Facile Preparations of Electrochemically Exfoliated N-Doped Graphene Nanosheets from Spent Zn-Carbon Primary Batteries Recycled for Supercapacitors Using Natural Sea Water Electrolytes
Abstract
:1. Introduction
2. Experimental
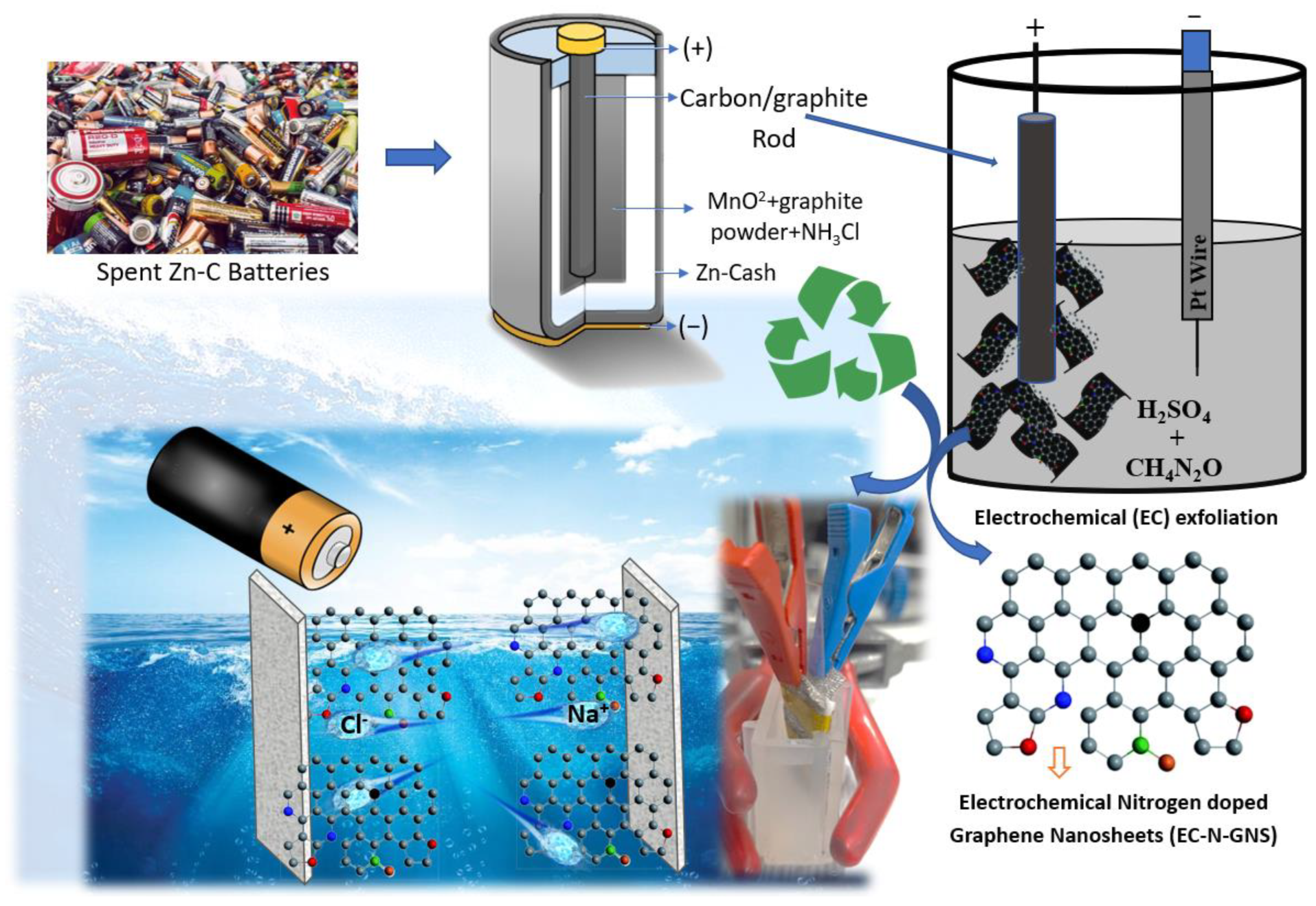
2.1. Electrochemical Synthesis of Nitrogen-Doped Graphene Nanosheets
2.2. Materials Characterization
2.3. Electrode Preparations of Slurry and Coating Process
3. Result and Discussions
3.1. Scanning Electron Microscopy (SEM) Analysis
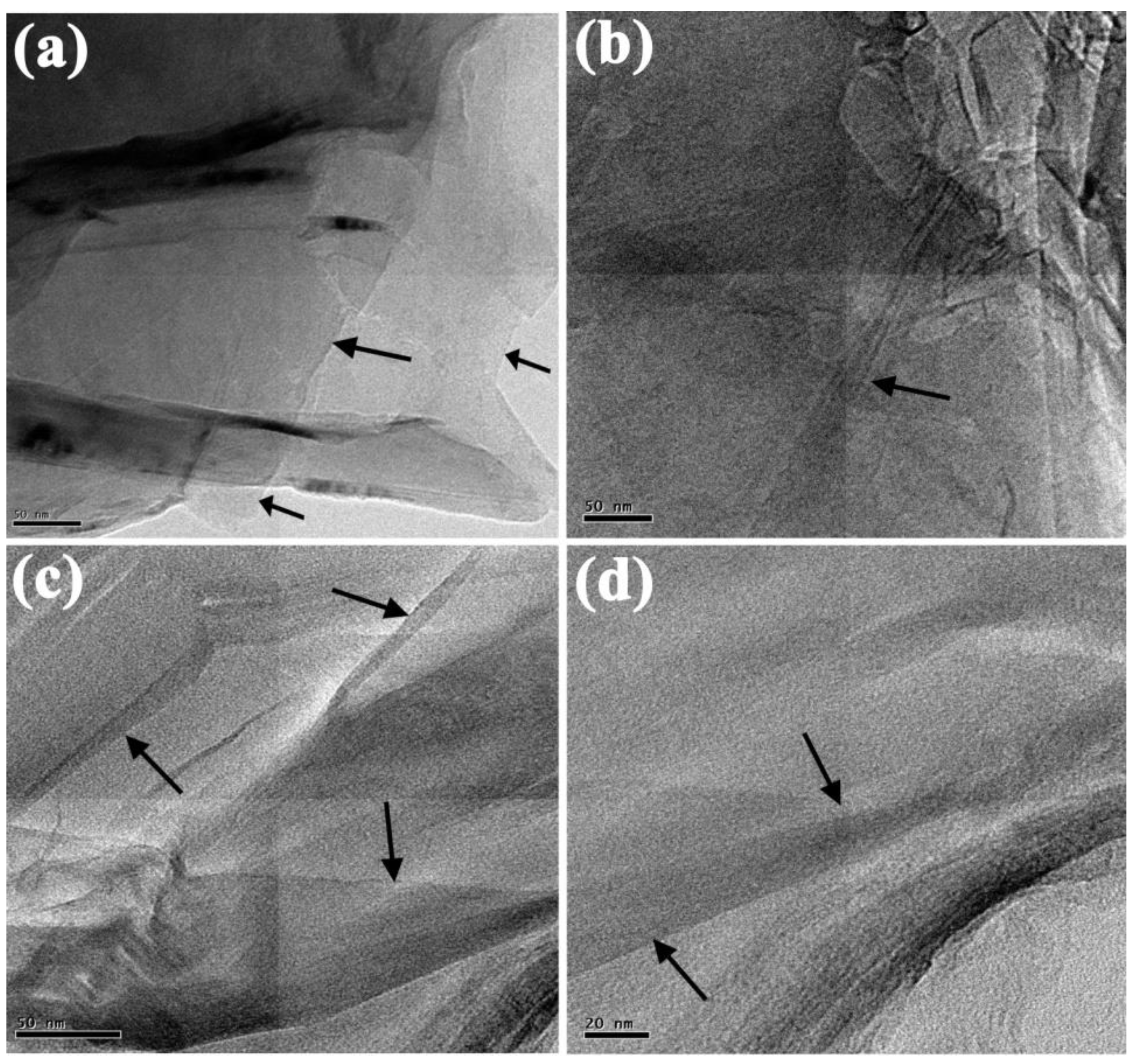
3.2. HR-TEM Analysis
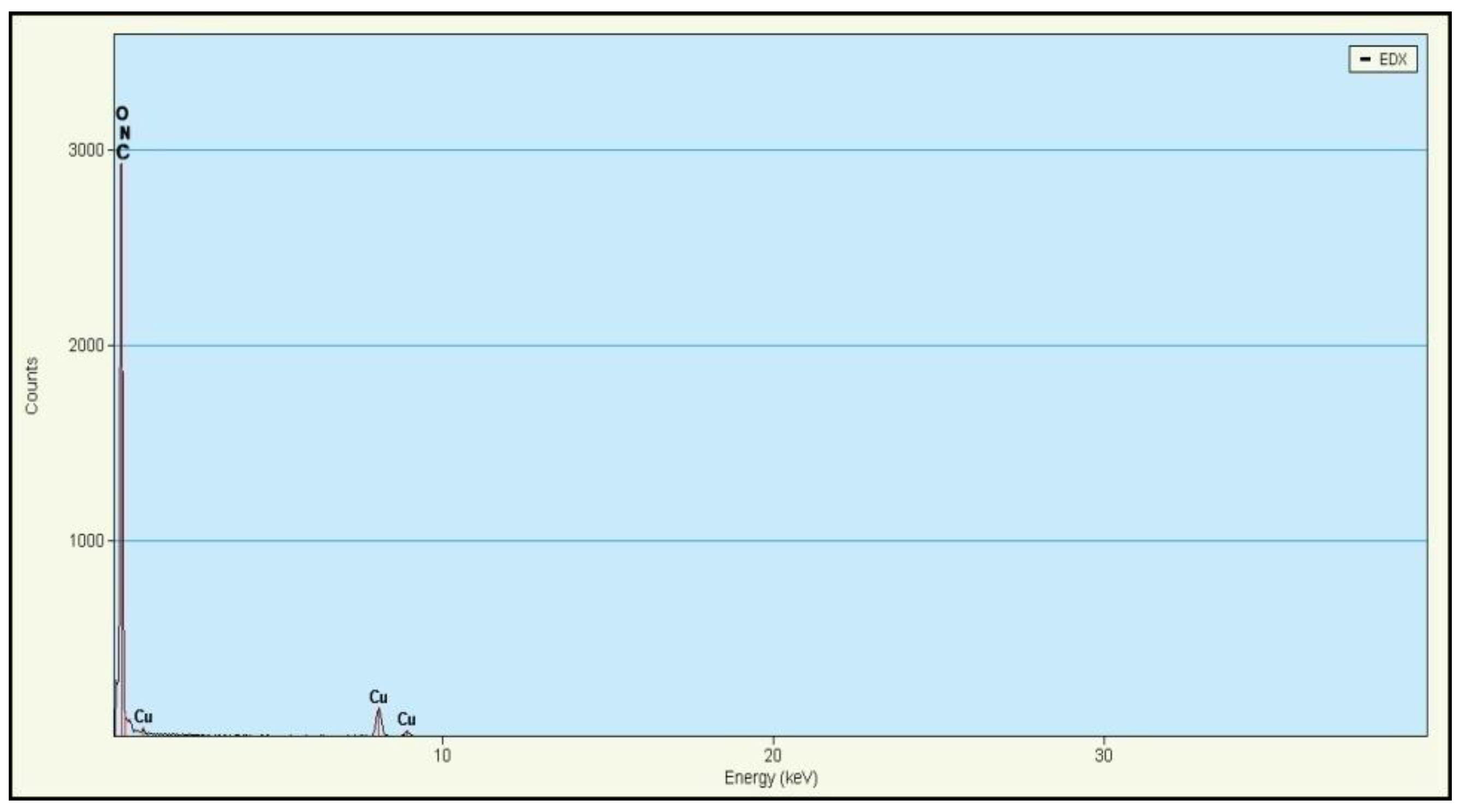
3.3. Energy Dispersive X-ray (EDX) Spectroscopy Analysis
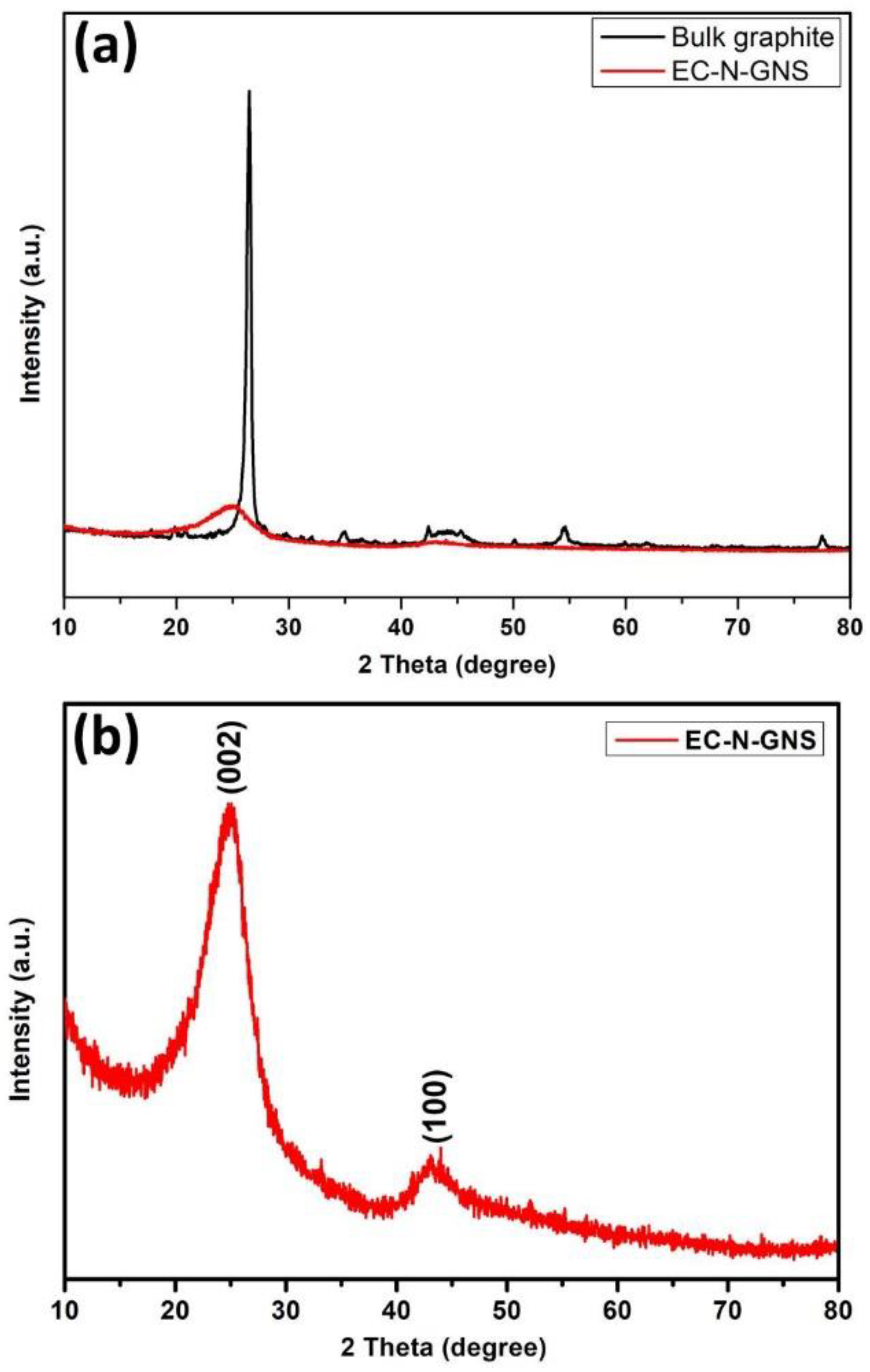
3.4. XRD Analysis
3.5. Raman Analysis
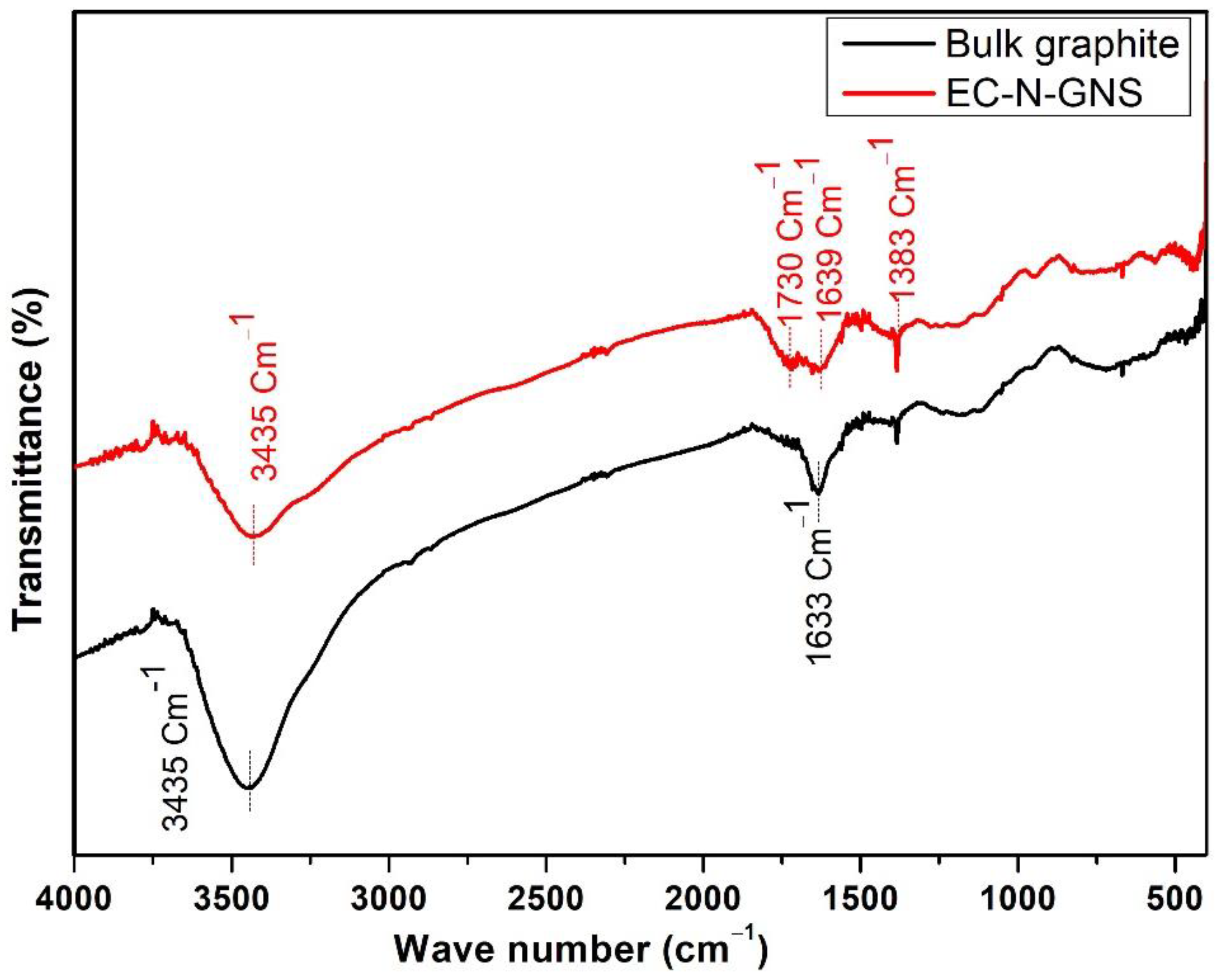
3.6. FT-IR Analysis
4. Electrochemical Performance—Sea Water Symmetric Supercapacitor
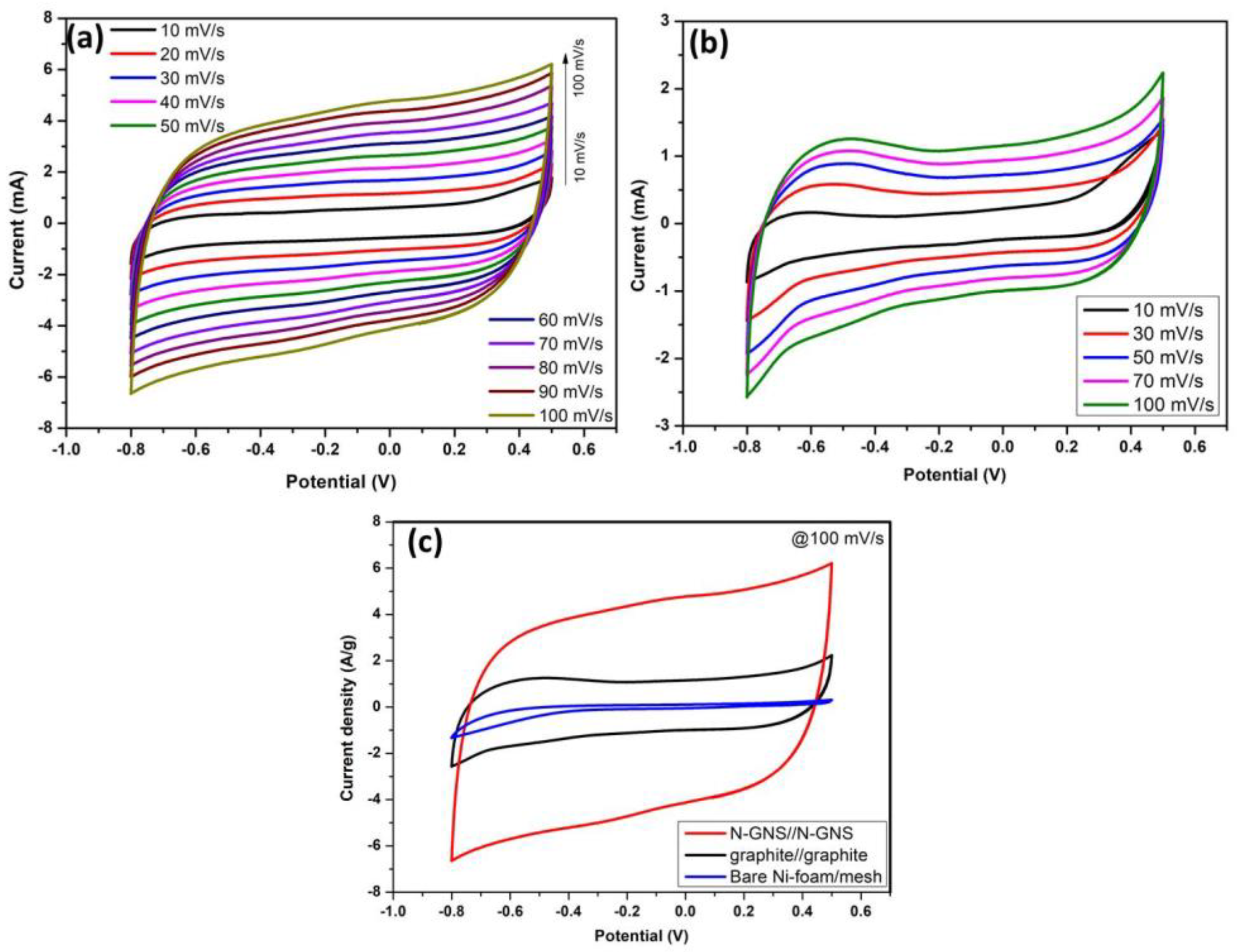
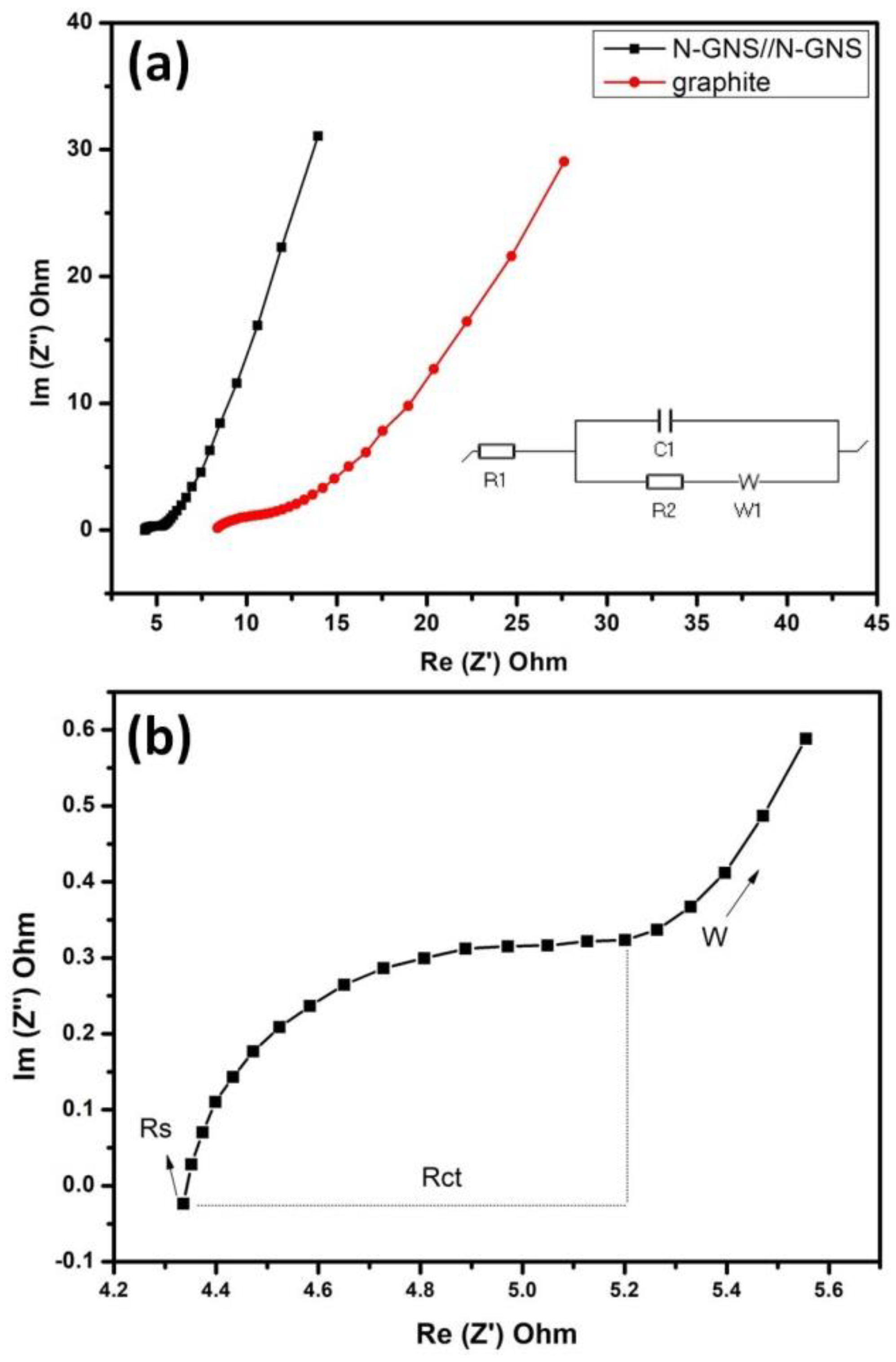
4.1. Cyclic Voltammetry—CV and EIS Analysis
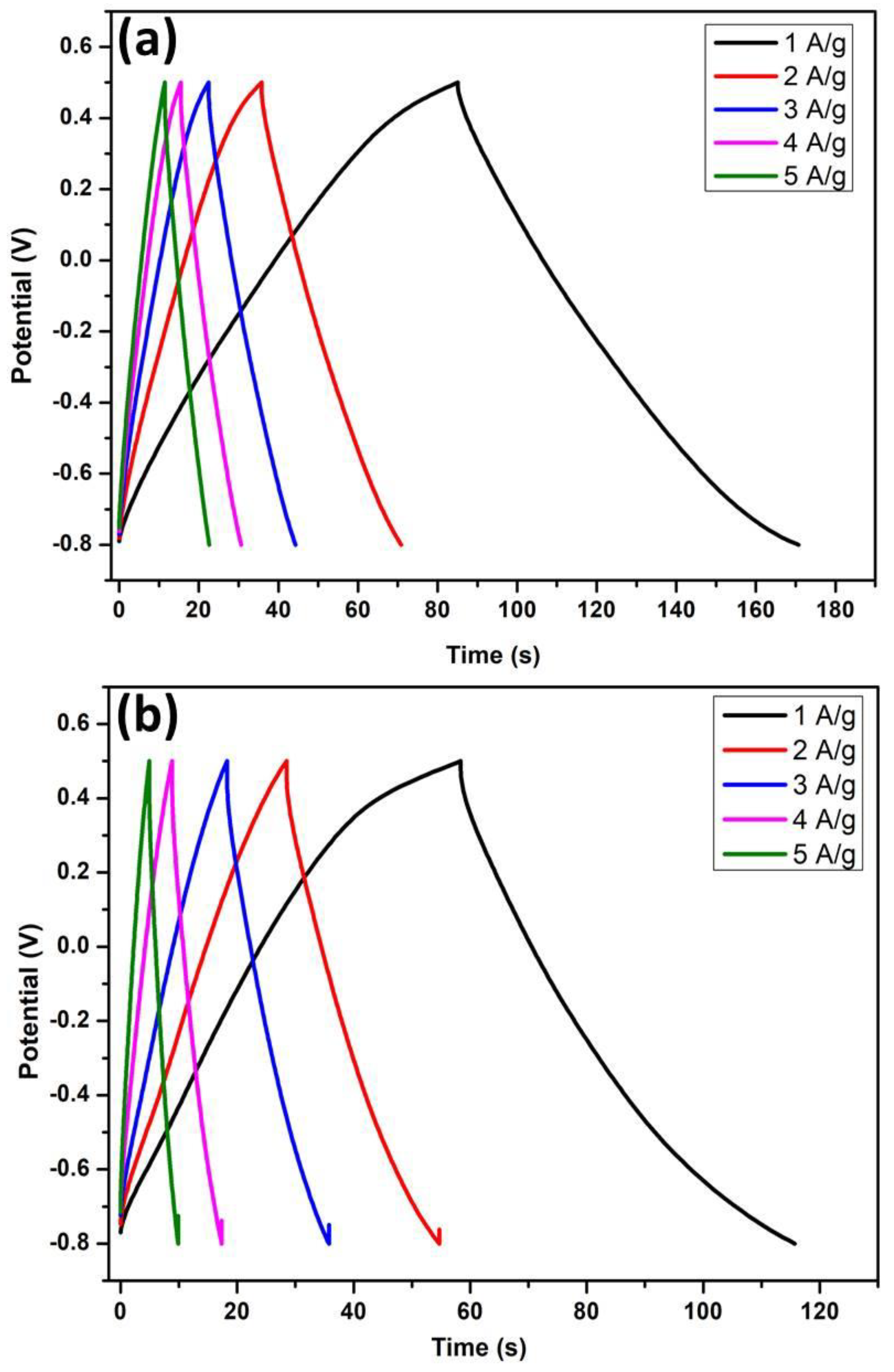
4.2. Charge/Discharge Studies for Sea Water-Supercapacitor Devices
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ferella, F.; Michelis, I.D.; Veglio, F. Process for the recycling of alkaline and zinc–carbon spent batteries. J. Power Sources 2008, 183, 805–811. [Google Scholar] [CrossRef]
- Vadivel, S.; Tejangkura, W.; Sawangphruk, M. Graphite/Graphene Composites from the Recovered Spent Zn/Carbon Primary Cell for the High-Performance Anode of Lithium-Ion Batteries. ACS Omega 2020, 25, 15240–15246. [Google Scholar] [CrossRef] [PubMed]
- Dimiev, A.M.; Tour, J.M. Mechanism of Graphene Oxide Formation. ACS Nano 2014, 8, 3060–3068. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wu, Q.; Shi, G. Graphene based new energy materials. Energy Envion. Sci. 2011, 4, 1113–1132. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Wang, X.; You, H.; Liu, F.; Li, M.; Wan, L.; Li, S.; Li, Q.; Xu, Y.; Tian, R.; Yu, Z.; et al. Large-scale synthesis of few-layered graphene using CVD. Chem. Vap. Depos. 2009, 15, 53–56. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Velamakanni, A.; Piner, R.D.; Ruoff, R.S. Microwave assisted exfoliation and reduction of graphite oxide for ultracapacitors. Carbon 2010, 48, 2118–2122. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Yanwu, Z.; An, J.; Ruoff, R.S. Graphene-Based ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.S.; Pei, S.; Ren, W.; Tang, D.; Gao, L.; Liu, B.; Li, F.; Liu, C.; Cheng, H.M. Field emission of single-layer graphene films prepared by electrophoretic deposition. Adv. Mater. 2009, 21, 1756–1760. [Google Scholar] [CrossRef]
- Thirumal, V.; Pandurangan, A.; Jayavel, R.; Krishnamoorthi, S.R.; Ilangovan, R. Synthesis of nitrogen doped coiled double walled carbon nanotubes by chemical vapor deposition method for supercapacitor applications. Curr. Appl. Phys. 2016, 16, 816–825. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang., X.; Yang, S. High performance supercapacitors based on highly conductive nitrogen-doped graphene sheets. Phys. Chem. Chem. Phys. 2011, 13, 12554–12558. [Google Scholar] [CrossRef] [PubMed]
- Terrones, H.; Lv, R.; Terrones, M.; Dresselhaus, M.S. The role of defects and doping in 2D graphene sheets and 1D nanoribbons. Rep. Prog. Phys. 2012, 75, 062501. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, X. Chemically Functionalized Graphene and Their Applications in Electrochemical Energy Conversion and Storage. In Advances in Graphene Science; Aliofkhazraei, M., Ed.; InTech: London, UK, 2013; Chapter-7. [Google Scholar]
- Wei, D.; Liu, Y.; Wang, Y.; Zhang, H.; Huang, L.; Yu, G. Synthesis of N-Doped Graphene by Chemical Vapor Deposition and Its Electrical Properties. Nano Lett. 2009, 9, 1752–1758. [Google Scholar] [CrossRef]
- Chen, P.; Yang, J.J.; Li, S.S.; Wang, Z.; Xiao, T.Y.; Qian, Y.H.; Yu, S.H. Hydrothermal synthesis of macroscopic nitrogen-doped graphene hydrogels for ultrafast supercapacitor. Nano Energy 2013, 2, 249–256. [Google Scholar] [CrossRef]
- Deng, D.; Pan, X.; Yu, L.; Cui, Y.; Jiang, Y.; Qi, J.; Li, W.X.; Fu, Q.; Ma, X.; Xue, Q.; et al. Toward N-Doped Graphene via Solvothermal Synthesis. Chem. Mater. 2011, 23, 1188–1193. [Google Scholar] [CrossRef]
- Jeong, H.M.; Lee, J.W.; Shin, W.H.; Choi, Y.J.; Shin, H.J.; Kang, J.K. Nitrogen-doped graphene for high-performance, ultracapacitors and the importance of nitrogen-doped sites at basal planes. Nano Lett. 2011, 11, 2472–2477. [Google Scholar] [CrossRef]
- Ewels, C.P.; Glerup, M. Nitrogen Doping in Carbon Nanotubes. J. Nanosci. Nanotechnol. 2005, 5, 1345–1363. [Google Scholar] [CrossRef]
- Shao, Y.; Zhang, S.; Engelhard, M.H.; Li, G.; Shao, G.; Wang, Y.; Liu, J.; Aksay, I.A.; Lin, Y. Nitrogen-doped graphene and its electrochemical applications. J. Mater. Chem. 2010, 20, 7491–7496. [Google Scholar] [CrossRef]
- Wen, Z.; Wang, X.; Mao, S.; Bo, Z.; Kim, H.; Cui, S.; Lu, G.; Feng, X.; Chen, J. Crumbeld Nitrogen doped graphene Nanosheets with ultra high pore volume for high performance supercapacitor. Adv. Mater. 2012, 24, 5610–5616. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.L.M.; Srivastava, A.; Gowda, S.R.; Gullapalli, H.; Dubey, M.; Ajayan, P.M. Synthesis of Nitrogen-Doped Graphene Films for Lithium Battery Application. ACS Nano 2010, 4, 6337–6342. [Google Scholar] [CrossRef] [Green Version]
- Xin, Y.; Liu, J.; Jie, X.; Liu, W.; Liu, F.; Yin, Y.; Gu, J.; Zou, Z. Preparation and electrochemical characterisation of nitrogen doped graphene by microwave as supporting materials for fuel cell catalysts. Electrochim. Acta 2012, 60, 354–358. [Google Scholar] [CrossRef]
- Cui, T.; Lv, R.; Huang, Z.H.; Zhu, H.; Zhang, J.; Li, Z.; Jia, Y.; Kang, F.; Wang, K.; Wu, D. Synthesis of nitrogen-doped carbon thin films and their applications in solar cells. Carbon 2011, 49, 5022–5028. [Google Scholar] [CrossRef]
- Lin, Z.; Song, M.K.; Ding, Y.; Liu, Y.; Liu, M.; Wong, C.P. Facile preparation of nitrogen-doped graphene as a metal-free catalyst for oxygen reduction reaction. Phys. Chem. Chem. Phys. 2012, 14, 3381–3387. [Google Scholar] [CrossRef]
- Zhang, Y.; Ge, J.; Wang, L.; Wang, D.; Ding, F.; Tao, X.; Chen, W. Manageable N-doped graphene for high performance oxygen reduction reaction. Sci. Rep. 2013, 3, 2771. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.R.; Li, X.L.; Zhang, L.; Yoon, Y.; Weber, P.K.; Wang, H.L.; Guo, J.; Dai, H. N-doping of graphene through electrothermal reactions with ammonia. J. Sci. 2009, 324, 768–777. [Google Scholar] [CrossRef]
- Long, D.; Li, W.; Ling, L.; Miyawaki, J.; Mochida, I.; Yoon, S.H. Preparation of nitrogen-doped graphene sheets by a combined chemical and hydrothermal reduction of graphene oxide. Langmuir 2010, 26, 16096. [Google Scholar] [CrossRef]
- Lin, Y.C.; Lin, C.Y.; Chiu, P.W. Controllable graphene N-doping with ammonia plasma. Appl. Phys. Lett. 2010, 96, 133110. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.X.; Wang, B.; Park, J.; Wang, Y.; Sun, B.; Yao, J. Highly efficient and large scale synthesis of graphene by electrolytic exfoliation. Carbon 2009, 47, 3242–3246. [Google Scholar] [CrossRef]
- Wei, D.; Grande, L.; Chundi, V.; White, R.; Bower, C.; Andrew, P.; Ryhanena, T. Graphene from electrochemical exfoliation and its direct applications in enhanced energy storage devices. Chem. Commun. 2012, 48, 1239–1241. [Google Scholar] [CrossRef] [PubMed]
- Parvez, K.; Li, R.; Puniredd, S.R.; Hernandez, Y.; Hinkel, F.; Wang, S.; Feng, X.; Mullen, K. Electrochemically Exfoliated Graphene as Solution Proces sable, Highly Conductive Electrodes for Organic Electronics. ACS Nano 2013, 7, 3598–3606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, H.; Pan, Q.; Song, Y.; Liu, X.X.; Liu, T. A Review on Nano-/Microstructured Materials Constructed by Electrochemical Technologies for Supercapacitors. Nano-Micro Lett. 2020, 12, 118. [Google Scholar] [CrossRef]
- Song, Y.; Pan, Q.; Lv, H.; Yang, D.; Qin, Z.; Zhang, M.Y.; Sun, X.; Liu, X.X. Ammonium-Ion Storage Using Electrodeposited Manganese Oxides. Angew. Chem. Int. Ed. 2021, 60, 5718–5722. [Google Scholar] [CrossRef]
- Zhao, C.; Zheng, W. A review for aqueous electrochemical supercapacitors. Front. Energy Res. 2015, 3, 23. [Google Scholar] [CrossRef] [Green Version]
- Millero, F.J. Sea Water as an Electrolyte. In Chemistry of Marine Water and Sediments; Gianguzza, A., Pelizzetti, E., Sammartano, S., Eds.; Environmental Science; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Kim, J.K.; Mueller, F.; Kim, H.; Bresser, D.; Park, J.S.; Lim, D.H.; Kim, G.T.; Passerini, S.; Kim, Y. Rechargeable-hybrid-seawater fuel cell. NPG Asia Mater. 2014, 6, e144. [Google Scholar] [CrossRef]
- Zhao, P.; Yao, M.; Zhang, Q.; Wang, N.; Hu, W.; Komarneni, S. Electrochemical behavior of representative electrode materials in artificial seawater for fabricating supercapacitors. Electrochim. Acta 2019, 318, 211–219. [Google Scholar] [CrossRef]
- Xia, Q.X.; Shinde, N.M.; Zhang, T.; Yun, J.M.; Zhou, A.; Mathur, S.; Mane, R.S.; Kim, K.H. Seawater Electrolyte-Mediated High Volumetric MXene-based Electrochemical Symmetric Supercapacitors. Dalton Trans. 2018, 47, 8676–8682. [Google Scholar] [CrossRef]
- Challagulla, N.V.; Vijayakumar, M.; Rohita, D.S.; Elsa, G.; Sankar, A.B.; Rao, T.N.; Karthik, M. Hierarchical Activated Carbon Fibers as a Sustainable Electrode and Natural Seawater as a Sustainable Electrolyte for High-Performance Supercapacitor. Energy Technol. 2020, 8, 2000417. [Google Scholar] [CrossRef]
- Feng, L.; Yang, L.; Huang, Z.; Luo, J.; Li, M.; Wang, D.; Chen., Y. Enhancing Electrocatalytic Oxygen Reduction on Nitrogen-Doped Graphene by Active Sites Implantation. Sci. Rep. 2013, 3, 3306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhoua, M.; Tang, J.; Cheng, Q.; Xu, G.; Cui, P.; Qin, L.C. Few-layer graphene obtained by electrochemical exfoliation of graphite cathode. Chem. Phys. Lett. 2013, 572, 61–65. [Google Scholar] [CrossRef]
- Hafiz, S.M.; Ritikos, R.; Whitcher, T.J.; Razib, N.M.; Bien, D.C.S.; Chanlek, N.; Nakajima, H.; Saisopa, T.; Songsiriritthigul, P.; Huang, N.M.; et al. A practical carbon dioxide gas sensor using room-temperature hydrogen plasma reduced graphene oxide. Sens. Actuators B Chem. 2014, 193, 692–700. [Google Scholar] [CrossRef]
- Betriu, X.D.; Garcia, S.A.; Botas, C.; Alvarez, P.; Marcos, J.S.; Prieto, C.; Menéndez, R.; Andres, A.D. Raman spectroscopy for the study of reduction mechanisms and optimization of conductivity in graphene oxide thin films. J. Mater. Chem. C 2013, 1, 6905–6912. [Google Scholar] [CrossRef]
- Chang, Y.Z.; Han, G.Y.; Yuan, J.P.; Fu, D.Y.; Liu, F.F.; Li, S.D. Using hydroxylamine asa reducer to prepare N-doped graphene hydrogels used in high-performance energy storage. J. Power Sources 2013, 238, 492–500. [Google Scholar] [CrossRef]
- Misra, A.; Tyagi, P.K.; Singh, M.K.; Misra, D.S. FTIR studies of nitrogen doped carbon nanotubes. Diam. Relat. Mater. 2006, 15, 385–388. [Google Scholar] [CrossRef]
- Jiang, B.J.; Tian, C.G.; Wang, L.; Sun, L.; Chen, C.; Nong, X.; Qiao, Y.; Fu, H. Highly concentrated, stable nitrogen-doped graphene for supercapacitors: Simultaneous doping and reduction. Appl. Surf. Sci. 2012, 258, 3438–3443. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thirumal, V.; Sreekanth, T.V.M.; Yoo, K.; Kim, J. Facile Preparations of Electrochemically Exfoliated N-Doped Graphene Nanosheets from Spent Zn-Carbon Primary Batteries Recycled for Supercapacitors Using Natural Sea Water Electrolytes. Energies 2022, 15, 8650. https://doi.org/10.3390/en15228650
Thirumal V, Sreekanth TVM, Yoo K, Kim J. Facile Preparations of Electrochemically Exfoliated N-Doped Graphene Nanosheets from Spent Zn-Carbon Primary Batteries Recycled for Supercapacitors Using Natural Sea Water Electrolytes. Energies. 2022; 15(22):8650. https://doi.org/10.3390/en15228650
Chicago/Turabian StyleThirumal, Vediyappan, T. V. M. Sreekanth, Kisoo Yoo, and Jinho Kim. 2022. "Facile Preparations of Electrochemically Exfoliated N-Doped Graphene Nanosheets from Spent Zn-Carbon Primary Batteries Recycled for Supercapacitors Using Natural Sea Water Electrolytes" Energies 15, no. 22: 8650. https://doi.org/10.3390/en15228650




