Fly Ash-Based Geopolymers as Lower Carbon Footprint Alternatives to Portland Cement for Well Cementing Applications
Abstract
1. Introduction
2. Materials
2.1. Cementitious Materials
2.2. Mixtures Preparation
3. Experimental Methods
3.1. Viscosity
3.2. Mechanical Properties
3.2.1. Curing Conditions
3.2.2. Unconfined Compressive Strength (UCS)
3.2.3. Tensile Strength
3.2.4. Bond Strength
3.3. Thickening Time
4. Results and Discussion
4.1. Viscosity
4.2. Mechanical Properties
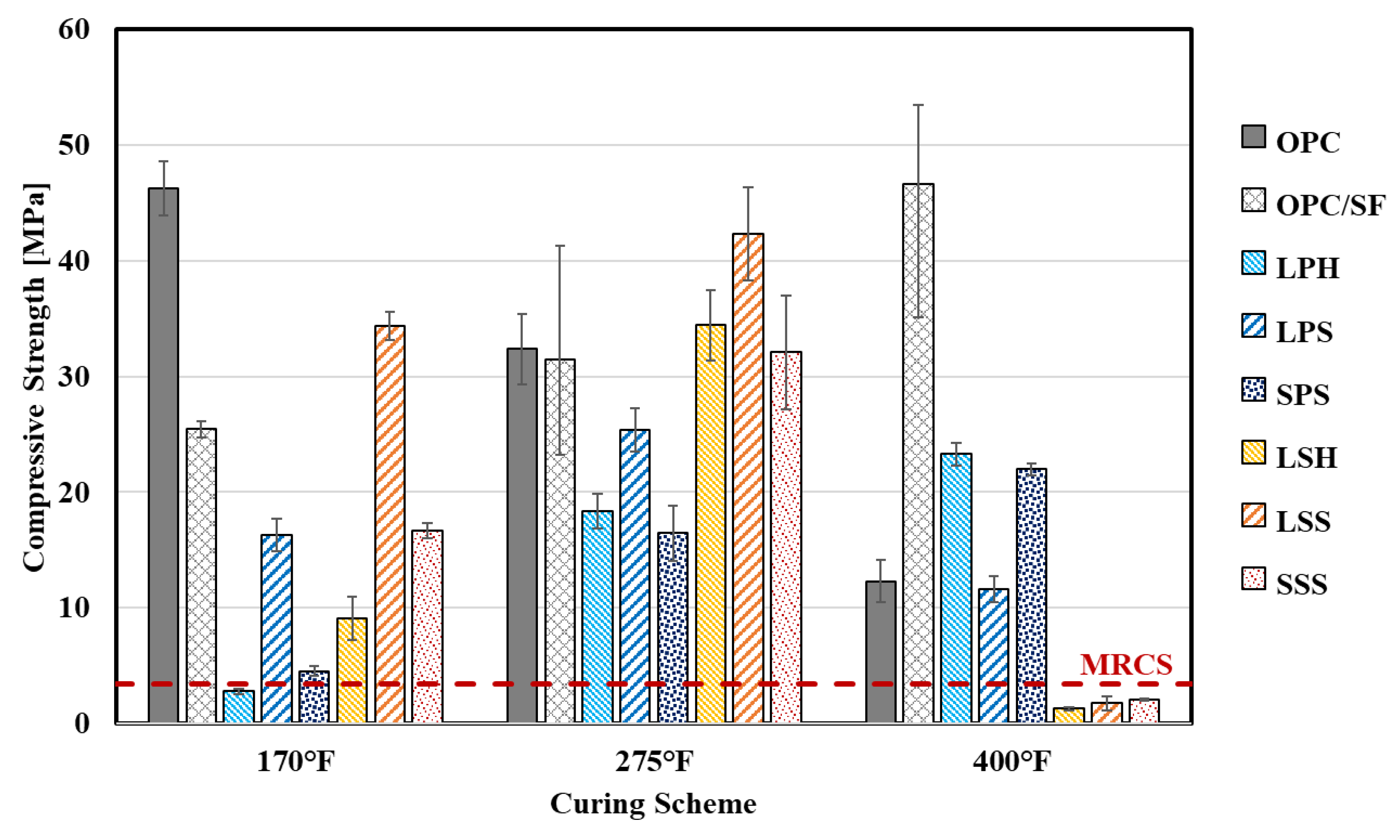
4.2.1. Unconfined Compressive Strength
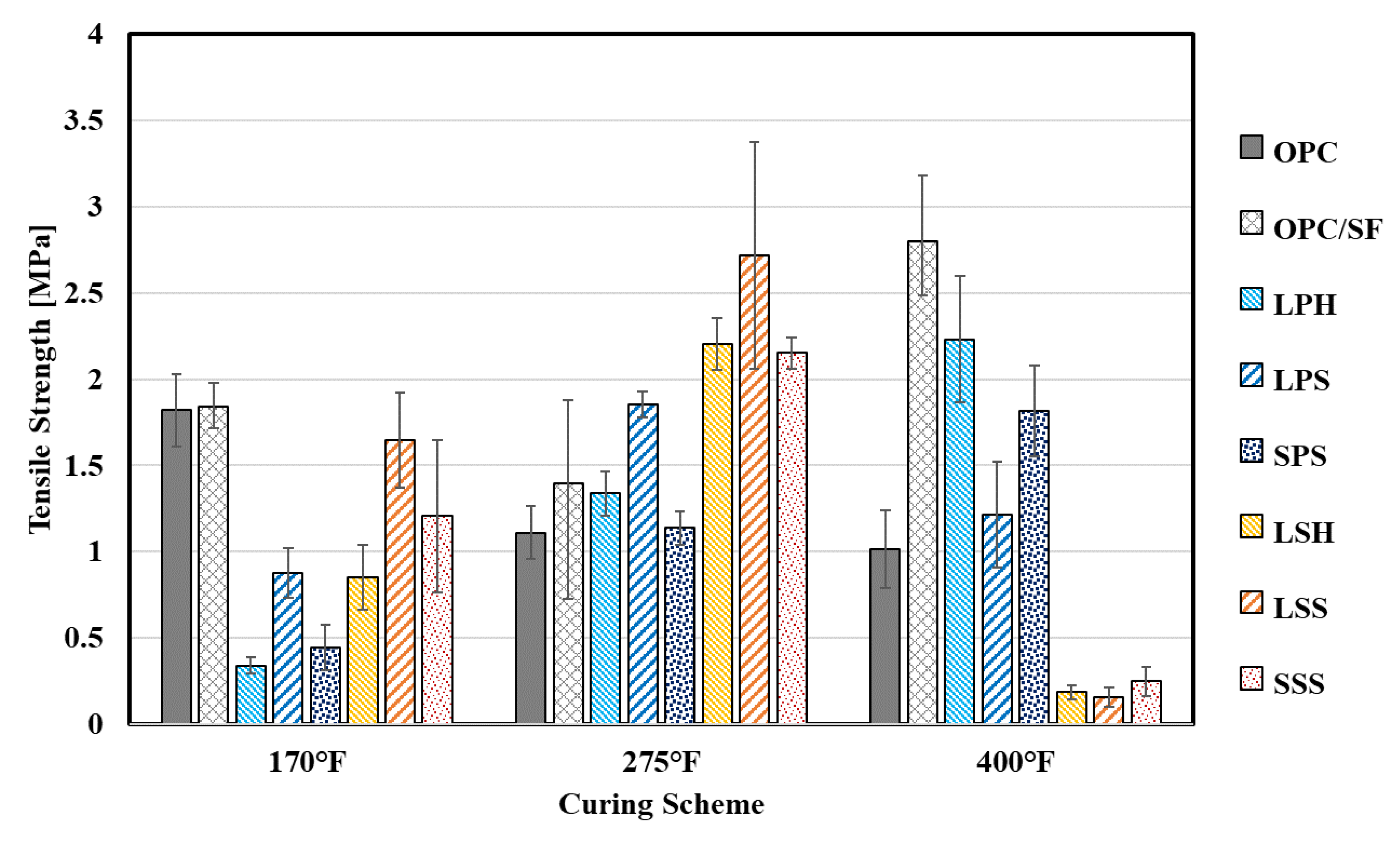
4.2.2. Tensile Strength
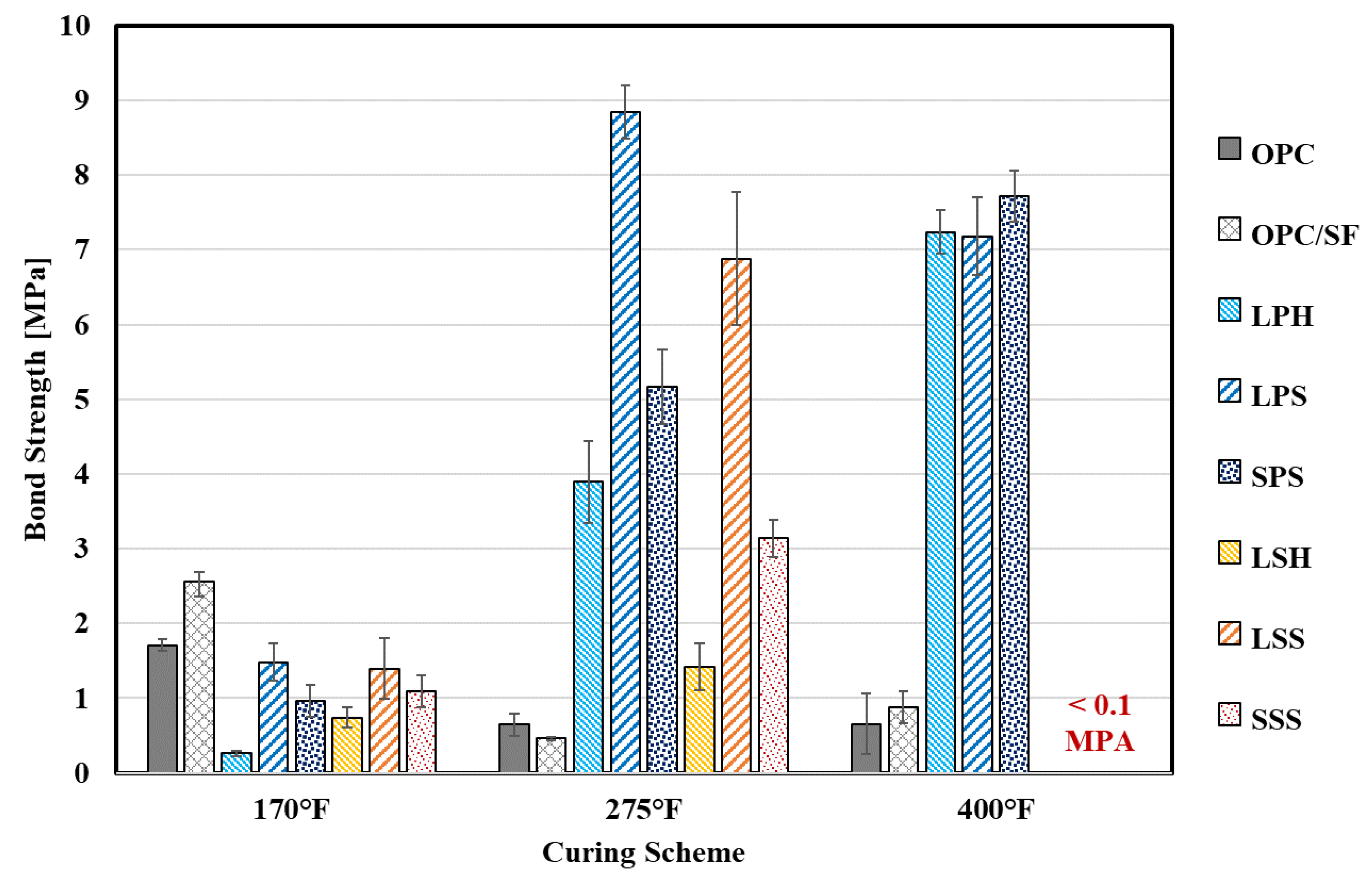
4.2.3. Bond Strength
4.3. Thickening Time
5. Discussion and Summary
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, A.C. Making Net Zero Matter. Wash. Lee Law Rev. 2022, 79, 679–768. [Google Scholar]
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Hastings, A.; Smith, P. Achieving Net Zero Emissions Requires the Knowledge and Skills of the Oil and Gas Industry. Front. Clim. 2020, 2, 601778. [Google Scholar] [CrossRef]
- Kang, M. CO2, Methane, and Brine Leakage through Subsurface Pathways: Exploring Modeling, Measurement, and Policy Options. Ph.D. Thesis, Princeton University, Princeton, MA, USA, 2014. [Google Scholar]
- Kang, M.; Mauzerall, D.L.; Ma, D.Z.; Celia, M.A. Reducing methane emissions from abandoned oil and gas wells: Strategies and costs. Energy Policy 2019, 132, 594–601. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S. Alkali Activated Materials: State-of-the-Art Report; RILEM TC 224-AAM; Springer Science & Business Media: Dordrecht, The Netherlands, 2013; Volume 13. [Google Scholar]
- Vrålstad, T.; Saasen, A.; Fjær, E.; Øia, T.; Ytrehus, J.D.; Khalifeh, M. Plug & abandonment of offshore wells: Ensuring long-term well integrity and cost-efficiency. J. Pet. Sci. Eng. 2019, 173, 478–491. [Google Scholar] [CrossRef]
- Khalifeh, M.; Saasen, A. Introduction to Permanent Plug and Abandonment of Wells; Springer Nature: Berlin, Germany, 2020. [Google Scholar]
- Khalifeh, M.; Hodne, H.; Saasen, A.; Integrity, O.; Eduok, E.I. Usability of Geopolymers for Oil Well Cementing Applications: Reaction Mechanisms, Pumpability, and Properties. In Proceedings of the SPE Asia Pacific Oil & Gas Conference and Exhibition, Perth, Australia, 10–12 October 2016. [Google Scholar] [CrossRef]
- Khalifeh, M.; Saasen, A.; Hodne, H.; Godøy, R.; Vrålstad, T. Geopolymers as an Alternative for Oil Well Cementing Applications: A Review of Advantages and Concerns. J. Energy Resour. Technol. 2018, 140, 092801. [Google Scholar] [CrossRef]
- Salehi, S.; Khattak, M.J.; Ali, N.; Rizvi, H.R. Development of Geopolymer-based Cement Slurries with Enhanced Thickening Time, Compressive and Shear Bond Strength and Durability. In Proceedings of the IADC/SPE Drilling Conference and Exhibition, Fort Worth, TX, USA, 1 March 2016. [Google Scholar] [CrossRef]
- Salehi, S.; Ezeakacha, C.P.; Khattak, M.J. Geopolymer Cements: How Can You Plug and Abandon a Well with New Class of Cheap Efficient Sealing Materials. In Proceedings of the SPE Oklahoma City Oil and Gas Symposium, Oklahoma City, OK, USA, 27–30 March 2017. [Google Scholar] [CrossRef]
- Salehi, S.; Khattak, J.; Saleh, F.K.; Igbojekwe, S. Investigation of mix design and properties of geopolymers for application as wellbore cement. J. Pet. Sci. Eng. 2019, 178, 133–139. [Google Scholar] [CrossRef]
- van Oort, E.; Juenger, M.; Liu, X.; McDonald, M. Silicate-Activated Geopolymer Alternatives to Portland Cement for Thermal Well Integrity. Society of Petroleum Engineers. In Proceedings of the SPE Thermal Well Integrity and Design Symposium, Banff, AB, Canada, 17 November 2019. [Google Scholar] [CrossRef]
- Genedy, M.; Juenger, M.; van Oort, E. Novel Cementing and Lost Circulation Solutions for Geothermal Wells based on Class F Fly Ash Geopolymers. GRC Trans. 2021, 45, 169–180. Available online: https://www.geothermal-library.org/index.php?mode=pubs&action=view&record=1034373 (accessed on 21 July 2022).
- Ahdaya, M.; Imqam, A. Investigating geopolymer cement performance in presence of water-based drilling fluid. J. Pet. Sci. Eng. 2019, 176, 934–942. [Google Scholar] [CrossRef]
- Liu, X.; Aughenbaugh, K.; Nair, S.; Shuck, M.; van Oort, E. Solidification of Synthetic-Based Drilling Mud Using Geopolymers. In Proceedings of the SPE Deepwater Drilling and Completions Conference, Galveston, TX, USA, 14–15 September 2016. [Google Scholar] [CrossRef]
- Liu, X.; Nair, S.; Aughenbaugh, K.; van Oort, E. Mud-to-cement conversion of non-aqueous drilling fluids using alkali-activated fly ash. J. Pet. Sci. Eng. 2019, 182, 106242. [Google Scholar] [CrossRef]
- Rostami, H.; Brendley, W. Alkali Ash Material: A Novel Fly Ash-Based Cement. Environ. Sci. Technol. 2003, 37, 3454–3457. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. Mud-to-Cement Conversion of Synthetic-Based Drilling Muds Using Geopolymers. Ph.D. Thesis, The University of Texas at Austin, Austin, TX, USA, 2017. [Google Scholar]
- Liu, X.; Ramos, M.J.; Nair, S.D.; Lee, H.; Espinoza, D.N.; van Oort, E. True Self-Healing Geopolymer Cements for Improved Zonal Isolation and Well Abandonment. In Proceedings of the SPE/IADC Drilling Conference and Exhibition, The Hague, The Netherlands, 14–15 March 2017. [Google Scholar] [CrossRef]
- Ross, J. An Evaluation of the Self-Healing Capabilities of Fly Ash-Based Geopolymers. Master’s Thesis, The University of Texas at Austin, Austin, TX, USA, 2020. [Google Scholar]
- Ross, J.H.; Genedy, M.; Juenger, M.C.; van Oort, E. Permeability Recovery by Self-Healing of Class F Fly Ash-Based Geopolymers. Cement 2022, 10, 100048. [Google Scholar] [CrossRef]
- Panchmatia, P.; Olvera, R.; Genedy, M.; Juenger, M.C.G.; van Oort, E. Shrinkage behavior of Portland and geopolymer cements at elevated temperature and pressure. J. Pet. Sci. Eng. 2020, 195, 107884. [Google Scholar] [CrossRef]
- Colangelo, F.; Cioffi, R.; Roviello, G.; Capasso, I.; Caputo, D.; Aprea, P.; Liguori, B.; Ferone, C. Thermal cycling stability of fly ash based geopolymer mortars. Compos. Part Eng. 2017, 129, 11–17. [Google Scholar] [CrossRef]
- Kong, D.L.Y.; Sanjayan, J.G. Effect of elevated temperatures on geopolymer paste, mortar and concrete. Cem. Concr. Res. 2010, 40, 334–339. [Google Scholar] [CrossRef]
- Denduluri, V.S.; Genedy, M.; Juenger, M.; McDonald, M.; van Oort, E. Using Geopolymers as a Sustainable Alternative Cementing Solution for Long-Term Zonal Isolation and Lost Circulation Challenges in Deep Geothermal Wells. GRC Trans. 2022, 46, 1438–1451. Available online: https://www.geothermal-library.org/index.php?mode=pubs&action=view&record=1034686 (accessed on 9 September 2022).
- Duxson, P.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S. The role of inorganic polymer technology in the development of ‘green concrete’. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Winnefeld, F.; Provis, J.L.; Ideker, J.H. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Alvi, M.A.A.; Khalifeh, M.; Agonafir, M.B. Effect of nanoparticles on properties of geopolymers designed for well cementing applications. J. Pet. Sci. Eng. 2020, 191, 107128. [Google Scholar] [CrossRef]
- Khalifeh, M.; Saasen, A.; Hodne, H.; Motra, H.B. Laboratory evaluation of rock-based geopolymers for zonal isolation and permanent P&A applications. J. Pet. Sci. Eng. 2019, 175, 352–362. [Google Scholar] [CrossRef]
- Salehi, S.; Khattak, M.J.; Rizvi, H.; Karbalaei, S.F.; Kiran, R. Sensitivity analysis of fly ash geopolymer cement slurries: Implications for oil and gas wells cementing applications. J. Nat. Gas Sci. Eng. 2017, 37, 116–125. [Google Scholar] [CrossRef]
- Grabowski, E.; Gillott, J.E. The effect of initial curing temperature on the performance of oilwell cements made with different types of silica. Cem. Concr. Res. 1989, 19, 703–714. [Google Scholar] [CrossRef]
- Pyatina, T.; Sugama, T. Thermal-Shock Resistant Cement for Heat Storage. GRC Trans. 2019, 43, 36–59. Available online: https://www.geothermal-library.org/index.php?mode=pubs&action=view&record=1034102 (accessed on 2 October 2022).
- Genedy, M.; Kandil, U.F.; Matteo, E.N.; Stormont, J.; Reda Taha, M.M. A new polymer nanocomposite repair material for restoring wellbore seal integrity. Int. J. Greenh. Gas Control 2017, 58, 290–298. [Google Scholar] [CrossRef]
- Xie, Z.; Yao, X. Influence of the particle size distribution of silica flour on the mechanical and microstructural properties of oil well cement paste exposed to HTHP conditions. Ceramics–Silikáty 2019, 63, 239–247. [Google Scholar] [CrossRef]
- Bezerra, U.T.; Martinelli, A.E.; Melo, D.M.A.; Melo, M.A.F.; Oliveira, V.G. The strength retrogression of special class Portland oilwell cement. Cerâmica 2011, 57, 150–154. [Google Scholar] [CrossRef]
- Li, L.; Kellum, M.; Doan, A. In-situ Tensile Strength Testing: Awareness of Variations with Testing Environment. In Proceedings of the SPE Deepwater Drilling and Completions Conference, Galveston, TX, USA, 14–15 September 2016. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; Elkatatny, S.; Ahmed, S.A.; Mahmoud, M. Nanoclay Content Influence on Cement Strength for Oil Wells Subjected to Cyclic Steam Injection and High-Temperature Conditions. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, UAE, 12 November 2018. [Google Scholar] [CrossRef]
- Teodoriu, C.; Yi, M.C.; Ichim, A.; Salehi, S. A novel view of cement failure with application to geothermal well construction. In Proceedings of the 43rd Workshop on Geothermal Reservoir Engineering, Stanford, CA, USA, 12–14 February 2018. [Google Scholar]
- Nelson, E.B.; Guillot, D. Well Cementing, 2nd ed.; Schlumberger: Sugarland, TX, USA, 2006. [Google Scholar]
- Salam, K.K.; Arinkoola, A.O.; Ajagbe, B.; Sanni, O. Evaluation of Thickening Time of Oil Field Class G Cement Slurry at High Temperature and Pressure using Experimental Design. Int. J. Eng. Sci. 2013, 2, 361–367. [Google Scholar]
- Fink, J. Petroleum Engineer’s Guide to Oil Field Chemicals and Fluids, 2nd ed.; Gulf Professional Publishing: Amsterdam, The Netherlands, 2021. [Google Scholar]

| Oxide | Content (wt%) | |
|---|---|---|
| Class H OPC | Class F FA | |
| SiO2 | 20.9 | 44.7 |
| Al2O3 | 3.6 | 23.2 |
| Fe2O3 | 6.3 | 24.2 |
| CaO | 63.3 | 3.2 |
| MgO | 1.0 | 0.8 |
| SO3 | 3.0 | 0.6 |
| Total alkali as sodium oxide, Na2Oeq | 0.55 | 1.0 |
| Loss on ignition (LOI) at 750 °C | 1.2 | 0.4 |
| Insoluble residue/other | 0.15 | 1.9 |
| Mix ID | Activator | W/S | M2O | SiO2/M2O | M2O/FA |
|---|---|---|---|---|---|
| OPC | DI Water | 0.385 | --- | --- | --- |
| OPC/SF | DI Water | 0.385 | --- | --- | --- |
| LSH | 8M Liquid Sodium Hydroxide | 0.33 | 8M | --- | --- |
| LPH | 8M Liquid Potassium Hydroxide | 0.33 | 8M | --- | --- |
| LSS | Liquid Sodium Silicate | 0.33 | 8M | 0.12 | 0.1 |
| SSS | Solid Sodium Silicate | 0.33 | 8M | 0.12 | 0.1 |
| LPS | Liquid Potassium Silicate | 0.33 | 8M | 0.12 | 0.2 |
| SPS | Solid Potassium Silicate | 0.33 | 8M | 0.12 | 0.2 |
| Scheme | Initial Temp. | BHCT | Ramp Time | BHST | Ramp Time | BHP |
|---|---|---|---|---|---|---|
| 1 | 23 °C (73.4 °F) | 52 °C (125 °F) | 120 min | 77 °C (170 °F) | 600 min | 20.7 MPa (3000 psi) |
| 2 | 23 °C (73.4 °F) | 100 °C (212 °F) | 150 min | 135 °C (275 °F) | 600 min | 20.7 MPa (3000 psi) |
| 3 | 23 °C (73.4 °F) | 135 °C (275 °F) | 180 min | 204 °C (400 °F) | 600 min | 20.7 MPa (3000 psi) |
| Mix ID | Apparent Viscosity at 300 rpm (Pa·s) | YPL Model Constants—See Equation (1) | ||
|---|---|---|---|---|
| Fluid Behavior Index n (Dimensionless) | Consistency Index K (Pa·sn) | |||
| OPC | 0.13 ± 0.03 | 6.70 ± 0.37 | 0.46 ± 0.06 | 3.87 ± 0.73 |
| OPC/SF | 0.12 ± 0.02 | 6.63 ± 2.91 | 0.61 ± 0.11 | 2.30 ± 0.72 |
| LSH | >0.32 | 2.87 ± 0.21 | 1.00 ± 0.05 | 0.84 ± 0.30 |
| LPH | 0.21 ± 0.06 | 2.62 ± 0.98 | 1.02 ± 0.08 | 0.18 ± 0.09 |
| LSS | >0.32 | 1.05 ± 0.72 | 1.00 ± 0.01 | 2.04 ± 0.48 |
| SSS | >0.32 | 4.31 ± 2.40 | 1.02 ± 0.05 | 1.69 ± 0.51 |
| LPS | 0.21 ± 0.09 | 0.57 ± 0.93 | 1.01 ± 0.10 | 0.19 ± 0.05 |
| SPS | 0.30 ± 0.03 | 3.20 ± 1.05 | 1.04 ± 0.04 | 0.22 ± 0.04 |
| Mix ID | BHCT | ||
|---|---|---|---|
| 52 °C (125 °F) | 100 °C (212 °F) | 135 °C (275 °F) | |
| OPC | 1:50 ± 2 | 1:22 ± 1 | 1:16 ± 4 |
| OPC/SF | 1:54 ± 4 | 1:28 ± 4 | 1:18 ± 3 |
| LSH | >24:00 | 1:42 ± 3 | N/A |
| LSS | 18:41 ± 54 * | 1:32 ± 3 * | N/A |
| SSS | 13:52 ± 98 * | 1:38 ± 7 * | N/A |
| LPH | >24:00 | 6:43 ± 14 | 2:35 ± 1 |
| LPS | >24:00 | 3:14 ± 4 | 2:35 ± 14 |
| SPS | >24:00 | 3:30 ± 9 | 2:32 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horan, C.; Genedy, M.; Juenger, M.; van Oort, E. Fly Ash-Based Geopolymers as Lower Carbon Footprint Alternatives to Portland Cement for Well Cementing Applications. Energies 2022, 15, 8819. https://doi.org/10.3390/en15238819
Horan C, Genedy M, Juenger M, van Oort E. Fly Ash-Based Geopolymers as Lower Carbon Footprint Alternatives to Portland Cement for Well Cementing Applications. Energies. 2022; 15(23):8819. https://doi.org/10.3390/en15238819
Chicago/Turabian StyleHoran, Cameron, Moneeb Genedy, Maria Juenger, and Eric van Oort. 2022. "Fly Ash-Based Geopolymers as Lower Carbon Footprint Alternatives to Portland Cement for Well Cementing Applications" Energies 15, no. 23: 8819. https://doi.org/10.3390/en15238819
APA StyleHoran, C., Genedy, M., Juenger, M., & van Oort, E. (2022). Fly Ash-Based Geopolymers as Lower Carbon Footprint Alternatives to Portland Cement for Well Cementing Applications. Energies, 15(23), 8819. https://doi.org/10.3390/en15238819






