Insights into Enhancing Electrochemical Performance of Li-Ion Battery Anodes via Polymer Coating
Abstract
1. Introduction
2. Polymer Coating on Anode Materials
- −
- Intercalation anodes, such as Graphite (Gr) and Titanium oxides.
- −
- Alloy anodes, for instance, Silicon (Si), Germanium (Ge), and Tin (Sn).
- −
- Conversion anodes, for example, transition metal oxides (Fe3O4, Co3O4, CuO, etc.).
3. Intercalation Anodes
3.1. Graphite (Gr)
- Rate capability: safety issues because of lithium (Li) plating on the electrode surface.
- First cycle irreversible capacity, or initial CE towing to the electrolyte decomposition, and therefore, the consumption of Li-ions as the charge carrier.
- Ageing, prompted by cycling and calendar ageing, and the resulting safety concerns because of the low (de)lithiation potential close to the Li plating.
3.2. Titanium Oxide Anodes
4. Alloy Anodes
4.1. Silicon-Based Anodes
4.2. Silicon@Graphite (Si@Gr)
4.3. Tin (Sn)-Based Anodes
4.4. SnO2
4.5. Other Tin (Sn)-Based Anodes
4.6. Germanium (Ge)
5. Conversion Anodes
5.1. Iron Oxides
5.2. MnO2
5.3. Copper Oxide (CuO)
5.4. Co3O4
5.5. ZnFe2O4
5.6. Vanadium-Based Anodes
6. Summary and Outlook
Funding
Data Availability Statement
Conflicts of Interest
References
- Kwade, A.; Haselrieder, W.; Leithoff, R.; Modlinger, A.; Dietrich, F.; Droeder, K. Current status and challenges for automotive battery production technologies. Nat. Energy 2018, 3, 290–300. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- Cavers, H.; Molaiyan, P.; Abdollahifar, M.; Lassi, U.; Kwade, A. Perspectives on Improving the Safety and Sustainability of High Voltage Lithium-Ion Batteries Through the Electrolyte and Separator Region. Adv. Energy Mater. 2022, 12, 2200147. [Google Scholar] [CrossRef]
- Peled, E.; Golodnitsky, D.; Ardel, G. Advanced model for solid electrolyte interphase electrodes in liquid and polymer electrolytes. J. Electrochem. Soc. 1997, 144, L208. [Google Scholar] [CrossRef]
- Aurbach, D.; Markovsky, B.; Levi, M.D.; Levi, E.; Schechter, A.; Moshkovich, M.; Cohen, Y. New insights into the interactions between electrode materials and electrolyte solutions for advanced nonaqueous batteries. J. Power Sources 1999, 81, 95–111. [Google Scholar] [CrossRef]
- Meda, U.S.; Lal, L.; Sushantha, M.; Garg, P. Solid Electrolyte Interphase (SEI), a boon or a bane for lithium batteries: A review on the recent advances. J. Energy Storage 2021, 47, 103564. [Google Scholar] [CrossRef]
- Kim, J.; Chae, O.B.; Lucht, B.L. Perspective—structure and stability of the solid electrolyte interphase on silicon anodes of lithium-ion batteries. J. Electrochem. Soc. 2021, 168, 30521. [Google Scholar] [CrossRef]
- Komaba, S.; Ozeki, T.; Okushi, K. Functional interface of polymer modified graphite anode. J. Power Sources 2009, 189, 197–203. [Google Scholar] [CrossRef]
- Wang, C.; Wu, H.; Chen, Z.; McDowell, M.T.; Cui, Y.; Bao, Z. Self-healing chemistry enables the stable operation of silicon microparticle anodes for high-energy lithium-ion batteries. Nat. Chem. 2013, 5, 1042. [Google Scholar] [CrossRef]
- Kim, J.-M.; Park, H.-S.; Park, J.-H.; Kim, T.-H.; Song, H.-K.; Lee, S.-Y. Conducting polymer-skinned electroactive materials of lithium-ion batteries: Ready for monocomponent electrodes without additional binders and conductive agents. ACS Appl. Mater. Interfaces 2014, 6, 12789–12797. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wu, C.-E.; Su, L.-Y.; Huang, S.-S.; Fang, C.-C.; Wu, Y.-S.; Chou, J.; Wu, N.-L. A proof-of-concept graphite anode with a lithium dendrite suppressing polymer coating. J. Power Sources 2018, 406, 63–69. [Google Scholar] [CrossRef]
- Carter, R.; Parker, J.F.; Sassin, M.B.; Klein, E.J.; Wolak, M.A.; Love, C.T.; Long, J.W. Initiated Chemical Vapor Deposition of Ultrathin Polymer Coatings at Graphite Electrodes for Enhanced Performance in Li-Ion Batteries. J. Electrochem. Soc. 2020, 167, 60510. [Google Scholar] [CrossRef]
- Li, C.; Zhang, H.P.; Fu, L.J.; Liu, H.; Wu, Y.P.; Rahm, E.; Holze, R.; Wu, H.Q. Cathode materials modified by surface coating for lithium ion batteries. Electrochim. Acta 2006, 51, 3872–3883. [Google Scholar] [CrossRef]
- Mauger, A.; Julien, C. Surface modifications of electrode materials for lithium-ion batteries: Status and trends. Ionics 2014, 20, 751–787. [Google Scholar] [CrossRef]
- Oriňáková, R.; Fedorková, A.; Oriňák, A. Effect of PPy/PEG conducting polymer film on electrochemical performance of LiFePO4 cathode material for Li-ion batteries. Chem. Pap. 2013, 67, 860–875. [Google Scholar] [CrossRef]
- Li, F.-S.; Wu, Y.-S.; Chou, J.; Winter, M.; Wu, N.-L. A Mechanically Robust and Highly Ion-Conductive Polymer-Blend Coating for High-Power and Long-Life Lithium-Ion Battery Anodes. Adv. Mater. 2015, 27, 130–137. [Google Scholar] [CrossRef]
- Cao, Z.; Xu, P.; Zhai, H.; Du, S.; Mandal, J.; Dontigny, M.; Zaghib, K.; Yang, Y. Ambient-Air Stable Lithiated Anode for Rechargeable Li-Ion Batteries with High Energy Density. Nano Lett. 2016, 16, 7235–7240. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, G.; Liu, K.; Cui, Y. Design of Complex Nanomaterials for Energy Storage: Past Success and Future Opportunity. Acc. Chem. Res. 2017, 50, 2895–2905. [Google Scholar] [CrossRef]
- Wang, F.; Chen, G.; Zhang, N.; Liu, X.; Ma, R. Engineering of carbon and other protective coating layers for stabilizing silicon anode materials. Carbon Energy 2019, 1, 219–245. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, S.; Liu, H.; Liu, P. Protective coatings for lithium metal anodes: Recent progress and future perspectives. J. Power Sources 2020, 450, 227632. [Google Scholar] [CrossRef]
- Fedorov, R.G.; Maletti, S.; Heubner, C.; Michaelis, A.; Ein-Eli, Y. Molecular Engineering Approaches to Fabricate Artificial Solid-Electrolyte Interphases on Anodes for Li-Ion Batteries: A Critical Review. Adv. Energy Mater. 2021, 11, 2101173. [Google Scholar] [CrossRef]
- Pham, Q.-T.; Chern, C.-S. Applications of polymers in lithium-ion batteries with enhanced safety and cycle life. J. Polym. Res. 2022, 29, 124. [Google Scholar] [CrossRef]
- Chen, Z.; Soltani, A.; Chen, Y.; Zhang, Q.; Davoodi, A.; Hosseinpour, S.; Peukert, W.; Liu, W. Emerging Organic Surface Chemistry for Si Anodes in Lithium-Ion Batteries: Advances, Prospects, and Beyond. Adv. Energy Mater. 2022, 12, 2200924. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Z.; Pan, F.; Cui, Y.; Amine, K. High-performance anode materials for rechargeable lithium-ion batteries. Electrochem. Energy Rev. 2018, 1, 35–53. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, J.; Chew, S.Y.; Chen, J.; Guo, Z.P.; Zhao, L.; Konstantinov, K.; Liu, H.-K. Synthesis and characterization of SnO2–polypyrrole composite for lithium-ion battery. J. Power Sources 2007, 174, 1183–1187. [Google Scholar] [CrossRef]
- Shao, Q.-G.; Chen, W.-M.; Wang, Z.-H.; Qie, L.; Yuan, L.-X.; Zhang, W.-X.; Hu, X.-L.; Huang, Y.-H. SnO2-based composite coaxial nanocables with multi-walled carbon nanotube and polypyrrole as anode materials for lithium-ion batteries. Electrochem. Commun. 2011, 13, 1431–1434. [Google Scholar] [CrossRef]
- Liu, R.; Li, D.; Wang, C.; Li, N.; Li, Q.; Lü, X.; Spendelow, J.S.; Wu, G. Core–shell structured hollow SnO2–polypyrrole nanocomposite anodes with enhanced cyclic performance for lithium-ion batteries. Nano Energy 2014, 6, 73–81. [Google Scholar] [CrossRef]
- Cao, Z.; Yang, H.; Dou, P.; Wang, C.; Zheng, J.; Xu, X. Synthesis of three-dimensional hollow SnO2@PPy nanotube arrays via template-assisted method and chemical vapor-phase polymerization as high performance anodes for lithium-ion batteries. Electrochim. Acta 2016, 209, 700–708. [Google Scholar] [CrossRef]
- Guan, B.Y.; Yu, L.; Li, J.; Lou, X.W. A universal cooperative assembly-directed method for coating of mesoporous TiO2 nanoshells with enhanced lithium storage properties. Sci. Adv. 2016, 2, e1501554. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, D.; Zhong, H.; Zhang, Q.; Yang, J.; Zhang, L. Facile synthesis of nanostructured Li4Ti5O12/PEDOT:PSS composite as anode material for lithium-ion batteries. RSC Adv. 2016, 6, 95512–95517. [Google Scholar] [CrossRef]
- Sun, X.; Lu, X.; Huang, S.; Xi, L.; Liu, L.; Liu, B.; Weng, Q.; Zhang, L.; Schmidt, O.G. Reinforcing Germanium Electrode with Polymer Matrix Decoration for Long Cycle Life Rechargeable Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 38556–38566. [Google Scholar] [CrossRef]
- Xu, D.; Wang, P.; Yang, R. Conducting polythiophene-wrapped Li4Ti5O12 spinel anode material for ultralong cycle-life Li-ion batteries. Ceram. Int. 2017, 43, 4712–4715. [Google Scholar] [CrossRef]
- Zhou, Y.; Jin, X.; Ni, J.; Zhang, S.; Yang, J.; Liu, P.; Wang, Z.; Lei, J. Evaporation induced uniform polypyrrole coating on CuO arrays for free-standing high lithium storage anode. J. Solid State Electrochem. 2019, 23, 1829–1836. [Google Scholar] [CrossRef]
- Ding, Z.; Zhang, Q.; Chen, Y.; Liu, G.; Xin, X.; He, H.; Cai, B.; Wu, J.; Yao, X. PEDOT-PSS coated VS2 nanosheet anodes for high rate and ultrastable lithium-ion batteries. New J. Chem. 2019, 43, 1681–1687. [Google Scholar] [CrossRef]
- Tolganbek, N.; Mentbayeva, A.; Serik, N.; Batyrgali, N.; Naizakarayev, M.; Kanamura, K.; Bakenov, Z. Design and preparation of thin film gel polymer electrolyte for 3D Li-ion battery. J. Power Sources 2021, 493, 229686. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, D.; Jiang, L.; Liang, F.; Rui, Y.; Tang, B. One-step synthesis based on non-aqueous sol-gel conductive polymer-coated SnO2 nanoparticles as advanced anode materials for lithium-ion batteries. J. Alloys Compd. 2022, 899, 163274. [Google Scholar] [CrossRef]
- Abdollahifar, M.; Vinograd, A.; Lu, C.-Y.; Chang, S.-J.; Müller, J.; Frankenstein, L.; Placke, T.; Kwade, A.; Winter, M.; Chao, C.-Y. Enabling Long-Cycling Life of Si-on-Graphite Composite Anodes via Fabrication of a Multifunctional Polymeric Artificial Solid–Electrolyte Interphase Protective Layer. ACS Appl. Mater. Interfaces 2022, 34, 38824–38834. [Google Scholar] [CrossRef]
- Seo, J.; Hyun, S.; Moon, J.; Lee, J.Y.; Kim, C. High Performance of a Polydopamine-Coated Graphite Anode with a Stable SEI Layer. ACS Appl. Energy Mater. 2022, 5, 5610–5616. [Google Scholar] [CrossRef]
- Liu, Y.; Matsumura, T.; Imanishi, N.; Hirano, A.; Ichikawa, T.; Takeda, Y. Preparation and Characterization of Si/C Composite Coated with Polyaniline as Novel Anodes for Li-Ion Batteries. Electrochem. Solid State Lett. 2005, 8, A599. [Google Scholar] [CrossRef]
- Xiao, W.; Chen, J.S.; Lu, Q.; Lou, X.W. Porous spheres assembled from polythiophene (PTh)-coated ultrathin MnO2 nanosheets with enhanced lithium storage capabilities. J. Phys. Chem. C 2010, 114, 12048–12051. [Google Scholar] [CrossRef]
- Jeong, J.-M.; Choi, B.G.; Lee, S.C.; Lee, K.G.; Chang, S.-J.; Han, Y.-K.; Lee, Y.B.; Lee, H.U.; Kwon, S.; Lee, G. Hierarchical hollow spheres of Fe2O3@ polyaniline for lithium ion battery anodes. Adv. Mater. 2013, 25, 6250–6255. [Google Scholar] [CrossRef]
- Han, F.; Li, D.; Li, W.-C.; Lei, C.; Sun, Q.; Lu, A.-H. Nanoengineered Polypyrrole-Coated Fe2O3@ C Multifunctional Composites with an Improved Cycle Stability as Lithium-Ion Anodes. Adv. Funct. Mater. 2013, 23, 1692–1700. [Google Scholar] [CrossRef]
- Du, F.-H.; Li, B.; Fu, W.; Xiong, Y.-J.; Wang, K.-X.; Chen, J.-S. Surface binding of polypyrrole on porous Silicon hollow nanospheres for Li-ion battery anodes with high structure Stability. Adv. Mater. 2014, 26, 6145–6150. [Google Scholar] [CrossRef]
- Luo, L.; Zhao, P.; Yang, H.; Liu, B.; Zhang, J.-G.; Cui, Y.; Yu, G.; Zhang, S.; Wang, C.-M. Surface Coating Constraint Induced Self-Discharging of Silicon Nanoparticles as Anodes for Lithium Ion Batteries. Nano Lett. 2015, 15, 7016–7022. [Google Scholar] [CrossRef]
- Yin, Z.; Fan, W.; Ding, Y.; Li, J.; Guan, L.; Zheng, Q. Shell Structure Control of PPy-Modified CuO Composite Nanoleaves for Lithium Batteries with Improved Cyclic Performance. ACS Sustain. Chem. Eng. 2015, 3, 507–517. [Google Scholar] [CrossRef]
- Ma, H.; Liu, X.; Zhang, D.; Xiang, J. Synthesis of polyaniline shell on nickel oxide nanoflake arrays for enhanced lithium ion storage. Mater. Res. Bull. 2017, 96, 301–305. [Google Scholar] [CrossRef]
- Gu, H.; Chen, F.; Liu, C.; Qian, J.; Ni, M.; Liu, T. Scalable fabrication of core-shell structured Li4Ti5O12/PPy particles embedded in N-doped graphene networks as advanced anode for lithium-ion batteries. J. Power Sources 2017, 369, 42–49. [Google Scholar] [CrossRef]
- Attia, E.N.; Hassan, F.M.; Li, M.; Batmaz, R.; Elkamel, A.; Chen, Z. Tailoring the chemistry of blend copolymers boosting the electrochemical performance of Si-based anodes for lithium ion batteries. J. Mater. Chem. A 2017, 5, 24159–24167. [Google Scholar] [CrossRef]
- Fu, R.; Nie, P.; Shi, M.; Wang, J.; Jiang, J.; Zhang, Y.; Wu, Y.; Fang, S.; Dou, H.; Zhang, X. Rigid Polyimide Buffering Layer Enabling Silicon Nanoparticles Prolonged Cycling Life for Lithium Storage. ACS Appl. Energy Mater. 2018, 1, 948–955. [Google Scholar] [CrossRef]
- Shen, B.H.; Wang, S.; Tenhaeff, W.E. Ultrathin conformal polycyclosiloxane films to improve silicon cycling stability. Sci. Adv. 2019, 5, eaaw4856. [Google Scholar] [CrossRef]
- Shi, W.; Wu, H.B.; Baucom, J.; Li, X.; Ma, S.; Chen, G.; Lu, Y. Covalently Bonded Si–Polymer Nanocomposites Enabled by Mechanochemical Synthesis as Durable Anode Materials. ACS Appl. Mater. Interfaces 2020, 12, 39127–39134. [Google Scholar] [CrossRef]
- Hou, L.; Bao, R.; kionga Denis, D.; Sun, X.; Zhang, J.; uz Zaman, F.; Yuan, C. Synthesis of ultralong ZnFe2O4@ polypyrrole nanowires with enhanced electrochemical Li-storage behaviors for lithium-ion batteries. Electrochim. Acta 2019, 306, 198–208. [Google Scholar] [CrossRef]
- Li, Z.-F.; Zhang, H.; Liu, Q.; Liu, Y.; Stanciu, L.; Xie, J. Novel pyrolyzed polyaniline-grafted silicon nanoparticles encapsulated in graphene sheets as Li-ion battery anodes. ACS Appl. Mater. Interfaces 2014, 6, 5996–6002. [Google Scholar] [CrossRef]
- Chen, X.; Cao, Z.; Xing, L.; Liao, Y.; Qiu, Y.; Li, W. Improved Li-storage performance with PEDOT-decorated MnO2 nanoboxes. Nanoscale 2017, 9, 18467–18473. [Google Scholar] [CrossRef]
- Yue, H.; Du, T.; Wang, Q.; Shi, Z.; Dong, H.; Cao, Z.; Qiao, Y.; Yin, Y.; Xing, R.; Yang, S. Biomimetic Synthesis of Polydopamine Coated ZnFe2O4 Composites as Anode Materials for Lithium-Ion Batteries. ACS Omega 2018, 3, 2699–2705. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, S.H.; Kim, G.; Heo, S.; Mun, J.; Han, S.; Jung, H.; Kyoung, Y.K.; Yun, D.J.; Baek, W.J.; et al. Protective Oxide Coating for Ionic Conductive Solid Electrolyte Interphase. ACS Appl. Mater. Interfaces 2016, 8, 30980–30984. [Google Scholar] [CrossRef]
- Li, F.-S.; Wu, Y.-S.; Chou, J.; Wu, N.-L. A dimensionally stable and fast-discharging graphite–silicon composite Li-ion battery anode enabled by electrostatically self-assembled multifunctional polymer-blend coating. Chem. Commun. 2015, 51, 8429–8431. [Google Scholar] [CrossRef]
- Liu, J.; Xu, J.; Chen, Y.; Sun, W.; Zhou, X.; Ke, J. Synthesis and electrochemical performance of a PEDOT: pSS@ Ge composite as the anode materials for lithium-ion batteries. Int. J. Electrochem. Sci. 2019, 14, 359–370. [Google Scholar] [CrossRef]
- Wu, Y.S.; Lyu, P.R. Double-Coated Hard Carbon as an Anode Material for High C-Rate Lithium Ion Batteries. Mater. Sci. Forum 2018, 921, 105–110. [Google Scholar] [CrossRef]
- Nieradko, M.; Eskandarian, L.; Semenikhin, O.A. Aluminum anodes coated with polymer electrolyte show improved reversibility and cycling ability in Li-Ion batteries. Electrochim. Acta 2019, 327, 135023. [Google Scholar] [CrossRef]
- Abdollahifar, M.; Doose, S.; Cavers, H.; Kwade, A. Graphite Recycling from End-of-Life Lithium-Ion Batteries: Processes and Applications. Adv. Mater. Technol. 2022, 2200368. [Google Scholar] [CrossRef]
- Abdollahifar, M.; Molaiyan, P.; Lassi, U.; Wu, N.L.; Kwade, A. Multifunctional behaviour of graphite in lithium–sulfur batteries. Renew. Sustain. Energy Rev. 2022, 169, 112948. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Ren, D.; Wang, L.; He, X. Graphite as anode materials: Fundamental mechanism, recent progress and advances. Energy Storage Mater. 2021, 36, 147–170. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Xie, S.; Chen, C.H. Pyrolytic polyurea encapsulated natural graphite as anode material for lithium ion batteries. Electrochim. Acta 2005, 50, 4728–4735. [Google Scholar] [CrossRef]
- Pan, Q.; Wang, H.; Jiang, Y. Natural graphite modified with nitrophenyl multilayers as anode materials for lithium ion batteries. J. Mater. Chem. 2007, 17, 329–334. [Google Scholar] [CrossRef]
- Chao, D.; Xia, X.; Liu, J.; Fan, Z.; Ng, C.F.; Lin, J.; Zhang, H.; Shen, Z.X.; Fan, H.J. A V2O5/conductive-polymer core/shell nanobelt array on three-dimensional graphite foam: A high-rate, ultrastable, and freestanding cathode for lithium-ion batteries. Adv. Mater. 2014, 26, 5794–5800. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material–fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Ui, K.; Kikuchi, S.; Mikami, F.; Kadoma, Y.; Kumagai, N. Improvement of electrochemical characteristics of natural graphite negative electrode coated with polyacrylic acid in pure propylene carbonate electrolyte. J. Power Sources 2007, 173, 518–521. [Google Scholar] [CrossRef][Green Version]
- Shi, Q.; Liu, W.; Qu, Q.; Gao, T.; Wang, Y.; Liu, G.; Battaglia, V.S.; Zheng, H. Robust solid/electrolyte interphase on graphite anode to suppress lithium inventory loss in lithium-ion batteries. Carbon 2017, 111, 291–298. [Google Scholar] [CrossRef]
- Müller, J.; Abdollahifar, M.; Vinograd, A.; Nöske, M.; Nowak, C.; Chang, S.-J.; Placke, T.; Haselrieder, W.; Winter, M.; Kwade, A. Si-on-Graphite fabricated by fluidized bed process for high-capacity anodes of Li-ion batteries. Chem. Eng. J. 2021, 407, 126603. [Google Scholar] [CrossRef]
- Müller, J.; Abdollahifar, M.; Doose, S.; Michalowski, P.; Wu, N.-L.; Kwade, A. Effects of carbon coating on calendered nano-silicon graphite composite anodes of LiB. J. Power Sources 2022, 548, 232000. [Google Scholar] [CrossRef]
- Komaba, S.; Okushi, K.; Groult, H. Polyacrylate as modifier for graphite/electrolyte interface. ECS Trans. 2008, 11, 63. [Google Scholar] [CrossRef]
- Nie, M.; Abraham, D.P.; Seo, D.M.; Chen, Y.; Bose, A.; Lucht, B.L. Role of solution structure in solid electrolyte interphase formation on graphite with LiPF6 in propylene carbonate. J. Phys. Chem. C 2013, 117, 25381–25389. [Google Scholar] [CrossRef]
- Xu, K.; Zhang, S.; Poese, B.A.; Jow, T.R. Lithium bis (oxalato) borate stabilizes graphite anode in propylene carbonate. Electrochem. Solid State Lett. 2002, 5, A259. [Google Scholar] [CrossRef]
- Luo, J.; Fang, C.-C.; Wu, N.-L. High polarity poly (vinylidene difluoride) thin coating for dendrite-free and high-performance lithium metal anodes. Adv. Energy Mater. 2018, 8, 1701482. [Google Scholar] [CrossRef]
- Lau, K.K.S.; Gleason, K.K. Initiated chemical vapor deposition (iCVD) of poly (alkyl acrylates): An experimental study. Macromolecules 2006, 39, 3688–3694. [Google Scholar] [CrossRef]
- Martin, T.P.; Lau, K.K.S.; Chan, K.; Mao, Y.; Gupta, M.; O’Shaughnessy, W.S.; Gleason, K.K. Initiated chemical vapor deposition (iCVD) of polymeric nanocoatings. Surf. Coat. Technol. 2007, 201, 9400–9405. [Google Scholar] [CrossRef]
- Subramanian, V.; Karki, A.; Gnanasekar, K.I.; Eddy, F.P.; Rambabu, B. Nanocrystalline TiO2 (anatase) for Li-ion batteries. J. Power Sources 2006, 159, 186–192. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Xu, H.; Wang, L.; Lu, X.; He, X. Li4Ti5O12 spinel anode: Fundamentals and advances in rechargeable batteries. InfoMat 2022, 4, e12228. [Google Scholar] [CrossRef]
- Chen, Z.; Belharouak, I.; Sun, Y.-K.; Amine, K. Titanium-based anode materials for safe lithium-ion batteries. Adv. Funct. Mater. 2013, 23, 959–969. [Google Scholar] [CrossRef]
- Yi, T.-F.; Zhu, Y.-R.; Tao, W.; Luo, S.; Xie, Y.; Li, X.-F. Recent advances in the research of MLi2Ti6O14 (M= 2Na, Sr, Ba, Pb) anode materials for Li-ion batteries. J. Power Sources 2018, 399, 26–41. [Google Scholar] [CrossRef]
- Lai, C.; Li, G.R.; Dou, Y.Y.; Gao, X.P. Mesoporous polyaniline or polypyrrole/anatase TiO2 nanocomposite as anode materials for lithium-ion batteries. Electrochim. Acta 2010, 55, 4567–4572. [Google Scholar] [CrossRef]
- Zheng, H.; Ncube, N.M.; Raju, K.; Mphahlele, N.; Mathe, M. The effect of polyaniline on TiO2 nanoparticles as anode materials for lithium ion batteries. SpringerPlus 2016, 5, 630. [Google Scholar] [CrossRef]
- Lee, J.-K.; Kung, M.C.; Trahey, L.; Missaghi, M.N.; Kung, H.H. Nanocomposites derived from phenol-functionalized Si nanoparticles for high performance lithium ion battery anodes. Chem. Mater. 2009, 21, 6–8. [Google Scholar] [CrossRef]
- Li, J.; Huang, J. A nanofibrous polypyrrole/silicon composite derived from cellulose substance as the anode material for lithium-ion batteries. Chem. Commun. 2015, 51, 14590–14593. [Google Scholar] [CrossRef]
- Yasar-Inceoglu, O.; Zhong, L.; Mangolini, L. Core/shell silicon/polyaniline particles via in-flight plasma-induced polymerization. J. Phys. D Appl. Phys. 2015, 48, 314009. [Google Scholar] [CrossRef]
- Mu, T.; Zhao, Y.; Zhao, C.; Holmes, N.G.; Lou, S.; Li, J.; Li, W.; He, M.; Sun, Y.; Du, C.; et al. Stable Silicon Anodes by Molecular Layer Deposited Artificial Zincone Coatings. Adv. Funct. Mater. 2021, 31, 2010526. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, Q.; Zhao, Y.; He, R.; Xu, M.; Feng, S.; Li, S.; Zhou, L.; Mai, L. Silicon oxides: A promising family of anode materials for lithium-ion batteries. Chem. Soc. Rev. 2019, 48, 285–309. [Google Scholar] [CrossRef]
- Chou, C.-Y.; Hwang, G.S. On the origin of the significant difference in lithiation behavior between silicon and germanium. J. Power Sources 2014, 263, 252–258. [Google Scholar] [CrossRef]
- Zheng, H.; Fang, S.; Tong, Z.; Dou, H.; Zhang, X. Porous Silicon@Polythiophene Core–Shell Nanospheres for Lithium-Ion Batteries. Part. Part. Syst. Charact. 2016, 33, 75–81. [Google Scholar] [CrossRef]
- Wu, H.; Cui, Y. Designing nanostructured Si anodes for high energy lithium ion batteries. Nano Today 2012, 7, 414–429. [Google Scholar] [CrossRef]
- Jin, Y.; Zhu, B.; Lu, Z.; Liu, N.; Zhu, J. Challenges and recent progress in the development of Si anodes for lithium-ion battery. Adv. Energy Mater. 2017, 7, 1700715. [Google Scholar] [CrossRef]
- Wang, G.X.; Ahn, J.H.; Yao, J.; Bewlay, S.; Liu, H.K. Nanostructured Si–C composite anodes for lithium-ion batteries. Electrochem. Commun. 2004, 6, 689–692. [Google Scholar] [CrossRef]
- Dou, F.; Shi, L.; Chen, G.; Zhang, D. Silicon/carbon composite anode materials for lithium-ion batteries. Electrochem. Energy Rev. 2019, 2, 149–198. [Google Scholar] [CrossRef]
- Wu, H.; Yu, G.; Pan, L.; Liu, N.; McDowell, M.T.; Bao, Z.; Cui, Y. Stable Li-ion battery anodes by in-situ polymerization of conducting hydrogel to conformally coat silicon nanoparticles. Nat. Commun. 2013, 4, 1943. [Google Scholar] [CrossRef]
- Huang, Y.; Li, H.; Wang, Z.; Zhu, M.; Pei, Z.; Xue, Q.; Huang, Y.; Zhi, C. Nanostructured polypyrrole as a flexible electrode material of supercapacitor. Nano Energy 2016, 22, 422–438. [Google Scholar] [CrossRef]
- Song, H.-K.; Palmore, G.T.R. Redox-active polypyrrole: Toward polymer-based batteries. Adv. Mater. 2006, 18, 1764–1768. [Google Scholar] [CrossRef]
- Wang, G.X.; Yang, L.; Chen, Y.; Wang, J.Z.; Bewlay, S.; Liu, H.K. An investigation of polypyrrole-LiFePO4 composite cathode materials for lithium-ion batteries. Electrochim. Acta 2005, 50, 4649–4654. [Google Scholar] [CrossRef]
- Fu, Y.; Su, Y.-S.; Manthiram, A. Sulfur-polypyrrole composite cathodes for lithium-sulfur batteries. J. Electrochem. Soc. 2012, 159, A1420. [Google Scholar] [CrossRef]
- Karimi, A.; Kazeminezhad, I.; Naderi, L.; Shahrokhian, S. Construction of a Ternary Nanocomposite, Polypyrrole/Fe–Co Sulfide-Reduced Graphene Oxide/Nickel Foam, as a Novel Binder-Free Electrode for High-Performance Asymmetric Supercapacitors. J. Phys. Chem. C 2020, 124, 4393–4407. [Google Scholar] [CrossRef]
- Guo, Z.P.; Wang, J.Z.; Liu, H.K.; Dou, S.X. Study of silicon/polypyrrole composite as anode materials for Li-ion batteries. J. Power Sources 2005, 146, 448–451. [Google Scholar] [CrossRef]
- Chew, S.Y.; Guo, Z.P.; Wang, J.Z.; Chen, J.; Munroe, P.; Ng, S.H.; Zhao, L.; Liu, H.K. Novel nano-silicon/polypyrrole composites for lithium storage. Electrochem. Commun. 2007, 9, 941–946. [Google Scholar] [CrossRef]
- Magasinski, A.; Dixon, P.; Hertzberg, B.; Kvit, A.; Ayala, J.; Yushin, G. High-performance lithium-ion anodes using a hierarchical bottom-up approach. Nat. Mater. 2010, 9, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, I.; Zdyrko, B.; Magasinski, A.; Hertzberg, B.; Milicev, Z.; Burtovyy, R.; Luzinov, I.; Yushin, G. A major constituent of brown algae for use in high-capacity Li-ion batteries. Science 2011, 334, 75–79. [Google Scholar] [CrossRef]
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-C.; Liu, W.-M.; Li, H.-L. Titanium dioxide doped polyaniline. Mater. Sci. Eng. C 2005, 25, 444–447. [Google Scholar] [CrossRef]
- Mu, G.; Ding, Z.; Mu, D.; Wu, B.; Bi, J.; Zhang, L.; Yang, H.; Wu, H.; Wu, F. Hierarchical void structured Si/PANi/C hybrid anode material for high-performance lithium-ion batteries. Electrochim. Acta 2019, 300, 341–348. [Google Scholar] [CrossRef]
- Pan, S.; Han, J.; Wang, Y.; Li, Z.; Chen, F.; Guo, Y.; Han, Z.; Xiao, K.; Yu, Z.; Yu, M. Integrating SEI into Layered Conductive Polymer Coatings for Ultrastable Silicon Anodes. Adv. Mater. 2022, 34, 2203617. [Google Scholar] [CrossRef]
- Yoon, J.H.; Lee, G.; Li, P.; Baik, H.; Yi, G.-R.; Park, J.H. Expandable crosslinked polymer coatings on silicon nanoparticle anode toward high-rate and long-cycle-life lithium-ion battery. Appl. Surf. Sci. 2022, 571, 151294. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, M.; Chen, G.; Dudko, N.; Li, Y.; Liu, H.; Shi, L.; Wu, G.; Zhang, D. High-Performance Microsized Si Anodes for Lithium-Ion Batteries: Insights into the Polymer Configuration Conversion Mechanism. Adv. Mater. 2022, 34, 2109658. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Wang, Y.; Zeng, Y.; Yu, M.; Liu, T.; Chen, J.; Wang, K.; Xie, J.; Li, L. Boosting Cyclability and Rate Capability of SiOx via Dopamine Polymerization-Assisted Hybrid Graphene Coating for Advanced Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 17388–17395. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-W.; Wang, J.-Z.; Chou, S.-L.; Liu, H.-K. Synthesis and electrochemical performance of LiV3O8/polyaniline as cathode material for the lithium battery. J. Power Sources 2012, 220, 47–53. [Google Scholar] [CrossRef]
- Gittleson, F.S.; Hwang, J.; Sekol, R.C.; Taylor, A.D. Polymer coating of vanadium oxide nanowires to improve cathodic capacity in lithium batteries. J. Mater. Chem. A 2013, 1, 7979–7984. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Shin, W.-K.; Kannan, A.G.; Koo, S.M.; Kim, D.-W. Improvement of the Cycling Performance and Thermal Stability of Lithium-Ion Cells by Double-Layer Coating of Cathode Materials with Al2O3 Nanoparticles and Conductive Polymer. ACS Appl. Mater. Interfaces 2015, 7, 13944–13951. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Cho, J.-H.; Kim, J.-S.; Shim, E.-G.; Lee, S.-Y. High-voltage cell performance and thermal stability of nanoarchitectured polyimide gel polymer electrolyte-coated LiCoO2 cathode materials. Electrochim. Acta 2012, 86, 346–351. [Google Scholar] [CrossRef]
- Jiang, B.; He, Y.; Li, B.; Zhao, S.; Wang, S.; He, Y.-B.; Lin, Z. Polymer-Templated Formation of Polydopamine-Coated SnO2 Nanocrystals: Anodes for Cyclable Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2017, 56, 1869–1872. [Google Scholar] [CrossRef]
- Fan, X.; Jiang, A.; Dou, P.; Ma, D.; Xu, X. Three-dimensional ultrathin Sn/polypyrrole nanosheet network as high performance lithium-ion battery anode. RSC Adv. 2014, 4, 52074–52082. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Yu, J.; Han, Y.; Lu, Z. Understanding the role of conductive polymer in thermal lithiation and battery performance of Li-Sn alloy anode. Energy Storage Mater. 2019, 20, 7–13. [Google Scholar] [CrossRef]
- Cao, Z.; Meng, H.; Dou, P.; Wang, C.; Zheng, J.; Xu, X. Effects of solid polymer electrolyte coating on the composition and morphology of the solid electrolyte interphase on Sn anodes. J. Solid State Electrochem. 2017, 21, 955–966. [Google Scholar] [CrossRef]
- Cui, L.; Shen, J.; Cheng, F.; Tao, Z.; Chen, J. SnO2 nanoparticles@ polypyrrole nanowires composite as anode materials for rechargeable lithium-ion batteries. J. Power Sources 2011, 196, 2195–2201. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, Y.; Chen, J.; Yang, X. Double shelled hollow SnO2/polymer microsphere as a high-capacity anode material for superior reversible lithium ion storage. J. Mater. Chem. A 2015, 3, 1068–1076. [Google Scholar] [CrossRef]
- Li, S.; Wi, T.-U.; Ji, M.; Cui, Z.; Lee, H.-W.; Lu, Z. The Role of Polymer and Inorganic Coatings to Enhance Interparticle Connections Diagnosed by In Situ Techniques. Nano Lett. 2021, 21, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Bi, R.; Yang, M.; Gao, W.; Wang, J. Coating conductive polypyrrole layers on multiple shells of hierarchical SnO2 spheres and their enhanced cycling stability as lithium-ion battery anode. Appl. Surf. Sci. 2022, 586, 152836. [Google Scholar] [CrossRef]
- Sher Shah, M.S.A.; Muhammad, S.; Park, J.H.; Yoon, W.-S.; Yoo, P.J. Incorporation of PEDOT:PSS into SnO2/reduced graphene oxide nanocomposite anodes for lithium-ion batteries to achieve ultra-high capacity and cyclic stability. RSC Adv. 2015, 5, 13964–13971. [Google Scholar] [CrossRef]
- Li, H.; Zhang, B.; Ou, X.; Zhou, Q.; Wang, C.; Peng, C.; Zhang, J. Core-Shell Structure of SnO2@C/PEDOT: PSS Microspheres with Dual Protection Layers for Enhanced Lithium Storage Performance. ChemElectroChem 2019, 6, 2182–2188. [Google Scholar] [CrossRef]
- Guo, J.; Chen, L.; Wang, G.; Zhang, X.; Li, F. In situ synthesis of SnO2–Fe2O3@ polyaniline and their conversion to SnO2–Fe2O3@ C composite as fully reversible anode material for lithium-ion batteries. J. Power Sources 2014, 246, 862–867. [Google Scholar] [CrossRef]
- Fan, X.; Dou, P.; Jiang, A.; Ma, D.; Xu, X. One-Step Electrochemical Growth of a Three-Dimensional Sn–Ni@PEO Nanotube Array as a High Performance Lithium-Ion Battery Anode. ACS Appl. Mater. Interfaces 2014, 6, 22282–22288. [Google Scholar] [CrossRef]
- Gowda, S.R.; Reddy, A.L.M.; Shaijumon, M.M.; Zhan, X.; Ci, L.; Ajayan, P.M. Conformal coating of thin polymer electrolyte layer on nanostructured electrode materials for three-dimensional battery applications. Nano Lett. 2011, 11, 101–106. [Google Scholar] [CrossRef]
- Dou, P.; Cao, Z.; Zheng, J.; Wang, C.; Xu, X. Solid polymer electrolyte coating three-dimensional Sn/Ni bimetallic nanotube arrays for high performance lithium-ion battery anodes. J. Alloys Compd. 2016, 685, 690–698. [Google Scholar] [CrossRef]
- Xiao, X.; Li, X.; Zheng, S.; Shao, J.; Xue, H.; Pang, H. Nanostructured germanium anode materials for advanced rechargeable batteries. Adv. Mater. Interfaces 2017, 4, 1600798. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, S.; Zhang, C.; Cui, G. High performance germanium-based anode materials. Coord. Chem. Rev. 2016, 326, 34–85. [Google Scholar] [CrossRef]
- Li, X.; Yang, Z.; Fu, Y.; Qiao, L.; Li, D.; Yue, H.; He, D. Germanium anode with excellent lithium storage performance in a germanium/lithium–cobalt oxide lithium-ion battery. ACS Nano 2015, 9, 1858–1867. [Google Scholar] [CrossRef]
- Seng, K.H.; Park, M.-H.; Guo, Z.P.; Liu, H.K.; Cho, J. Self-assembled germanium/carbon nanostructures as high-power anode material for the lithium-ion battery. Angew. Chem. 2012, 124, 5755–5759. [Google Scholar] [CrossRef]
- Liu, X.; Wu, X.-Y.; Chang, B.; Wang, K.-X. Recent progress on germanium-based anodes for lithium ion batteries: Efficient lithiation strategies and mechanisms. Energy Storage Mater. 2020, 30, 146–169. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Wang, Y.; Li, H.; Peng, Y. The application of nanostructured transition metal sulfides as anodes for lithium ion batteries. J. Energy Chem. 2018, 27, 1536–1554. [Google Scholar] [CrossRef]
- Nam, K.-H.; Jeon, K.-J.; Park, C.-M. Layered germanium phosphide-based anodes for high-performance lithium-and sodium-ion batteries. Energy Storage Mater. 2019, 17, 78–87. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, C.; Li, Z.; Liang, Q.; Zhu, B.; Chai, J.; Cheng, X.; Zheng, P.; Zheng, Y.; Liu, Z. Layered Tin Phosphide Composites as Promising Anodes for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 11306–11313. [Google Scholar] [CrossRef]
- Tseng, K.-W.; Huang, S.-B.; Chang, W.-C.; Tuan, H.-Y. Synthesis of mesoporous germanium phosphide microspheres for high-performance lithium-ion and sodium-ion battery anodes. Chem. Mater. 2018, 30, 4440–4447. [Google Scholar] [CrossRef]
- Lin, H.-P.; Chen, K.-T.; Chang, C.-B.; Tuan, H.-Y. Aluminum phosphide as a high-performance lithium-ion battery anode. J. Power Sources 2020, 465, 228262. [Google Scholar] [CrossRef]
- Chae, S.; Park, S.; Ahn, K.; Nam, G.; Lee, T.; Sung, J.; Kim, N.; Cho, J. Gas phase synthesis of amorphous silicon nitride nanoparticles for high-energy LIBs. Energy Environ. Sci. 2020, 13, 1212–1221. [Google Scholar] [CrossRef]
- De Guzman, R.C.; Yang, J.; Cheng, M.M.-C.; Salley, S.O.; Ng, K.S. High capacity silicon nitride-based composite anodes for lithium ion batteries. J. Mater. Chem. A 2014, 2, 14577–14584. [Google Scholar] [CrossRef]
- Zhang, M.; Qiu, Y.; Han, Y.; Guo, Y.; Cheng, F. Three-dimensional tungsten nitride nanowires as high performance anode material for lithium ion batteries. J. Power Sources 2016, 322, 163–168. [Google Scholar] [CrossRef]
- Li, C.; Zhu, L.; Qi, S.; Ge, W.; Ma, W.; Zhao, Y.; Huang, R.; Xu, L.; Qian, Y. Ultrahigh-areal-capacity battery anodes enabled by free-standing vanadium nitride@ N-doped carbon/graphene architecture. ACS Appl. Mater. Interfaces 2020, 12, 49607–49616. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, Y.; Murali, S.; Stoller, M.D.; Ruoff, R.S. Nanostructured reduced graphene oxide/Fe2O3 composite as a high-performance anode material for lithium ion batteries. ACS Nano 2011, 5, 3333–3338. [Google Scholar] [CrossRef]
- Jiang, T.; Bu, F.; Feng, X.; Shakir, I.; Hao, G.; Xu, Y. Porous Fe2O3 nanoframeworks encapsulated within three-dimensional graphene as high-performance flexible anode for lithium-ion battery. ACS Nano 2017, 11, 5140–5147. [Google Scholar] [CrossRef]
- Reddy, M.V.; Yu, T.; Sow, C.-H.; Shen, Z.X.; Lim, C.T.; Subba Rao, G.V.; Chowdari, B.V.R. α-Fe2O3 nanoflakes as an anode material for Li-ion batteries. Adv. Funct. Mater. 2007, 17, 2792–2799. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, D.; Li, Y.; Yuan, T.; Bahlawane, N.; Liang, C.; Sun, W.; Lu, Y.; Yan, M. Amorphous Fe2O3 as a high-capacity, high-rate and long-life anode material for lithium ion batteries. Nano Energy 2014, 4, 23–30. [Google Scholar] [CrossRef]
- He, C.; Wu, S.; Zhao, N.; Shi, C.; Liu, E.; Li, J. Carbon-encapsulated Fe3O4 nanoparticles as a high-rate lithium ion battery anode material. ACS Nano 2013, 7, 4459–4469. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, D.-W.; Li, F.; Zhang, L.; Li, N.; Wu, Z.-S.; Wen, L.; Lu, G.Q.; Cheng, H.-M. Graphene-wrapped Fe3O4 anode material with improved reversible capacity and cyclic stability for lithium ion batteries. Chem. Mater. 2010, 22, 5306–5313. [Google Scholar] [CrossRef]
- Kopuklu, B.B.; Tasdemir, A.; Gursel, S.A.; Yurum, A. High stability graphene oxide aerogel supported ultrafine Fe3O4 particles with superior performance as a Li-ion battery anode. Carbon 2021, 174, 158–172. [Google Scholar] [CrossRef]
- Salimi, P.; Norouzi, O.; Pourhosseini, S.E.M. Two-step synthesis of nanohusk Fe3O4 embedded in 3D network pyrolytic marine biochar for a new generation of anode materials for Lithium-Ion batteries. J. Alloys Compd. 2019, 786, 930–937. [Google Scholar] [CrossRef]
- Ma, J.; Kong, Y.; Liu, S.; Li, Y.; Jiang, J.; Zhou, Q.; Huang, Y.; Han, S. Flexible phosphorus-doped graphene/metal–organic framework-derived porous Fe2O3 anode for lithium-ion battery. ACS Appl. Energy Mater. 2020, 3, 11900–11906. [Google Scholar] [CrossRef]
- Park, J.; Yoo, H.; Choi, J. 3D ant-nest network of α-Fe2O3 on stainless steel for all-in-one anode for Li-ion battery. J. Power Sources 2019, 431, 25–30. [Google Scholar] [CrossRef]
- Wang, Z.; Luan, D.; Madhavi, S.; Li, C.M.; Lou, X.W.D. α-Fe2O3 nanotubes with superior lithium storage capability. Chem. Commun. 2011, 47, 8061–8063. [Google Scholar] [CrossRef]
- Zhou, W.; Cheng, C.; Liu, J.; Tay, Y.Y.; Jiang, J.; Jia, X.; Zhang, J.; Gong, H.; Hng, H.H.; Yu, T. Epitaxial growth of branched α-Fe2O3/SnO2 nano-heterostructures with improved lithium-ion battery performance. Adv. Funct. Mater. 2011, 21, 2439–2445. [Google Scholar] [CrossRef]
- Wang, Z.; Luan, D.; Madhavi, S.; Hu, Y.; Lou, X.W.D. Assembling carbon-coated α-Fe2O3 hollow nanohorns on the CNT backbone for superior lithium storage capability. Energy Environ. Sci. 2012, 5, 5252–5256. [Google Scholar] [CrossRef]
- Xu, X.; Cao, R.; Jeong, S.; Cho, J. Spindle-like mesoporous α-Fe2O3 anode material prepared from MOF template for high-rate lithium batteries. Nano Lett. 2012, 12, 4988–4991. [Google Scholar] [CrossRef]
- Zou, Y.; Kan, J.; Wang, Y. Fe2O3-graphene rice-on-sheet nanocomposite for high and fast lithium ion storage. J. Phys. Chem. C 2011, 115, 20747–20753. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, W.; Lai, L.; Yang, H.; Lim, S.H.; Zhen, Y.; Yu, T.; Shen, Z.; Lin, J. Three dimensionals α-Fe2O3/polypyrrole (Ppy) nanoarray as anode for micro lithium ion batteries. Nano Energy 2013, 2, 726–732. [Google Scholar] [CrossRef]
- Applestone, D.; Manthiram, A. Cu 6 Sn 5–TiC–C nanocomposite alloy anodes with high volumetric capacity for lithium ion batteries. RSC Adv. 2012, 2, 5411–5417. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, S.; Liu, W.; Du, Z.; Fang, H. Fe3O4/PPy composite nanospheres as anode for lithium-ion batteries with superior cycling performance. Electrochim. Acta 2014, 121, 428–433. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Han, H.; Zhao, Y.; Ma, W.; Sun, H. Polyaniline coated Fe3O4 hollow nanospheres as anode materials for lithium ion batteries. Sustain. Energy Fuels 2017, 1, 915–922. [Google Scholar] [CrossRef]
- Wei, W.; Cui, X.; Chen, W.; Ivey, D.G. Manganese oxide-based materials as electrochemical supercapacitor electrodes. Chem. Soc. Rev. 2011, 40, 1697–1721. [Google Scholar] [CrossRef]
- Reddy, A.L.M.; Shaijumon, M.M.; Gowda, S.R.; Ajayan, P.M. Coaxial MnO2/carbon nanotube array electrodes for high-performance lithium batteries. Nano Lett. 2009, 9, 1002–1006. [Google Scholar] [CrossRef]
- Gu, X.; Yue, J.; Li, L.; Xue, H.; Yang, J.; Zhao, X. General synthesis of MnOx (MnO2, Mn2O3, Mn3O4, MnO) hierarchical microspheres as lithium-ion battery anodes. Electrochim. Acta 2015, 184, 250–256. [Google Scholar] [CrossRef]
- Wang, D.; Wang, L.; Liang, G.; Li, H.; Liu, Z.; Tang, Z.; Liang, J.; Zhi, C. A superior δ-MnO2 cathode and a self-healing Zn-δ-MnO2 battery. ACS Nano 2019, 13, 10643–10652. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, X.; Meng, Y.; Yu, M.; Yi, J.; Wu, Y.; Lu, X.; Tong, Y. Achieving Ultrahigh Energy Density and Long Durability in a Flexible Rechargeable Quasi-Solid-State Zn–MnO2 Battery. Adv. Mater. 2017, 29, 1700274. [Google Scholar] [CrossRef]
- Weng, Y.-T.; Huang, T.-Y.; Lim, C.-H.; Shao, P.-S.; Hy, S.; Kuo, C.-Y.; Cheng, J.-H.; Hwang, B.-J.; Lee, J.-F.; Wu, N.-L. An unexpected large capacity of ultrafine manganese oxide as a sodium-ion battery anode. Nanoscale 2015, 7, 20075–20081. [Google Scholar] [CrossRef]
- Wang, L.; Cao, X.; Xu, L.; Chen, J.; Zheng, J. Transformed akhtenskite MnO2 from Mn3O4 as cathode for a rechargeable aqueous zinc ion battery. ACS Sustain. Chem. Eng. 2018, 6, 16055–16063. [Google Scholar] [CrossRef]
- Akbari Garakani, M.; Abouali, S.; Cui, J.; Kim, J.-K. In situ TEM study of lithiation into a PPy coated α-MnO2/graphene foam freestanding electrode. Mater. Chem. Front. 2018, 2, 1481–1488. [Google Scholar] [CrossRef]
- Muhammad, N.; Yasin, G.; Li, A.; Chen, Y.; Saleem, H.M.; Liu, R.; Li, D.; Sun, Y.; Zheng, S.; Chen, X. Volumetric buffering of manganese dioxide nanotubes by employing ‘as is’ graphene oxide: An approach towards stable metal oxide anode material in lithium-ion batteries. J. Alloys Compd. 2020, 842, 155803. [Google Scholar] [CrossRef]
- Abdollahifar, M.; Liu, H.-W.; Lin, C.-H.; Weng, Y.-T.; Sheu, H.-S.; Lee, J.-F.; Lu, M.-L.; Liao, Y.-F.; Wu, N.-L. Enabling Extraordinary Rate Performance for Poorly Conductive Oxide Pseudocapacitors. Energy Environ. Mater. 2020, 3, 405–413. [Google Scholar] [CrossRef]
- Abdollahifar, M.; Huang, S.-S.; Lin, Y.-H.; Sheu, H.-S.; Lee, J.-F.; Lu, M.-L.; Liao, Y.-F.; Wu, N.-L. Tetragonal LiMn2O4 as dual-functional pseudocapacitor-battery electrode in aqueous Li-ion electrolytes. J. Power Sources 2019, 412, 545–551. [Google Scholar] [CrossRef]
- Abdollahifar, M.; Huang, S.-S.; Lin, Y.-H.; Lin, Y.-C.; Shih, B.-Y.; Sheu, H.-S.; Liao, Y.-F.; Wu, N.-L. High-performance carbon-coated ZnMn2O4 nanocrystallite supercapacitors with tailored microstructures enabled by a novel solution combustion method. J. Power Sources 2018, 378, 90–97. [Google Scholar] [CrossRef]
- Wang, C.; Higgins, D.; Wang, F.; Li, D.; Liu, R.; Xia, G.; Li, N.; Li, Q.; Xu, H.; Wu, G. Controlled synthesis of micro/nanostructured CuO anodes for lithium-ion batteries. Nano Energy 2014, 9, 334–344. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Xiong, H.; Qin, C.; Zhao, W.; Liu, X. Yucca fern shaped CuO nanowires on Cu foam for remitting capacity fading of Li-ion battery anodes. Sci. Rep. 2018, 8, 6530. [Google Scholar] [CrossRef]
- Zhu, C.; Chao, D.; Sun, J.; Bacho, I.M.; Fan, Z.; Ng, C.F.; Xia, X.; Huang, H.; Zhang, H.; Shen, Z.X. Enhanced lithium storage performance of CuO nanowires by coating of graphene quantum dots. Adv. Mater. Interfaces 2015, 2, 1400499. [Google Scholar] [CrossRef]
- Yin, D.; Huang, G.; Na, Z.; Wang, X.; Li, Q.; Wang, L. CuO nanorod arrays formed directly on Cu foil from MOFs as superior binder-free anode material for lithium-ion batteries. ACS Energy Lett. 2017, 2, 1564–1570. [Google Scholar] [CrossRef]
- Wang, X.; Tang, D.-M.; Li, H.; Yi, W.; Zhai, T.; Bando, Y.; Golberg, D. Revealing the conversion mechanism of CuO nanowires during lithiation–delithiation by in situ transmission electron microscopy. Chem. Commun. 2012, 48, 4812–4814. [Google Scholar] [CrossRef]
- Wu, R.; Qian, X.; Yu, F.; Liu, H.; Zhou, K.; Wei, J.; Huang, Y. MOF-templated formation of porous CuO hollow octahedra for lithium-ion battery anode materials. J. Mater. Chem. A 2013, 1, 11126–11129. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, N.; Sun, K. Facile fabrication of CuO mesoporous nanosheet cluster array electrodes with super lithium-storage properties. J. Mater. Chem. 2012, 22, 13637–13642. [Google Scholar] [CrossRef]
- Xue, X.; Deng, P.; Yuan, S.; Nie, Y.; He, B.; Xing, L.; Zhang, Y. CuO/PVDF nanocomposite anode for a piezo-driven self-charging lithium battery. Energy Environ. Sci. 2013, 6, 2615–2620. [Google Scholar] [CrossRef]
- Yin, Z.; Ding, Y.; Zheng, Q.; Guan, L. CuO/polypyrrole core–shell nanocomposites as anode materials for lithium-ion batteries. Electrochem. Commun. 2012, 20, 40–43. [Google Scholar] [CrossRef]
- Yan, N.; Hu, L.; Li, Y.; Wang, Y.; Zhong, H.; Hu, X.; Kong, X.; Chen, Q. Co3O4 nanocages for high-performance anode material in lithium-ion batteries. J. Phys. Chem. C 2012, 116, 7227–7235. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Xiao, F.; Huang, Z.; Wang, Y.; Richardson, C.; Chen, Z.; Jiao, L.; Yuan, H. Facile synthesis of nanocage Co3O4 for advanced lithium-ion batteries. J. Power Sources 2015, 298, 203–208. [Google Scholar] [CrossRef]
- Li, Y.; Tan, B.; Wu, Y. Mesoporous Co3O4 nanowire arrays for lithium ion batteries with high capacity and rate capability. Nano Lett. 2008, 8, 265–270. [Google Scholar] [CrossRef]
- Liu, Y.; Mi, C.; Su, L.; Zhang, X. Hydrothermal synthesis of Co3O4 microspheres as anode material for lithium-ion batteries. Electrochim. Acta 2008, 53, 2507–2513. [Google Scholar] [CrossRef]
- Huang, G.; Xu, S.; Lu, S.; Li, L.; Sun, H. Micro-/nanostructured Co3O4 anode with enhanced rate capability for lithium-ion batteries. ACS Appl. Mater. Interfaces 2014, 6, 7236–7243. [Google Scholar] [CrossRef]
- Zhan, L.; Chen, H.; Fang, J.; Wang, S.; Ding, L.-X.; Li, Z.; Ashman, P.J.; Wang, H. Coaxial Co3O4@polypyrrole core-shell nanowire arrays for high performance lithium ion batteries. Electrochim. Acta 2016, 209, 192–200. [Google Scholar] [CrossRef]
- Ding, Z.; Yao, B.; Feng, J.; Zhang, J. Enhanced rate performance and cycling stability of a CoCO3–polypyrrole composite for lithium ion battery anodes. J. Mater. Chem. A 2013, 1, 11200–11209. [Google Scholar] [CrossRef]
- Mukkabla, R.; Deepa, M.; Srivastava, A.K. Enhanced Lithium-Ion Storage Capability of a Bismuth Sulfide/Graphene Oxide/Poly(3,4-ethylenedioxythiophene) Composite. ChemPhysChem 2015, 16, 3242–3253. [Google Scholar] [CrossRef]
- Li, X.; Han, X.; Liu, R.; Zhang, S.; Zhang, Y.; Cao, Y.; Wang, X.; Wang, R.; Yang, Z.; Sun, J. Tannic acid-polypyrrole multifunctional coating layer enhancing the interface effect and efficient Li-ion transport of a phosphorus anode. Nanoscale 2022, 14, 3625–3631. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, W.; Chen, Z.; Zhang, C.; Jiang, L.; Yu, F. A lithiophilic hyperbranched polymer-decorated three-dimensional carbon skeleton boosting highly reversible lithium metal anode. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129104. [Google Scholar] [CrossRef]
- Yin, W.; Chai, W.; Wang, K.; Ye, W.; Rui, Y.; Tang, B. A highly Meso@Microporous carbon-supported Antimony sulfide nanoparticles coated by conductive polymer for high-performance lithium and sodium ion batteries. Electrochim. Acta 2019, 321, 134699. [Google Scholar] [CrossRef]
- Wang, H.; Zeng, Y.; Huang, K.; Liu, S.; Chen, L. Improvement of cycle performance of lithium ion cell LiMn2O4/LixV2O5 with aqueous solution electrolyte by polypyrrole coating on anode. Electrochim. Acta 2007, 52, 5102–5107. [Google Scholar] [CrossRef]
- Wang, H.; Huang, K.; Zeng, Y.; Zhao, F.; Chen, L. Stabilizing Cyclability of an Aqueous Lithium-Ion Battery LiNi1/3Mn1/3Co1/3O2/ Li x V2O5 by Polyaniline Coating on the Anode. Electrochem. Solid State Lett. 2007, 10, A199. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.; Yang, J.; Tian, J.; Xu, H.; Yang, J.; Fan, W. Conductive Polymer-Coated VS4 Submicrospheres As Advanced Electrode Materials in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 18797–18805. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, R. Synthesis and electrochemical performance of CuV2O6/PEDOT:PSS composite nanobelts as anode materials for lithium-ion batteries. Mater. Lett. 2016, 176, 131–134. [Google Scholar] [CrossRef]
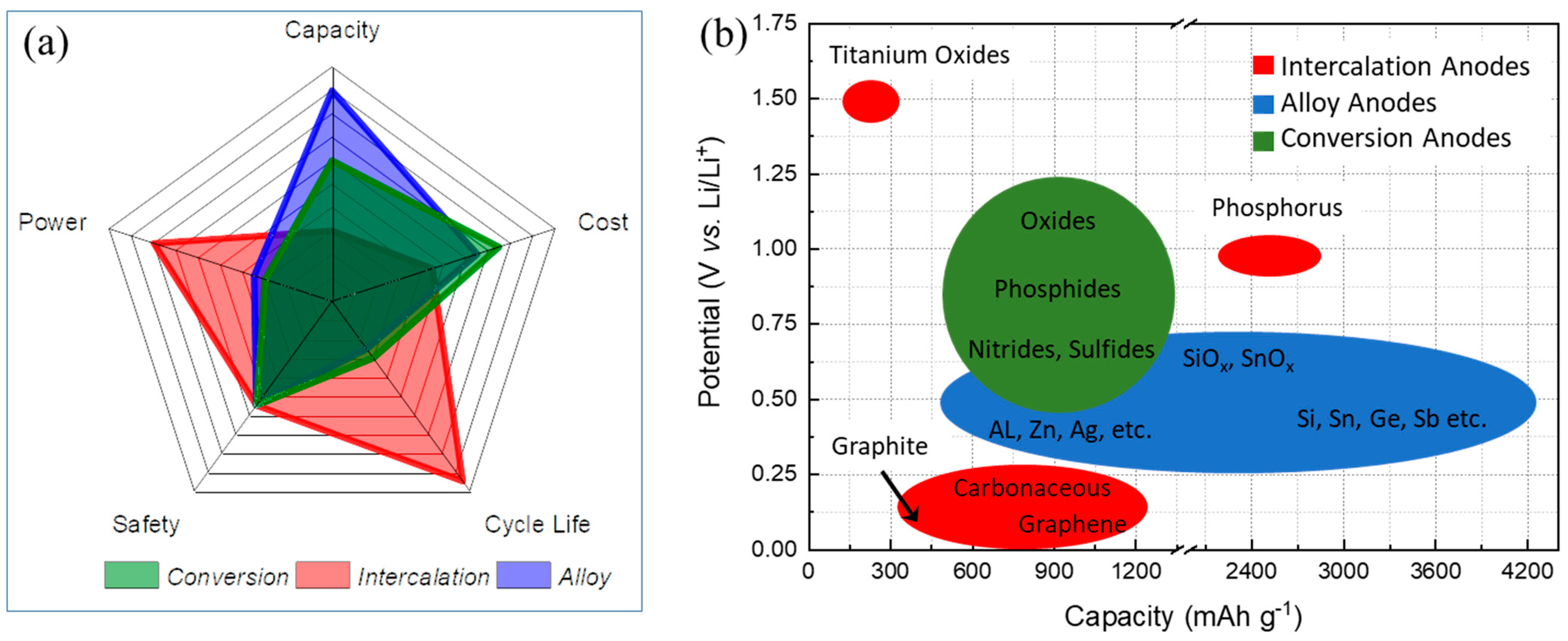
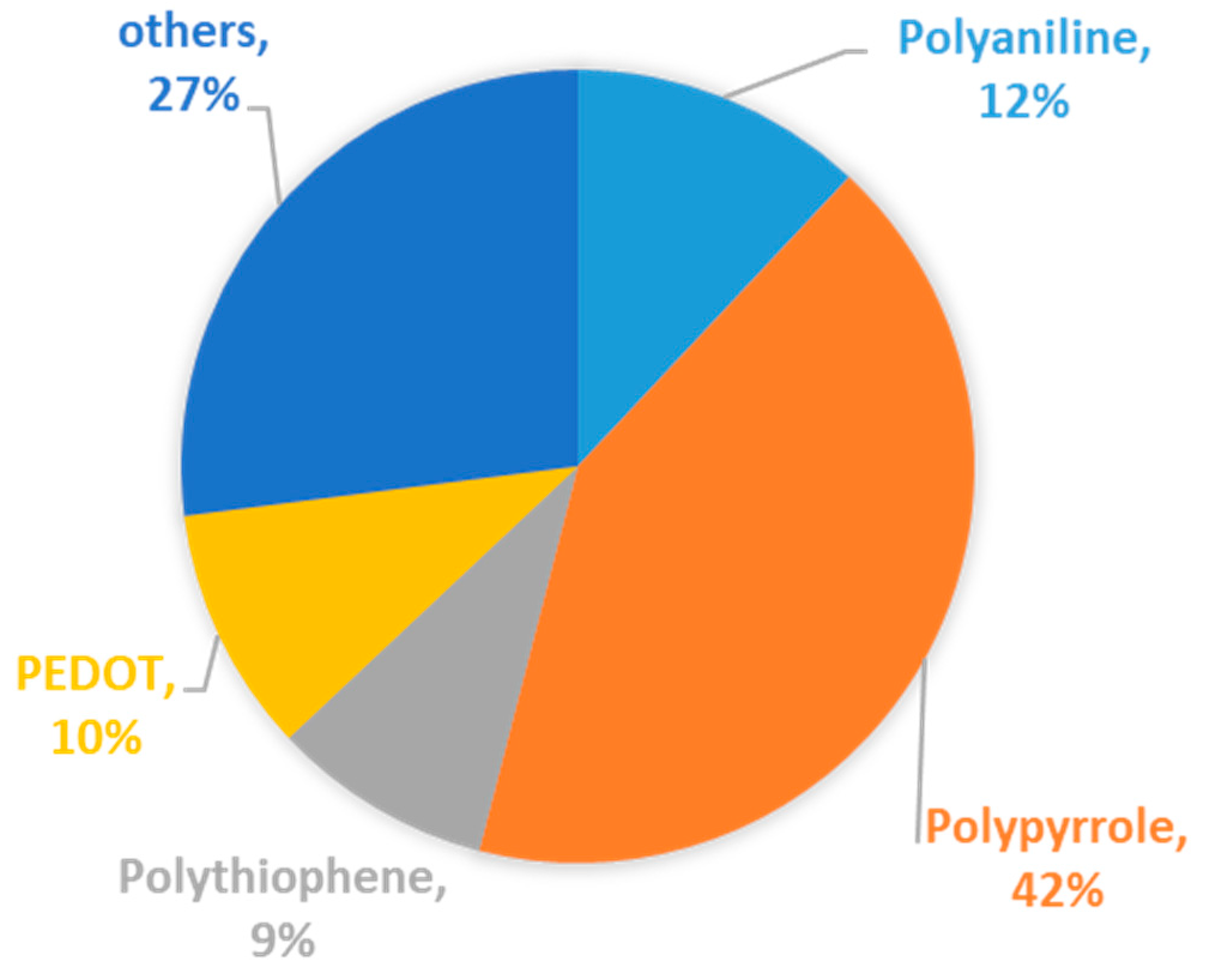

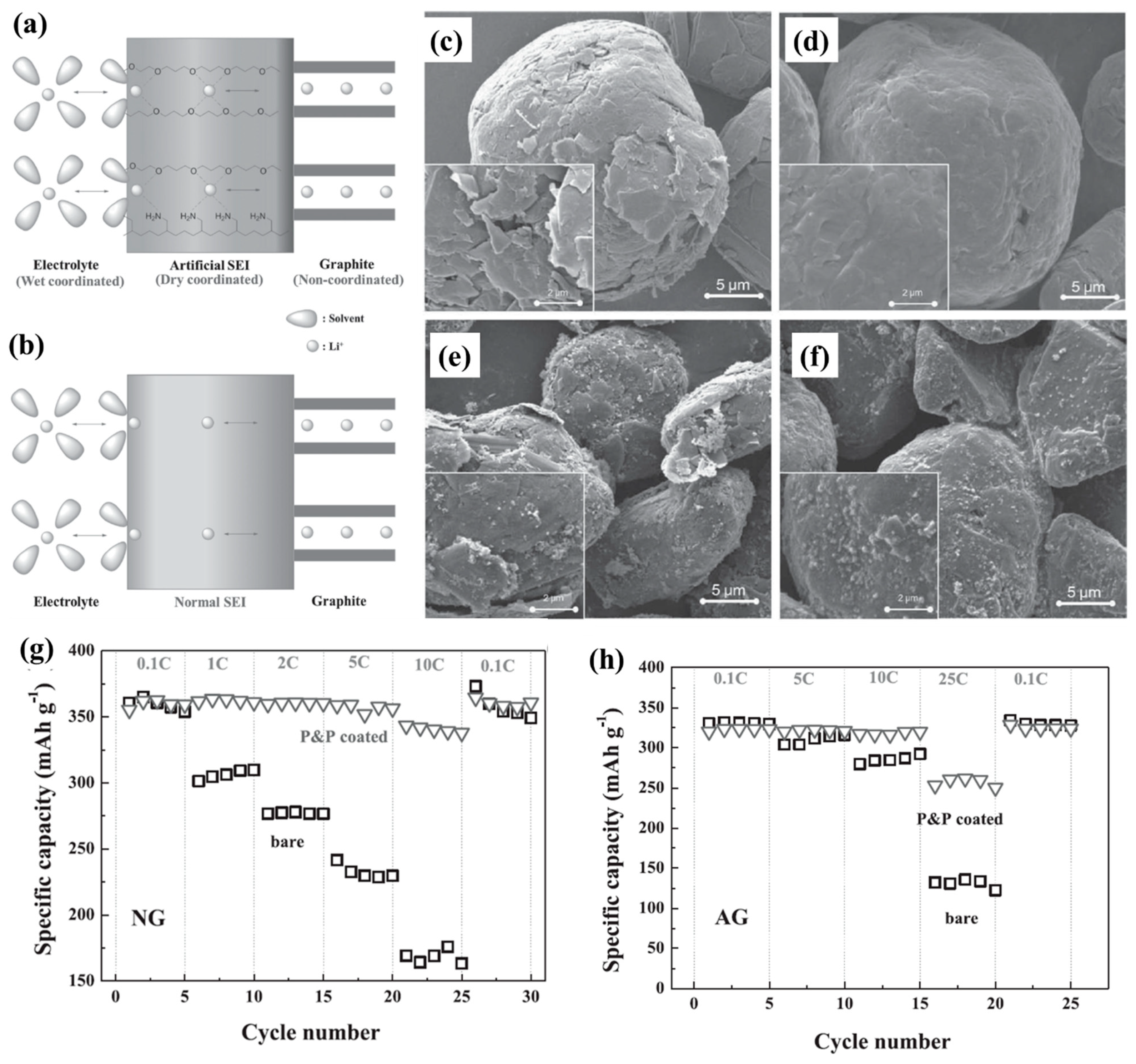
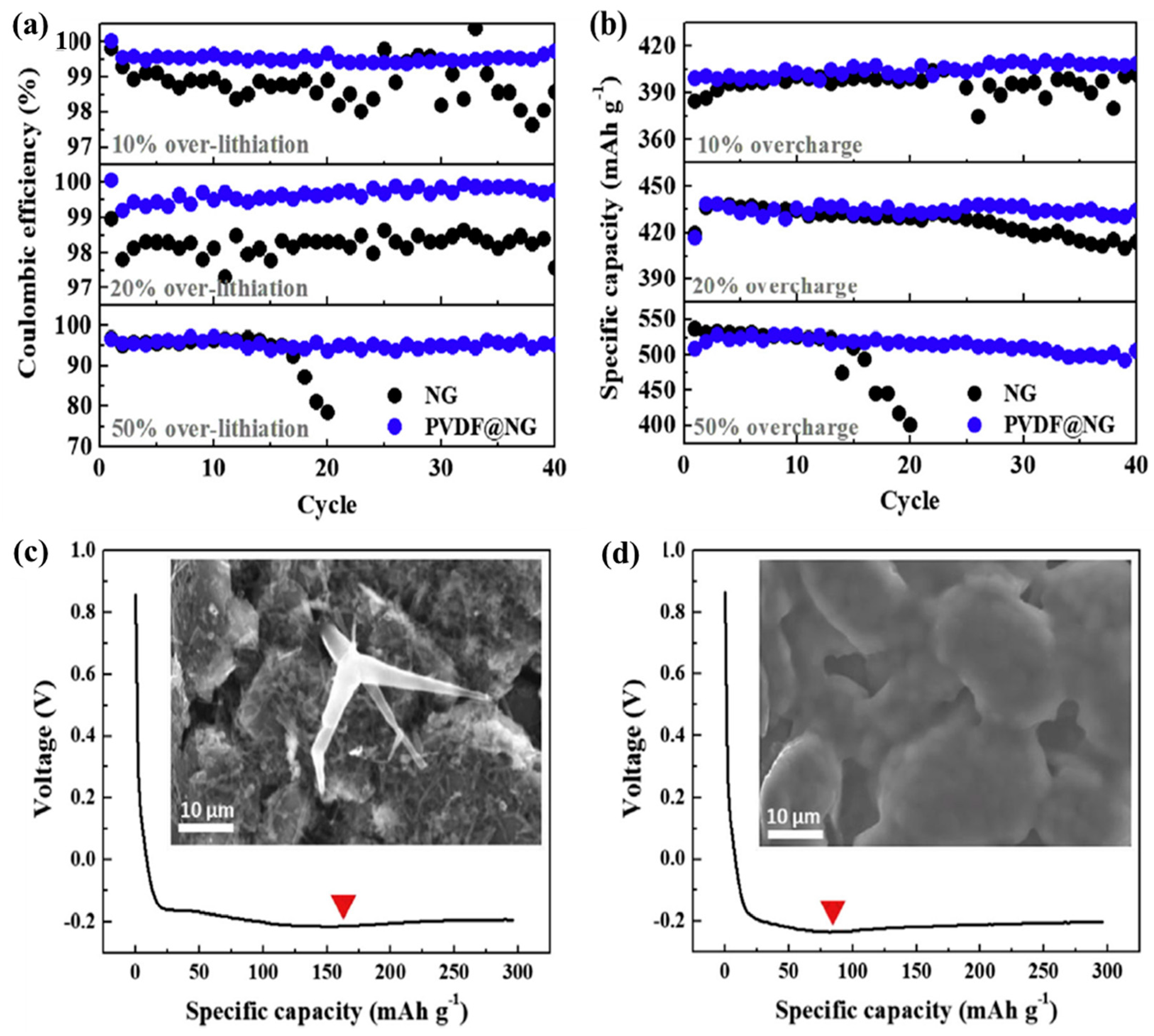
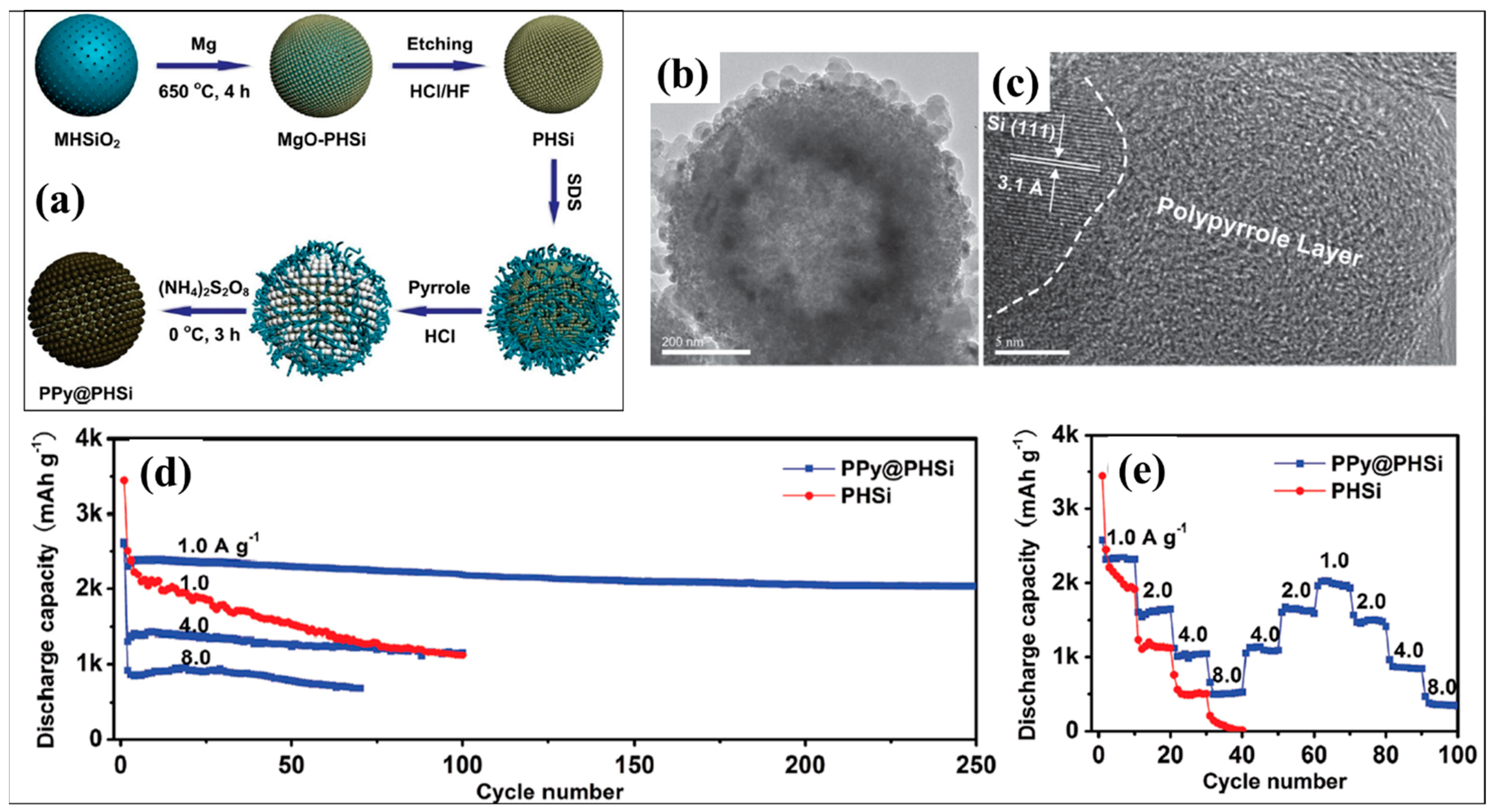
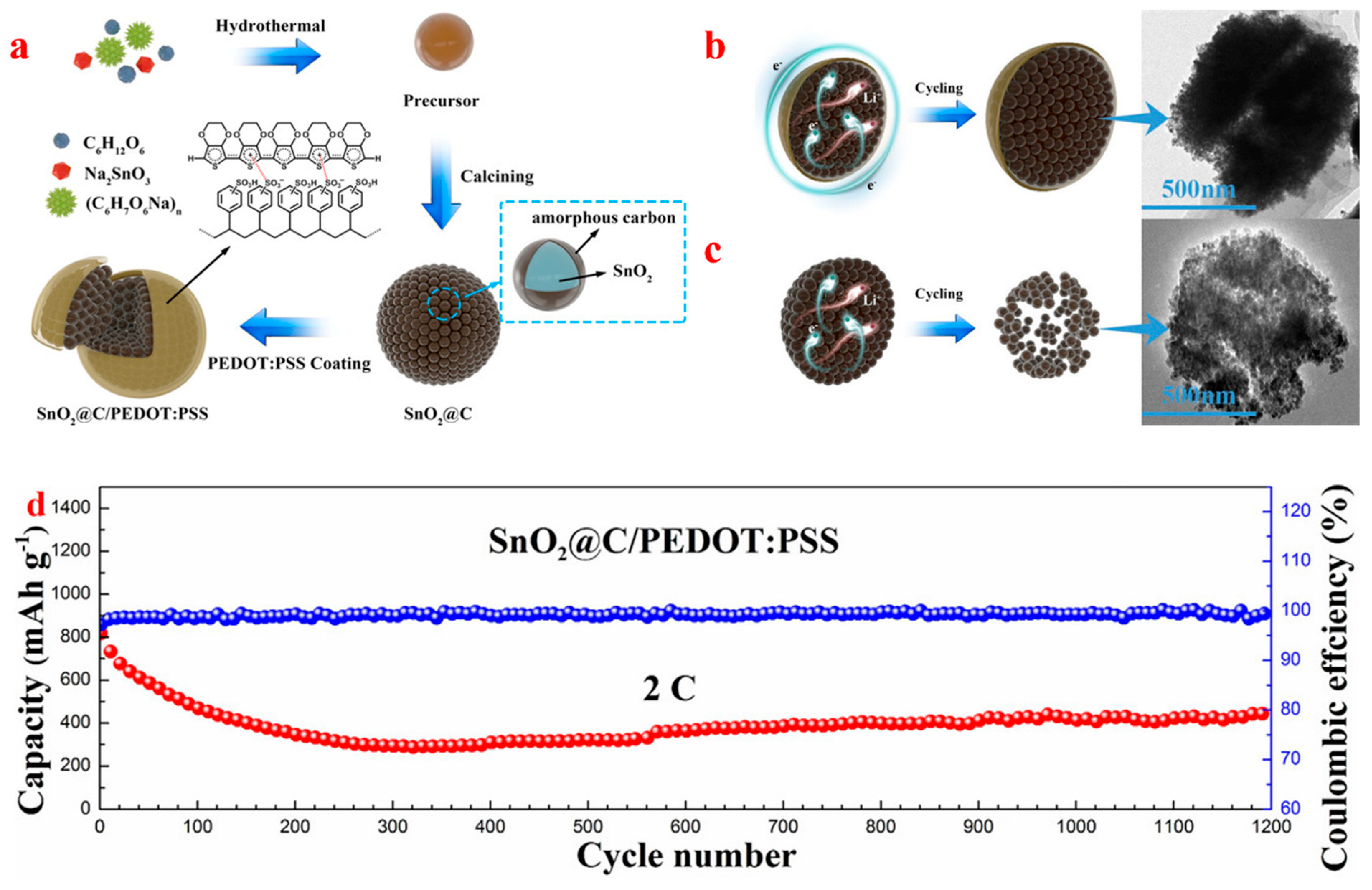


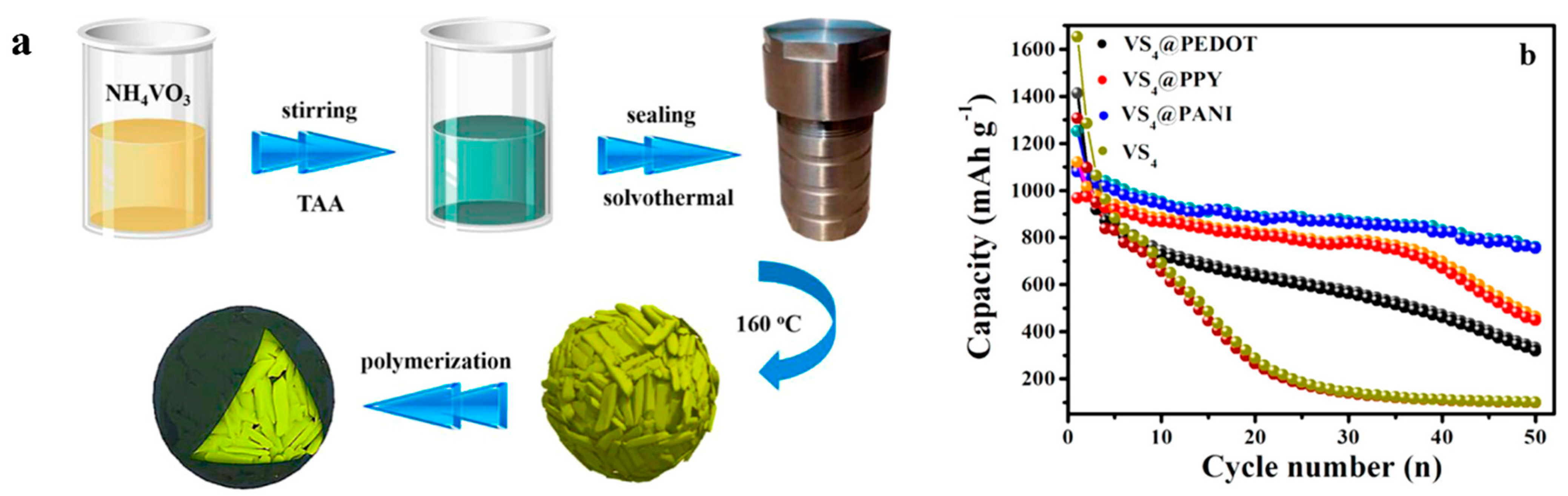
| Types of Anodes | Benefits | Drawbacks |
|---|---|---|
| Intercalation-type anodes | Low-cost materials, high electronic conductivity, good safety for Ti-based anodes, long cycle life, high power capability | Low specific capacity, safety issue for Gr, low energy density |
| Alloy-type anodes | High specific capacity, good safety, low-cost materials and abundance for Si, high energy density | Large volume changes, low electronic conductivity, poor CE, poor cycling |
| Conversion-type anodes | High specific capacity, low-cost materials, low operation potential | Poor CE, unstable SEI, poor cycling |
| Polymer | Synthesis Method | Coating on Particle/Electrode | Thickness | Cycling Performance (mAh g−1)/Current Rate (mA g−1) | Ref. |
|---|---|---|---|---|---|
| Polypyrrole (PPy) | Chemical polymerization method | Electrode | - | 450 (20 cycles)/50 250 (20 cycles for bare SnO2)/50 | [26] |
| Chemical polymerization method | Particle | - | 600 (30 cycles)/100 | [27] | |
| In situ chemical polymerization | Electrode | - | 430 (20 cycles)/50 | [121] | |
| Hydrothermal method and in situ chemical-polymerization | Particle | 25 nm | 448.4 (100 cycles)/78 | [28] | |
| Chemical vapor-phase polymerization | Electrode | 20 nm | 646 (150 cycles)/100 | [29] | |
| In situ coating method | Particle | 20 nm | 760 (400 cycles)/0.1 C | [123] | |
| Poly(3,4-ethylenedioxythiophene-poly(styrenesulfonate) (PEDOT:PSS) | Wet chemical method | Particle | 2–3 nm | 980 (160 cycles)/80 | [125] |
| Hydrothermal method and polymerization | Particle | 6 nm | 441.5 (1200 cycles)/2 C | [126] | |
| Poly(ethyleneglycol dimethacrylate-co-methacrylic acid) | Hydrothermal method and polymerization | Particle | 24.5 nm | 711.9 (430 cycles)/200 | [122] |
| Polydopamine (PDA) | Wet chemical method | Electrode | 5 nm | 1502 (300 cycles)/160 | [117] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdollahifar, M.; Molaiyan, P.; Perovic, M.; Kwade, A. Insights into Enhancing Electrochemical Performance of Li-Ion Battery Anodes via Polymer Coating. Energies 2022, 15, 8791. https://doi.org/10.3390/en15238791
Abdollahifar M, Molaiyan P, Perovic M, Kwade A. Insights into Enhancing Electrochemical Performance of Li-Ion Battery Anodes via Polymer Coating. Energies. 2022; 15(23):8791. https://doi.org/10.3390/en15238791
Chicago/Turabian StyleAbdollahifar, Mozaffar, Palanivel Molaiyan, Milena Perovic, and Arno Kwade. 2022. "Insights into Enhancing Electrochemical Performance of Li-Ion Battery Anodes via Polymer Coating" Energies 15, no. 23: 8791. https://doi.org/10.3390/en15238791
APA StyleAbdollahifar, M., Molaiyan, P., Perovic, M., & Kwade, A. (2022). Insights into Enhancing Electrochemical Performance of Li-Ion Battery Anodes via Polymer Coating. Energies, 15(23), 8791. https://doi.org/10.3390/en15238791








