sector. Int. J. Fluids 2022, 15, 100161. [Google Scholar] [CrossRef]
- Laimon, M.; Mai, T.; Goh, S.; Yusaf, T. Energy sector development: System dynamics analysis. Appl. Sci. 2019, 10, 134. [Google Scholar] [CrossRef]
- ACCC. Inquiry into the National Electricity Market; ACCC: Canberra, ACT, Australia, 2022. [Google Scholar]
- Jesus Junior, M.M.; De Avila Rodrigues, F.; Moreira Da Costa, M.; Guirardello, R. Economic Evaluation for Bioproducts Production from Carbohydrates Obtained from Hydrolysis of Sugarcane Bagasse. Chem. Eng. Trans. 2022, 92, 703–708. [Google Scholar]
- Yadav, P.; Kumar Tiwari, S.; Kumar, V.; Singh, D.; Kumar, S.; Malik, V.; Singh, B. Sugarcane bagasse: An important lignocellulosic substrate for production of enzymes and biofuels. Biomass Conv. Bioref. 2022. [Google Scholar] [CrossRef]
- Haghdan, S.; Renneckar, S.; Smith, G.D. 1—Sources of lignin. In Lignin in Polymer Composites; Faruk, O., Sain, M., Eds.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 1–11. [Google Scholar]
- Zhao, X.; Peng, F.; Cheng, K.; Liu, D. Enhancement of the enzymatic digestibility of sugarcane bagasse by alkali–peracetic acid pretreatment. Enzym. Microb. Technol. 2009, 44, 17–23. [Google Scholar] [CrossRef]
- Doherty, W.; Halley, P.; Edye, L.; Rogers, D.; Cardona, F.; Park, Y.; Woo, T. Studies on polymers and composites from lignin and fiber derived from sugarcane. Polym. Adv. Technol. 2007, 18, 673–678. [Google Scholar] [CrossRef]
- Chandel, A.K.; da Silva, S.S.; Carvalho, W.; Singh, O.V. Sugarcane bagasse and leaves: Foreseeable biomass of biofuel and bio-products. J. Chem. Technol. Biotechnol. 2012, 87, 11–20. [Google Scholar] [CrossRef]
- Chandel, A.K.; Chan, E.; Rudravaram, R.; Narasu, M.L.; Rao, L.V.; Ravindra, P. Economics, and environmental impact of bioethanol production technologies: An appraisal. Biotechnol. Mol. Biol. Rev. 2007, 2, 14–32. [Google Scholar]
- Canilha, L.; Kumar Chandel, A.; dos Santos Milessi, T.S.; Fernandes Antunes, F.A.; da Costa Freitas, W.L.; das Gracas Almeida Felipe, M.; da Silva, S.S. Bioconversion of Sugarcane Biomass into Ethanol: An Overview about Composition, Pretreatment Methods, Detoxification of Hydrolysates, Enzymatic Saccharification, and Ethanol Fermentation. J. Biomed. Biotechnol. 2012, 2012, 989572. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, N.; Ezeji, T.C.; Ebener, J.; Dien, B.S.; Cotta, M.A.; Blaschek, H.P. Butanol production by Clostridium beijerinckii. Part I: Use of acid and enzyme hydrolyzed corn fiber. Bioresour. Technol. 2008, 99, 5915–5922. [Google Scholar] [CrossRef]
- Qureshi, N.; Saha, B.C.; Hector, R.E.; Hughes, S.R.; Cotta, M.A. Butanol production from wheat straw by simultaneous saccharification and fermentation using Clostridium beijerinckii: Part I—Batch fermentation. Biomass Bioenergy 2008, 32, 168–175. [Google Scholar] [CrossRef]
- Efremenko, E.; Nikolskaya, A.B.; Lyagin, I.V.; Sen’Ko, O.V.; Makhlis, T.A.; Stepanov, N.A.; Maslova, O.V.; Mamedova, F.; Varfolomeev, S.D. Production of biofuels from pretreated microalgae biomass by anaerobic fermentation with immobilized Clostridium acetobutylicum cells. Bioresour. Technol. 2012, 114, 342–348. [Google Scholar] [CrossRef]
- Bankar, S.B.; Survase, S.A.; Singhal, R.S.; Granström, T. Continuous two stage acetone–butanol–ethanol fermentation with integrated solvent removal using Clostridium acetobutylicum B 5313. Bioresour. Technol. 2012, 106, 110–116. [Google Scholar] [CrossRef]
- Yen, H.-W.; Li, R.-J.; Ma, T.-W. The development process for a continuous acetone–butanol–ethanol (ABE) fermentation by immobilized Clostridium acetobutylicum. J. Taiwan Inst. Chem. Eng. 2011, 42, 902–907. [Google Scholar] [CrossRef]
- Jonglertjunya, W.; Chinwatpaiboon, P.; Thambaramee, H.; Prayoonyong, P. Butanol, Ethanol and Acetone Production from Sugarcane Bagasses by Acid Hydrolysis and Fermentation Using Clostridium sp. Adv. Mater. Res. 2014, 931–932, 1602–1607. [Google Scholar] [CrossRef]
- Lu, C.; Zhao, J.; Yang, S.-T.; Wei, D. Fed-batch fermentation for n-butanol production from cassava bagasse hydrolysate in a fibrous bed bioreactor with continuous gas stripping. Bioresour. Technol. 2012, 104, 380–387. [Google Scholar] [CrossRef]
- Wang, L.; Chen, H. Increased fermentability of enzymatically hydrolyzed steam-exploded corn stover for butanol production by removal of fermentation inhibitors. Process Biochem. 2011, 46, 604–607. [Google Scholar] [CrossRef]
- Qureshi, N.; Saha, B.C.; Dien, B.; Hector, R.E.; Cotta, M.A. Production of butanol (a biofuel) from agricultural residues: Part I—Use of barley straw hydrolysate. Biomass Bioenergy 2010, 34, 559–565. [Google Scholar] [CrossRef]
- Rodríguez-Chong, A.; Ramírez, J.A.; Garrote, G.; Vázquez, M. Hydrolysis of sugarcane bagasse using nitric acid: A kinetic assessment. J. Food Eng. 2004, 61, 143–152. [Google Scholar] [CrossRef]
- Dalena, F.; Senatore, A.; Iulianelli, A.; Di Paola, L.; Basile, M.; Basile, A. Ethanol from Biomass: Future and Perspectives. In Ethanol; Basile, A., Iulianelli, A., Dalena, F., Veziroğlu, T.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 2; pp. 25–59. [Google Scholar]
- Pattra, S.; Sangyoka, S.; Boonmee, M.; Reungsang, A. Bio-hydrogen production from the fermentation of sugarcane bagasse hydrolysate by Clostridium butyricum. Int. J. Hydrogen Energy 2008, 33, 5256–5265. [Google Scholar] [CrossRef]
- Mohapatra, S.; Ray, R.C.; Ramachandran, S. Bioethanol from Biorenewable Feedstocks: Technology, Economics, and Challenges. In Bioethanol Production from Food Crops; Ray, R.C., Ramachandran, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; Chapter 1; pp. 3–27. [Google Scholar]
- Dias, M.O.S.; Junqueira, T.L.; Jesus, C.D.F.; Rossell, C.E.V.; Maciel Filho, R.; Bonomi, A. Improving second generation ethanol production through optimization of first-generation production process from sugarcane. Energy 2012, 43, 246–252. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H.; Öz, C. Progress in bioethanol processing. Prog. Energy Combust. Sci. 2008, 34, 551–573. [Google Scholar] [CrossRef]
- Vasić, K.; Knez, Ž.; Leitgeb, M. Bioethanol Production by Enzymatic Hydrolysis from Different Lignocellulosic Sources. Molecules 2021, 26, 753. [Google Scholar] [CrossRef]
- Leite, G.B.; Abdelaziz, A.E.M.; Hallenbeck, P.C. Algal biofuels: Challenges and opportunities. Bioresour. Technol. 2013, 145, 134-41. [Google Scholar] [CrossRef]
- Bertrand, E.; Vandenberghe, L.P.S.; Soccol, C.R.; Sigoillot, J.-C.; Faulds, C. First generation bioethanol. In Green Fuels Technology: Biofuels; Soccol, C.R., Brar, S.K., Faulds, C., Ramos, L.P., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 175–212. [Google Scholar]
- Macedo, I.C.; Seabra, J.E.A.; Silva, J.E.A.R. Greenhouse gases emissions in the production and use of ethanol from sugarcane in Brazil: The 2005/2006 averages and a prediction for 2020. Biomass Bioenergy 2008, 32, 582–595. [Google Scholar] [CrossRef]
- Humbert, R.P. The Growing of Sugarcane; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Bell, M.J.; Garside, A.L. Shoot and stalk dynamics and the yield of sugarcane crops in tropical and subtropical Queensland, Australia. Field Crops Res. 2005, 92, 231–248. [Google Scholar] [CrossRef]
- Hemwong, S.; Cadisch, G.; Toomsan, B.; Limpinuntana, V.; Vityakon, P.; Patanothai, A. Dynamics of residue decomposition and N2 fixation of grain legumes upon sugarcane residue retention as an alternative to burning. Soil Tillage Res. 2008, 99, 84–97. [Google Scholar] [CrossRef]
- Peng, F.; Ren, J.-L.; Xu, F.; Bian, J.; Peng, P.; Sun, R.-C. Comparative Study of Hemicelluloses Obtained by Graded Ethanol Precipitation from Sugarcane Bagasse. J. Agric. Food Chem. 2009, 57, 6305–6317. [Google Scholar] [CrossRef]
- Li, X.; Kondo, R.; Sakai, K. Biodegradation of sugarcane bagasse with marine fungus Phlebia sp. MG-60. J. Wood Sci. 2002, 48, 159–162. [Google Scholar] [CrossRef]
- Guo, G.-L.; Hsu, D.-C.; Chen, W.-H.; Chen, W.-H.; Hwang, W.-S. Characterization of enzymatic saccharification for acid-pretreated lignocellulosic materials with different lignin composition. Enzym. Microb. Technol. 2009, 45, 80–87. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Bai, F.W. Mechanisms of yeast stress tolerance and its manipulation for efficient fuel ethanol production. J. Biotechnol. 2009, 144, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Khare, S.K.; Pandey, A.; Larroche, C. Current perspectives in enzymatic saccharification of lignocellulosic biomass. Biochem. Eng. J. 2015, 102, 38–44. [Google Scholar] [CrossRef]
- Hoang, A.T.; Ong, H.C.; Fattah, I.M.R.; Chong, C.T.; Cheng, C.K.; Sakthivel, R.; Ok, Y.S. Progress on the lignocellulosic biomass pyrolysis for biofuel production toward environmental sustainability. Fuel Process. Technol. 2021, 223, 106997. [Google Scholar] [CrossRef]
- Methrath Liyakathali, N.A.; Muley, P.D.; Aita, G.; Boldor, D. Effect of frequency and reaction time in focused ultrasonic pretreatment of energy cane bagasse for bioethanol production. Bioresour. Technol. 2016, 200, 262–271. [Google Scholar] [CrossRef]
- Jugwanth, Y.; Sewsynker-Sukai, Y.; Gueguim Kana, E.B. Valorization of sugarcane bagasse for bioethanol production through simultaneous saccharification and fermentation: Optimization and kinetic studies. Fuel 2020, 262, 116552. [Google Scholar] [CrossRef]
- Valladares-Diestra, K.K.; Porto de Souza Vandenberghe, L.; Zevallos Torres, L.A.; Nishida, V.S.; Zandoná Filho, A.; Woiciechowski, A.L.; Soccol, C.R. Imidazole green solvent pre-treatment as a strategy for second-generation bioethanol production from sugarcane bagasse. Chem. Eng. J. 2021, 420, 127708. [Google Scholar] [CrossRef]
- Ramadoss, G.; Muthukumar, K. Influence of dual salt on the pretreatment of sugarcane bagasse with hydrogen peroxide for bioethanol production. Chem. Eng. J. 2015, 260, 178–187. [Google Scholar] [CrossRef]
- Asada, C.; Sasaki, C.; Oka, C.; Nakamura, Y. Ethanol Production from Sugarcane Bagasse Using Pressurized Microwave Treatment with Inorganic Salts and Salt-Tolerant Yeast. Waste Biomass Valoriz. 2020, 11, 2001–2007. [Google Scholar] [CrossRef]
- Tura, A.; Fontana, R.C.; Camassola, M. Schizosaccharomyces pombe as an Efficient Yeast to Convert Sugarcane Bagasse Pretreated with Ionic Liquids in Ethanol. Appl. Biochem. Biotechnol. 2018, 186, 960–971. [Google Scholar] [CrossRef] [PubMed]
- Raj, K.; Krishnan, C. Improved high solid loading enzymatic hydrolysis of low-temperature aqueous ammonia-soaked sugarcane bagasse using laccase-mediator system and high concentration ethanol production. Ind. Crops Prod. 2019, 131, 32–40. [Google Scholar] [CrossRef]
- Bu, J.; Yan, X.; Wang, Y.-T.; Zhu, S.-M.; Zhu, M.-J. Co-production of high-gravity bioethanol and succinic acid from potassium peroxymonosulfate and deacetylation sequentially pretreated sugarcane bagasse by simultaneous saccharification and co-fermentation. Energy Convers. Manag. 2019, 186, 131–139. [Google Scholar] [CrossRef]
- Neves, P.V.; Pitarelo, A.P.; Ramos, L.P. Production of cellulosic ethanol from sugarcane bagasse by steam explosion: Effect of extractives content, acid catalysis and different fermentation technologies. Bioresour. Technol. 2016, 208, 184–194. [Google Scholar] [CrossRef]
- Wang, Z.; Dien, B.S.; Rausch, K.D.; Tumbleson, M.E.; Singh, V. Improving ethanol yields with deacetylated and two-stage pretreated corn stover and sugarcane bagasse by blending commercial xylose-fermenting and wild type Saccharomyces yeast. Bioresour. Technol. 2019, 282, 103–109. [Google Scholar] [CrossRef]
- Ninomiya, K.; Utami, A.R.I.; Tsuge, Y.; Kuroda, K.; Ogino, C.; Taima, T.; Saito, J.; Kimizu, M.; Takahashi, K. Pretreatment of bagasse with a minimum amount of cholinium ionic liquid for subsequent saccharification at high loading and co-fermentation for ethanol production. Chem. Eng. J. 2018, 334, 657–663. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, W.; Tan, X.; Zahoor; Chen, X.; Guo, Y.; Yu, Q.; Yuan, Z.; Zhuang, X. Low-temperature sodium hydroxide pretreatment for ethanol production from sugarcane bagasse without washing process. Bioresour. Technol. 2019, 291, 121844. [Google Scholar] [CrossRef]
- biofuel.org.uk. Major Biofuel Producers by Region. Available online: http://biofuel.org.uk/major-producers-by-region.html (accessed on 15 July 2022).
- Alternative Fuels Data Center. Maps and Data—Global Ethanol Production by Country or Region. 2021. Available online: https://afdc.energy.gov/data/10331 (accessed on 15 July 2022).
- Ong, H.C.; Tiong, Y.W.; Goh, B.H.H.; Gan, Y.Y.; Mofijur, M.; Fattah, I.M.R.; Chong, C.T.; Alam, M.A.; Lee, H.V.; Silitonga, A.S.; et al. Recent advances in biodiesel production from agricultural products and microalgae using ionic liquids: Opportunities and challenges. Energy Convers. Manag. 2021, 228, 113647. [Google Scholar] [CrossRef]
- Imtenan, S.; Varman, M.; Masjuki, H.H.; Kalam, M.A.; Sajjad, H.; Arbab, M.I.; Rizwanul Fattah, I.M. Impact of low temperature combustion attaining strategies on diesel engine emissions for diesel and biodiesels: A review. Energy Convers. Manag. 2014, 80, 329–356. [Google Scholar] [CrossRef]
- Shahir, S.A.; Masjuki, H.H.; Kalam, M.A.; Imran, A.; Rizwanul Fattah, I.M.; Sanjid, A. Feasibility of diesel–biodiesel–ethanol/bioethanol blend as existing CI engine fuel: An assessment of properties, material compatibility, safety and combustion. Renew. Sustain. Energy Rev. 2014, 32, 379–395. [Google Scholar] [CrossRef]
- Gupta, A.; Verma, J.P. Sustainable bio-ethanol production from agro-residues: A review. Renew. Sustain. Energy Rev. 2015, 41, 550–567. [Google Scholar] [CrossRef]
- Haankuku, C.; Epplin, F.M.; Kakani, V.G. Industrial sugar beets to biofuel: Field to fuel production system and cost estimates. Biomass Bioenergy 2015, 80, 267–277. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J.J. Dilute acid pretreatment of rye straw and bermudagrass for ethanol production. Bioresour. Technol. 2005, 96, 1599–1606. [Google Scholar] [CrossRef]
- Aiyejagbara, M.; Aderemi, B.; Ameh, A.; Ishidi, E.; Ibeneme, E.F.; Olakunle, M. Production of Bioethanol from Elephant Grass (Pennisetum purpureum) Stem. Int. J. Innov. Math. Stat. Energy Policies 2016, 4, 1–9. [Google Scholar]
- Semhaoui, I.; Zarguili, I.; Rezzoug, S.A.; Maugard, T.; Zhao, J.M.Q.; Toyir, J.; Nawdali, M.; Maache-Rezzoug, Z. Bioconversion of Moroccan Alfa (Stipa Tenacissima) by Thermomechanical Pretreatment Combined to Acid or Alkali Spraying for Ethanol Production. J. Mater. Environ. Sci. 2017, 8, 2619–2631. [Google Scholar]
- Cardona, E.; Rios, J.; Peña, J.; Peñuela, M.; Rios, L. King Grass: A very promising material for the production of second-generation ethanol in tropical countries. Biomass Bioenergy 2016, 95, 206–213. [Google Scholar] [CrossRef]
- Keshwani, D.R.; Cheng, J.J. Switchgrass for bioethanol and other value-added applications: A review. Bioresour. Technol. 2009, 100, 1515–1523. [Google Scholar] [CrossRef]
- Carpio, L.G.T.; de Souza, F.S. Optimal allocation of sugarcane bagasse for producing bioelectricity and second-generation ethanol in Brazil: Scenarios of cost reductions. Renew. Energy 2017, 111, 771–780. [Google Scholar] [CrossRef]
- Adsul, M.; Sandhu, S.K.; Sinhania, R.R.; Gupta, R.; Puri, S.K.; Mathur, A. Designing a cellulolytic enzyme cocktail for the efficient and economical conversion of lignocellulosic biomass to biofuels. Enzym. Microb. Technol. 2020, 133, 109442. [Google Scholar] [CrossRef]
- Prajapati, B.P.; Jana, U.K.; Suryawanshi, R.K.; Kango, N. Sugarcane bagasse saccharification using Aspergillus tubingensis enzymatic cocktail for 2G bio ethanol production. Renew. Energy 2020, 152, 653–663. [Google Scholar] [CrossRef]
- Raj, K.; Krishnan, C. Improved co-production of ethanol and xylitol from low temperature aqueous ammonia pretreated sugarcane bagasse using two-stage high solids enzymatic hydrolysis and Candida tropicalis. Renew. Energy 2020, 153, 392–403. [Google Scholar] [CrossRef]
- Unrean, P.; Ketsub, N. Integrated lignocellulosic bioprocess for co-production of ethanol and xylitol from sugarcane bagasse. Ind. Crop Prod. 2018, 123, 238–246. [Google Scholar] [CrossRef]
- Valladares-Diestra, K.K.; de Souza Vandenberghe, L.P.; Soccol, C.R. Integrated xylooligosaccharides production from imidazole-treated sugarcane bagasse with application of in house produced enzymes. Bioresour. Technol. 2022, 362, 127800. [Google Scholar] [CrossRef]
- Duan, X.; Xu, Z.; Sun, X.; Deng, B.; Liu, J. Effects of injection timing and EGR on combustion and emissions characteristics of the diesel engine fuelled with acetone–butanol–ethanol/diesel blend fuels. Energy 2021, 231, 121069. [Google Scholar] [CrossRef]
- Aguado-Deblas, L.; López-Tenllado, F.J.; Luna, D.; Bautista, F.M.; Romero, A.A.; Estevez, R. Advanced Biofuels from ABE(Acetone/Butanol/Ethanol) and Vegetable Oils (Castor or Sunflower Oil) for Using in Triple Blends with Diesel: Evaluation on a Diesel Engine. Materials 2022, 15, 6493. [Google Scholar] [CrossRef]
- Nilaphai, O.; Komanee, K.; Chuepeng, S. Expansion heat release and thermal efficiency of acetone-butanol-ethanol- diesel blended fuel (ABE20) combustion in piston engine. Fuel 2022, 309, 122214. [Google Scholar] [CrossRef]
- Dinesha, P.; Mohan, S.; Kumar, S. Experimental investigation of SI engine characteristics using Acetone-Butanol-Ethanol (ABE)—Gasoline blends and optimization using Particle Swarm Optimization. Int. J. Hydrogen Energy 2022, 47, 5692–5708. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, X.; Guo, Z.; Li, Y.; Zhang, J.; Liu, D. Study on Combustion and Emissions of a Spark Ignition Engine with Gasoline Port Injection Plus Acetone–Butanol–Ethanol (ABE) Direct Injection under Different Speeds and Loads. Energies 2022, 15, 7028. [Google Scholar] [CrossRef]
- Kunwer, R.; Pasupuleti, S.R.; Bhurat, S.S.; Gugulothu, S.K.; Rathore, N. Blending of ethanol with gasoline and diesel fuel—A review. Mater. Today Proc. 2022, in press. [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).