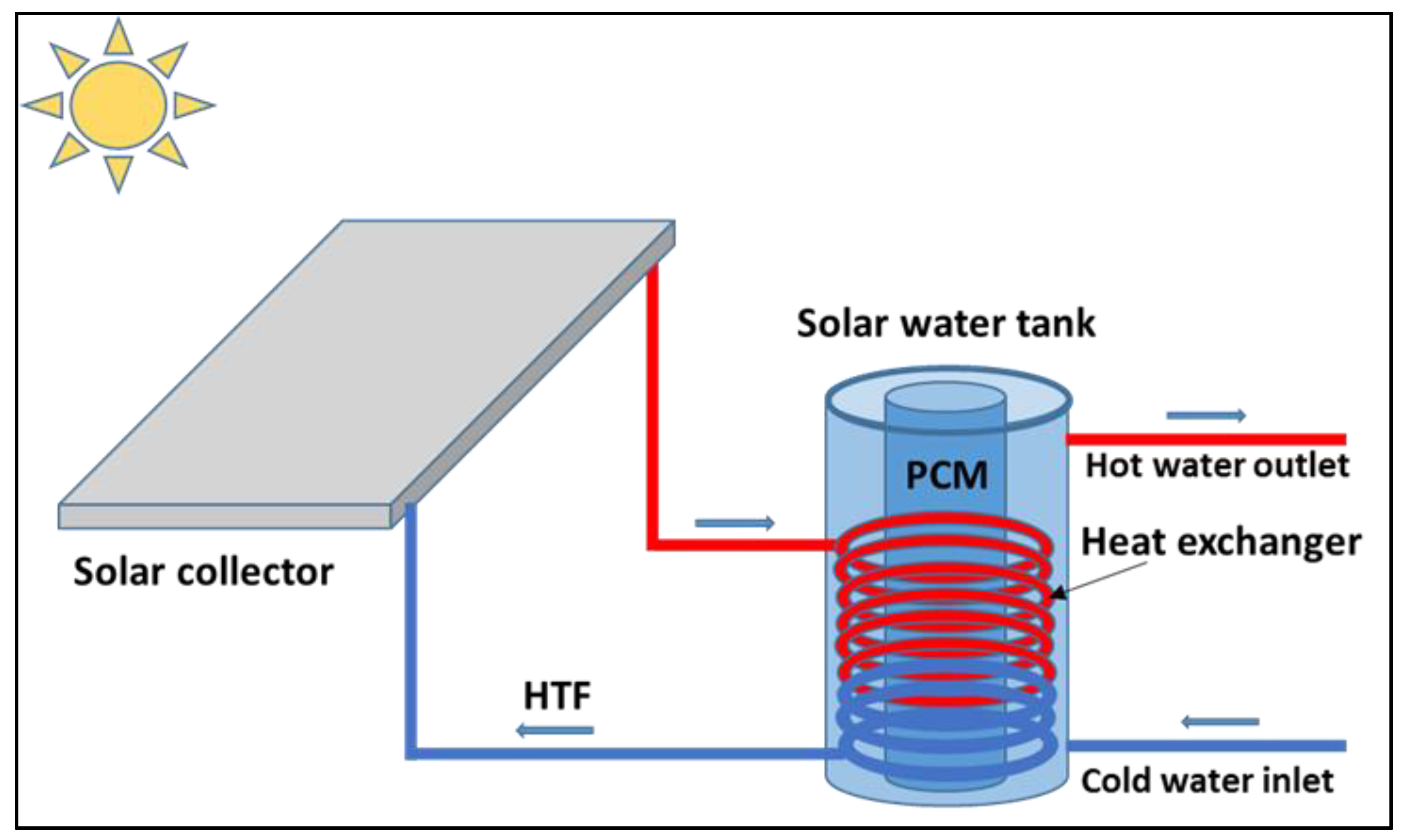
The current energy context is characterized by the depletion of fossil fuel resources on the one hand and global warming as a result of greenhouse gas emissions on the other, necessitating the development of alternative energy solutions. Among the alternatives being investigated is the effective utilization of renewable energy sources. Energy use in buildings, namely for air cooling and water heating, accounts for around two-thirds of total energy demands. Consequently, it is imperative to limit building energy use. Shifting a part of peak-period electricity usage to off-peak periods could have substantial economic, environmental, and social consequences. Solar energy has the greatest potential of all renewable energies [
1]. This kind of energy poses storage issues, as thermal energy can be stored as either sensible or latent heat. Storing surplus solar thermal energy and utilizing it during the night period would improve the operation and effectiveness of solar water heaters. Integrating thermal energy storage through latent heat thermal energy is a promising method for off-peak load shifting. Thermal energy storage systems can be used in conjunction with building walls, solar water collectors, and domestic hot water tanks. Domestic hot water production is receiving more attention at the moment due to concerns about its energy efficiency, as well as the size and number of storage tanks. The incorporation of phase change materials (PCM) enables the provision of solutions by storing thermal energy via latent heat rather than sensible heat, as indicated by the extensive literature in the domain [
1,
2,
3]. The research conducted focuses primarily on the precise investigation of heat transfer within various types of water heaters using computational [
4] and/or experimental [
5] methodologies. The following are the most frequently used designs for solar water heaters with latent heat storage: (i) the use of PCM capsules in a water tank powered by standard solar collectors, (ii) the combination of a standard solar collector and a separate PCM unit, and (iii) the use of integrated solar collectors and PCM [
2]. Solar thermal storage combined with a latent storage system based on PCMs is an intriguing solution for more efficient energy use. As a result, numerous studies have been conducted on the study and modeling of solar collectors [
3,
4,
5,
6,
7]. Sharma et al. [
3] summarized existing research on this topic by evaluating PCM-based storage devices and their applications in solar, building insulation, and aerospace. Additionally, they demonstrated techniques for measuring latent heat and temperature. Comparative studies on the usage of various types of storage in conjunction with PCM have been conducted. Kurklu et al. [
4] designed a novel type of solar collector in which a water tank is integrated with a PCM unit with a melting point of 45 to 50 °C. The results indicated that the reservoir temperature is maintained at 30 °C throughout the night on a sunny day. Additionally, the immediate thermal efficiency ranged from roughly 22% to 80%. Zalba [
5] investigates several PCM kinds. It lists approximately 150 materials that have been used and 45 that have been commercialized; therefore, there is a vast array of PCM materials available. The advantages of PCM systems are discussed, including the high-equivalent specific heat produced by phase change and the ability to regulate the water temperature. Nonetheless, important issues, such as stability, lifespan, storage capacity reduction based on the number of working cycles, and hazardous dangers in the event of a fire are noted. Eames and Griffiths [
6] investigated the collector and heat storage in a self-storing collector when water was replaced with PCM in various quantities. They noted that as long as the temperature inside the tank remains below the melting point (58–60 °C), the efficiency of the solar system with PCM is lower than that of a system filled entirely with water. Plantier et al. [
7] conducted research on a water tank filled with PCM spheres. It demonstrates that the use of PCM increases storage density and enables temperature control within the tank. The low thermal conductivity of PCM was mentioned as one of the issues. Additionally, the spheres were too massive to be extracted efficiently. To remedy this problem, Haillot et al. [
8] numerically and experimentally addressed the challenge of a solar collector incorporating a PCM/graphite mixture as a storage medium. This system enables greater storage and extraction of solar energy due to its storage capacity and the high thermal conductivity of graphite. During withdrawal, the efficiency of the solar collector integrating the storage is around 98%, demonstrating the high discharge capacity of the system. Other advantages are noted, such as the reduction in the stagnation temperature and storage volume. Mettaweea et al. [
9] conducted an experiment to evaluate the performance of a compact solar–PCM collector system. The results demonstrated that the average heat transfer coefficient increases with increasing thickness of the paraffin layer, owing to natural convection. The problems of solid–liquid phase change are of remarkable interest in many industries. Consequently, the use of numerical calculation methods becomes essential. Lacroix et al. [
10] devoted considerable attention to this sort of problem by undertaking a numerical and analytical evaluation of a thermal energy storage system with cylindrical tubes as the energy storage elements. Additionally, Laouadi et al. [
11] numerically investigated a system based on the PCM’s melting and cyclic solidification. Numerous types of storage units have been designed and studied, as indicated by the preceding studies [
3,
4,
5,
6,
7,
8,
9,
10,
11]. They all differ in terms of the location of the PCM, the system’s configuration, and the type of material used as the storage medium. Currently, the integration of a separate latent storage system is possible either with conventional solar water heaters (that operate via thermosiphon or forced circulation) or with auto-storage collectors [
12,
13]. However, these approaches are not satisfactory, neither from a thermal perspective (high losses), nor from an aesthetic perspective (tank visible on the outside) [
14]. Recent investigations have revealed that the integration of a thermal storage unit has a considerable impact on the efficiency of the solar collectors [
15,
16]. Dahou H. et al. [
15] experimentally examined the performance of an SWT integrated with nano-enhanced PCM and a Stirrer. The experimental results found that the completion time of the SWT/Ne-PCM system was reduced by 12.5% compared to a conventional tank.
To the authors’ knowledge, no published study has examined the impact of PCM’s thermophysical properties, such as density, enthalpy, and melting temperature, on the load shifting of a solar water heater (SWH) system integrated with PCM. Therefore, the objective of this study is to perform a parametric analysis of the SWH on the basis of PCM’s thermophysical properties. Finally, a comparison of SWH–PCM systems with different PCM’s thermophysical properties was conducted for introducing the performance of the SWH better, with a series of comparative simulations performed.