Pyrolytic Depolymerization Mechanisms for Post-Consumer Plastic Wastes †
Abstract
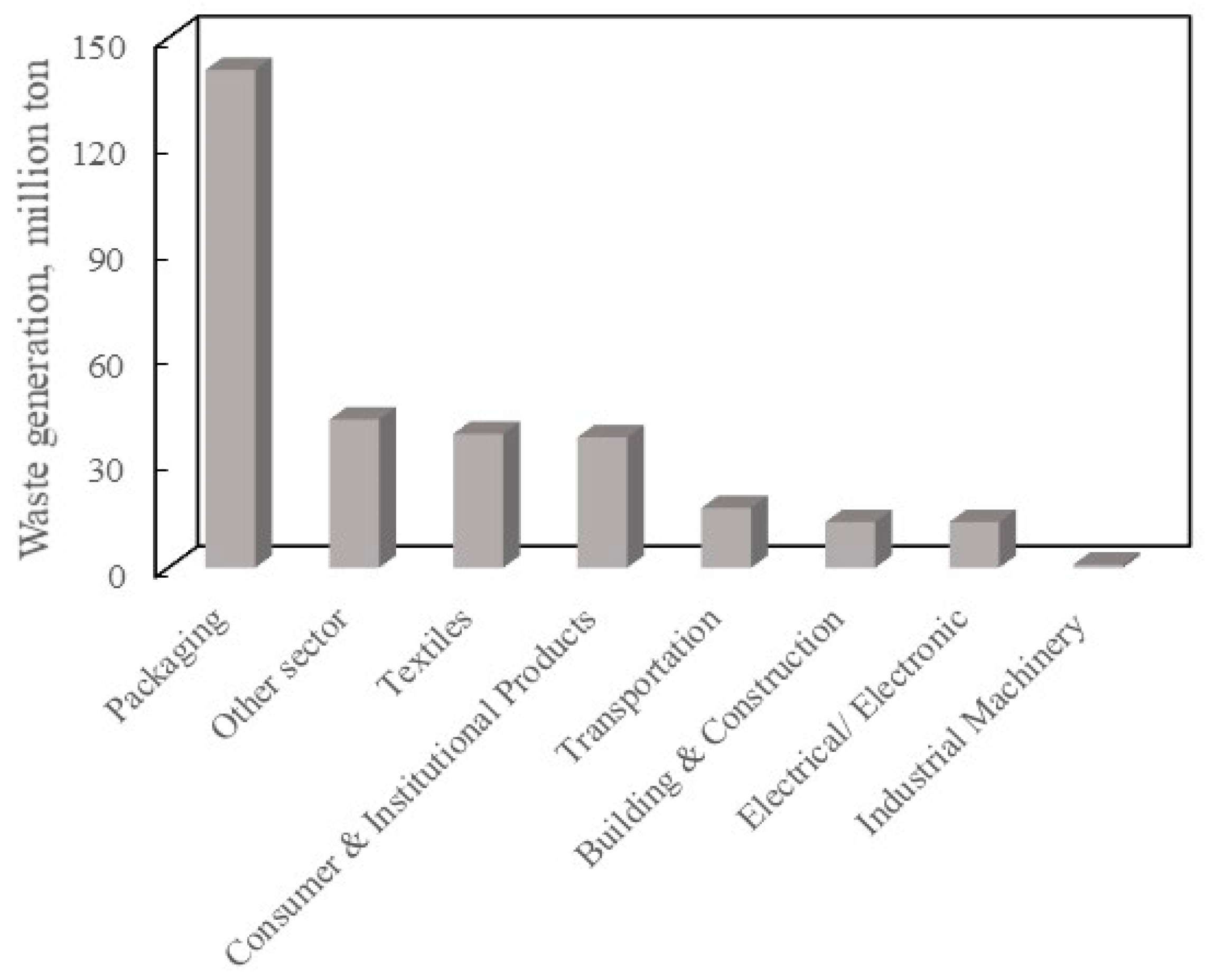
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pyrolysis-GC/MS Experiments
2.3. Batch-Scale Pyrolysis of Landfill Liner
2.3.1. Product Analysis
2.3.2. Fuel Properties Analysis
3. Results
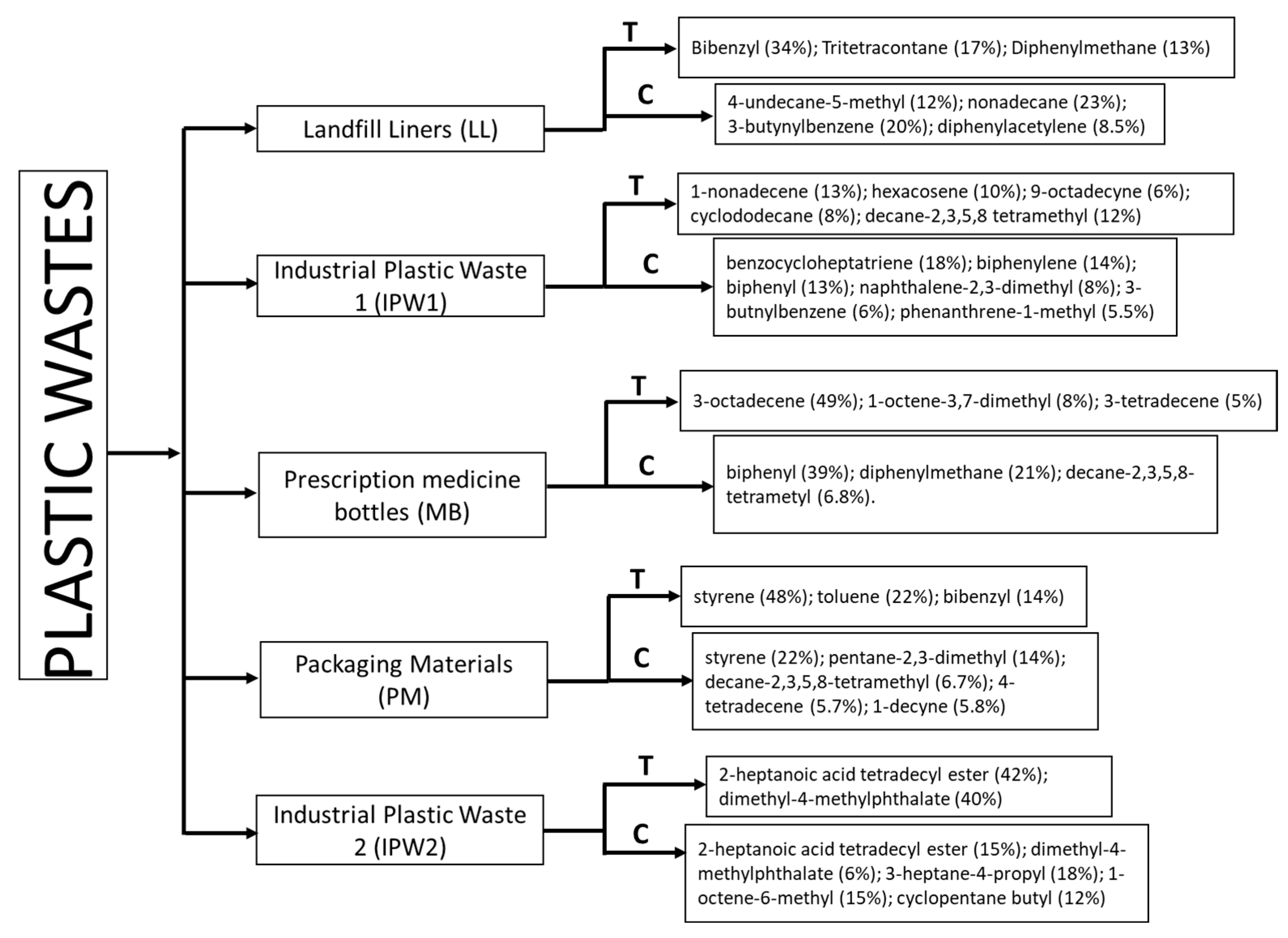
3.1. Landfill Liners (LL) Pyrolysis
3.2. Industrial Plastic Waste 1 (IPW1) Pyrolysis
3.3. Prescription Medicine Bottles (MB) Pyrolysis
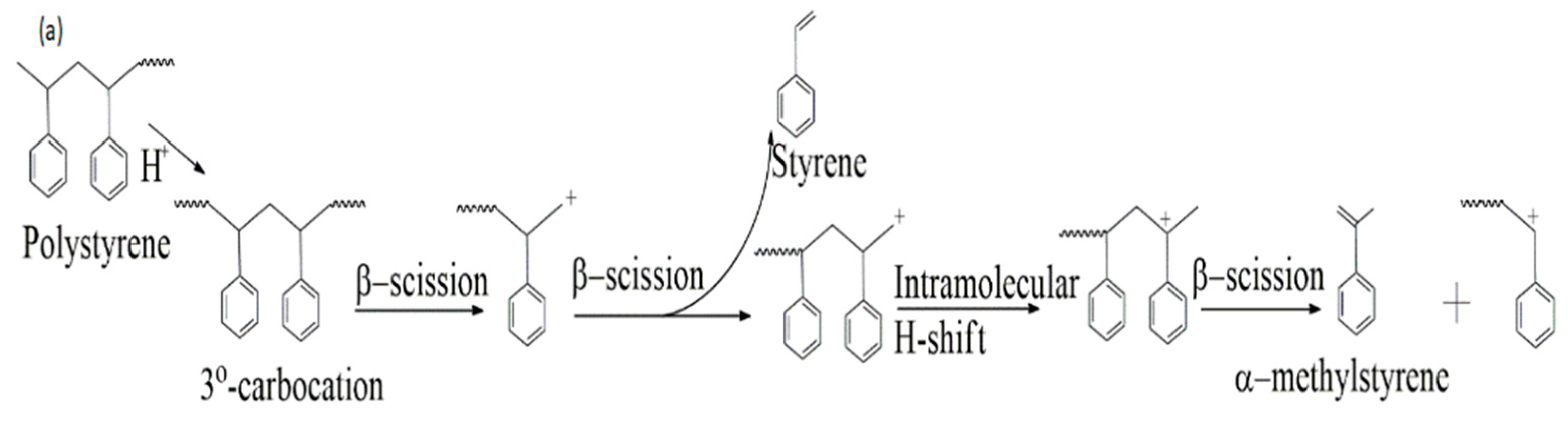
3.4. Packaging Materials (PM) Pyrolysis
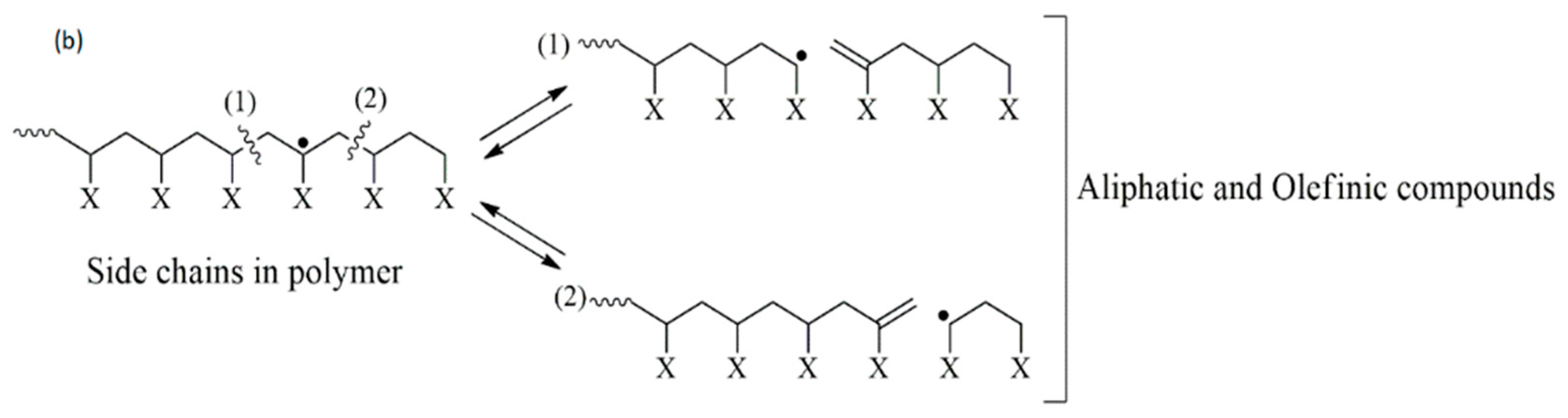
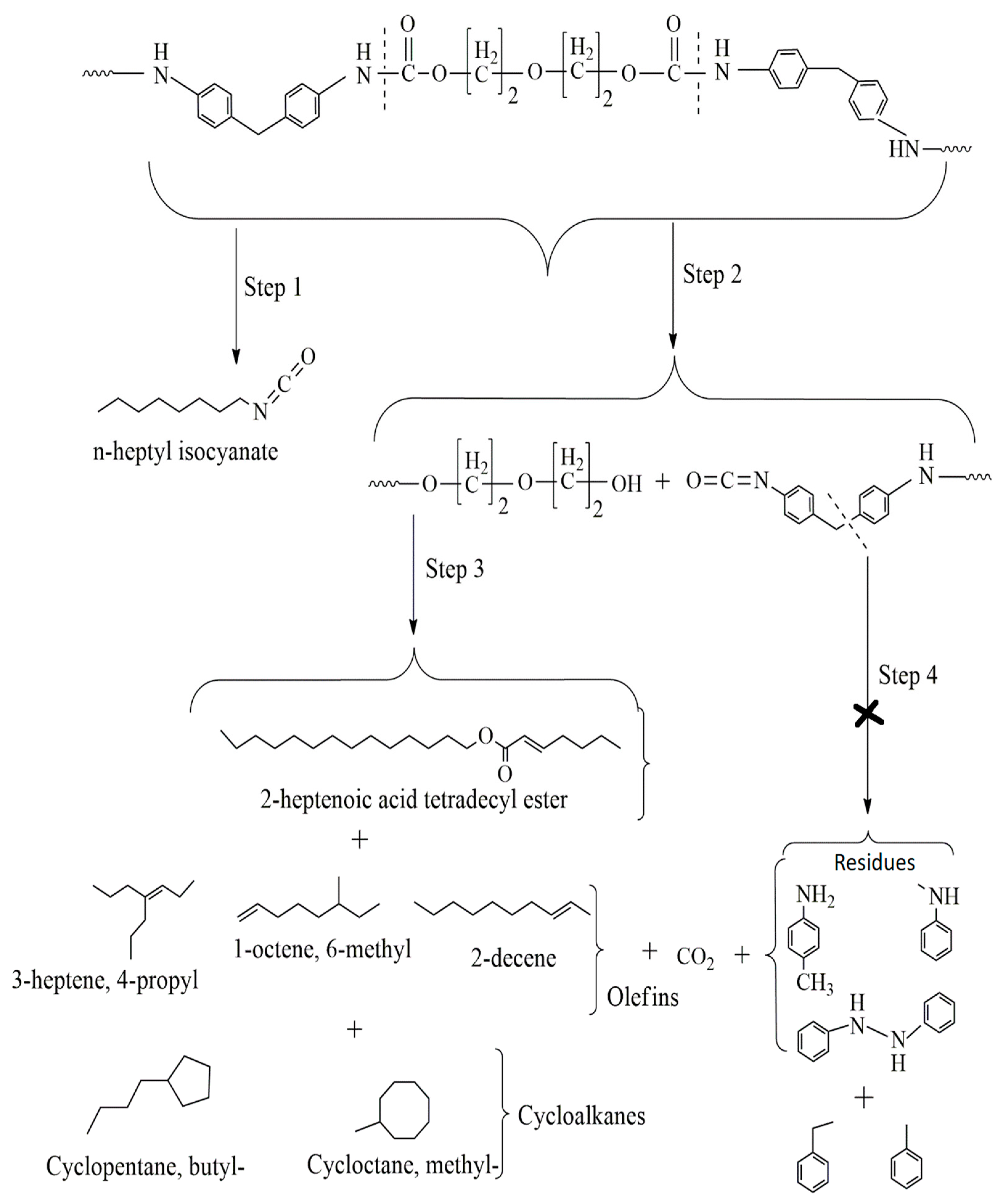
3.5. Industrial Plastic Waste 2 (IPW2) Pyrolysis
- Dissociation of urethane to isocyanate (R–NCO) and alcohol (OH–R1–),
- Dissociation to primary amine (R–NH2), olefin (CH2=CH=R1–), and carbon dioxide,
4. Batch-Scale Pyrolysis Validation of Landfill Liner (LL) Plastic Waste
4.1. Compositional Analysis of PCO Distillate Fractions
4.2. Fuel Properties of Jet Fuel Fraction
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guglielmi, G. In the next 30 years, we’ll make four times more plastic waste than we ever have. Science 2017. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, 1700782. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency Office of Solid Waste (5306P) Municipal Solid Waste in The United States, EPA530-R-13-001 (2013). Available online: https://archive.epa.gov/epawaste/nonhaz/municipal/web/html/msw99.html (accessed on 17 February 2022).
- Hoornweg, D.; Bhada-Tata, P.; Kennedy, C. Environment: Waste production must peak this century. Nature 2013, 502, 615–616. [Google Scholar] [CrossRef] [PubMed]
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2018; p. 29. ISBN 978-1-4648-1329-0. Available online: https://openknowledge.worldbank.org/handle/10986/30317 (accessed on 17 February 2022).
- Bagri, R.; Williams, P.T. Catalytic pyrolysis of polyethylene. J. Anal. Appl. Pyrolysis 2012, 63, 29–41. [Google Scholar] [CrossRef]
- Williams, P.T.; Williams, E.A. Fluidised bed pyrolysis of low-density polyethylene to produce petrochemical feedstock. J. Anal. Appl. Pyrolysis 1999, 51, 107–126. [Google Scholar] [CrossRef]
- Liu, Y.; Qian, J.; Wang, J. Pyrolysis of polystyrene waste in a fluidized-bed reactor to obtain styrene monomer and gasoline fraction. Fuel Process. Technol. 2000, 63, 45–55. [Google Scholar] [CrossRef]
- Williams, E.A.; Williams, P.T. Analysis of products derived from the fast pyrolysis of plastic waste. J. Anal. Appl. Pyrolysis 1997, 40, 347–363. [Google Scholar] [CrossRef]
- Williams, J.H.; Williams, P.T. Analysis of products from the pyrolysis of plastics recovered from the commercial scale recycling of waste electrical and electronic equipment. J. Anal. Appl. Pyrolysis 2007, 79, 375–386. [Google Scholar] [CrossRef]
- Miandad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Rehan, M.; Nizami, A.S. Catalytic pyrolysis of plastic waste: A review. Process Saf. Environ. Prot. 2016, 102, 822–838. [Google Scholar] [CrossRef]
- Adrados, A.; de Marco, I.; Caballero, B.M.; López, A.; Laresgoiti, M.F.; Torres, A. Pyrolysis of plastic packaging waste: A comparison of plastic residuals from material recovery facilities with simulated plastic waste. Waste Manag. 2012, 32, 826–832. [Google Scholar] [CrossRef]
- López, A.; de Marco, I.; Caballero, B.M.; Adrados, A.; Laresgoiti, M.F. Deactivation and regeneration of ZSM-5 zeolite in catalytic pyrolysis of plastic wastes. Waste Manag. 2011, 31, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.R.; Kunwar, B.; Moser, B.R.; Rajagopalan, N.; Sharma, B.K. Catalytic thermal cracking of postconsumer waste plastics to fuels. 1. Kinetics and Optimization. Energy Fuels 2015, 29, 6068–6077. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, H.; Yadavalli, G.; Zhu, L.; Wei, Y.; Liu, Y. Gasoline-range hydrocarbons produced from microwave-induced pyrolysis of low-density polyethylene over ZSM-5. Fuel 2015, 144, 33–42. [Google Scholar] [CrossRef]
- Auxilio, A.R.; Choo, W.; Kohli, I.; Srivatsa, S.C.; Bhattacharya, S. An experimental study on thermo-catalytic pyrolysis of plastic waste using a continuous pyrolyzer. Waste Manag. 2017, 67, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Park, Y.K. Catalytic pyrolysis of polyethylene and polypropylene over desilicated beta and Al-MSU-F. Catalysts 2018, 8, 501. [Google Scholar] [CrossRef]
- Jia, C.; Xie, S.; Zhang, W.; Intan, N.N.; Sampath, J.; Pfaendtner, J.; Lin, H. Deconstruction of high-density polyethylene into liquid hydrocarbon fuels and lubricants by hydrogenolyis over Ru catalyst. Chem Catal. 2021, 1, 437–455. [Google Scholar] [CrossRef]
- Tomasek, S.; Varga, Z.; Hancsok, J. Production of jet fuel from cracked fractions of waste polypropylene and polyethylene. Fuel Process. Technol. 2020, 197, 106197. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Ke, L.; Dai, L.; Wu, Q.; Cobb, K.; Zeng, Y.; Zou, R.; Liu, Y.; Ruan, R. A review on catalytic pyrolysis of plastic wastes to high-value products. Energy Convers. Manag. 2022, 254, 115243. [Google Scholar] [CrossRef]
- Dai, L.; Zhou, N.; Lv, Y.; Cheng, Y.; Wang, Y.; Liu, Y.; Cobb, K.; Chen, P.; Lei, H.; Ruan, R. Pyrolysis technology for plastic waste recycling: A state-of-the-art review. Prog. Energy Combust. Sci. 2022, 93, 101021. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Ampah, J.D.; Soudagar, E.M.; Veza, I.; Kingsley, U.; Afrane, S.; Jin, C.; Liu, H.; Elfasakhany, A.; Buyondo, K.A. Effect of hybrid nanoparticle additives in n-butanol/waste plastic oil/diesel blends on combustion, particulate and gaseous emissions from diesel engine evaluated with entropy-weighted PROMETHEE II and TOPSIS:Environmental and health risks of plastic waste. Energy Convers. Manag. 2022, 264, 115758. [Google Scholar] [CrossRef]
- Rocha-Santos, T.; Duarte, A.C. A critical overview of the analytical approaches to the occurrence, the fate and the behavior of microplastics in the environment. Trends Anal. Chem. 2015, 65, 47–53. [Google Scholar] [CrossRef]
- Frère, L.; Paul-Pont, I.; Moreau, J.; Soudant, P.; Lambert, C.; Huvet, A.; Rinnert, E. A semi-automated Raman micro-spectroscopy method for morphological and chemical characterizations of microplastic litter. Mar. Pollut. Bull. 2016, 113, 461–468. [Google Scholar] [CrossRef]
- Camacho, W.; Karlsson, S. NIR, DSC, and FTIR as quantitative methods for compositional analysis of blends of polymers obtained from recycled mixed plastic wastes. Polym. Eng. Sci. 2001, 41, 1626–1635. [Google Scholar] [CrossRef]
- Hummel, D.O.; Scholl, F.K. Atlas of Polymer and Plastics Analysis V.2: Plastics, Fibers, Rubbers, Resins, Starting and Auxiliary Materials, Degradation Products; Carl Hanser Verlag Chemie GmbH (Wiley-VCH GmbH): Weinheim, Germany, 1988. [Google Scholar] [CrossRef]
- Hallensleben, M.L.; Rambke, J. Photoresponsive polymers, 2.4-vinylazobenzene, a new polymerizable azo-monomer, Die Makromolekulare Chemie. Rapid Commun. 1989, 10, 559–562. [Google Scholar] [CrossRef]
- Kusch, P.; Knupp, G. Headspace-SPME-GC-MS identification of volatile organic compounds released from expanded polystyrene. J. Polym. Environ. 2004, 12, 83–87. [Google Scholar] [CrossRef]
- Wampler, T.P. Applied Pyrolysis Handbook; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar] [CrossRef]
- Moldoveanu, S. Analytical Pyrolysis of Synthetic Organic Polymers; Elsevier: Amsterdam, The Netherlands, 2005; Volume 25. [Google Scholar] [CrossRef]
- Yang, M.; Shibasaki, Y. Mechanisms of thermal degradation of polystryrene, polymethacrylonitrile, and their copolymers on flash pyrolysis. J. Polym. Sci. Part A Polym. Chem. 1998, 36, 2315–2330. [Google Scholar] [CrossRef]
- Sharma, B.K.; Moser, B.R.; Vermillion, K.E.; Doll, K.M.; Rajagopalan, N. Production, characterization and fuel properties of alternative diesel fuel from pyrolysis of waste plastic grocery bags. Fuel Process. Technol. 2014, 122, 79–90. [Google Scholar] [CrossRef]
- Kunwar, B.; Moser, B.R.; Chandrasekaran, S.R.; Rajagopalan, N.; Sharma, B.K. Catalytic and thermal depolymerization of low value post-consumer high density polyethylene plastic. Energy 2016, 111, 884–892. [Google Scholar] [CrossRef]
- Suarez, P.A.Z.; Moser, B.R.; Sharma, B.K.; Erhan, S.Z. Comparing the lubricity of biofuels obtained from pyrolysis and alcoholysis of soybean oil and their blends with petroleum diesel. Fuel 2009, 88, 1143–1147. [Google Scholar] [CrossRef]
- Moser, B.R.; Williams, A.; Haas, M.J.; McCormick, R.L. Exhaust emissions and fuel properties of partially hydrogenated soybean oil methyl esters blended with ultra-low sulfur diesel fuel. Fuel Process. Technol. 2009, 90, 1122–1128. [Google Scholar] [CrossRef]
- Moser, B.R. Impact of fatty ester composition on low temperature properties of biodiesel-petroleum diesel blends. Fuel 2014, 115, 500–506. [Google Scholar] [CrossRef]
- Doll, K.M.; Moser, B.R.; Erhan, S.Z. Surface tension studies of alkyl esters and epoxidized alkyl esters relevant to oleochemically based fuel additives. Energy Fuels 2007, 21, 3044–3048. [Google Scholar] [CrossRef]
- Manos, G.; Garforth, A.; Dwyer, J. Thermolysis of low-density polyethylene catalyzed by zeolities. J. Anal. Appl. Pyrolysis 1994, 29, 45–55. [Google Scholar]
- Costa, P.A.; Pinto, F.J.; Ramos, A.M.; Gulyurtlu, I.K.; Cabrita, I.A.; Bernardo, M.S. Kinetic evaluation of the pyrolysis of polyethylene waste. Energy Fuels 2007, 21, 2489–2498. [Google Scholar] [CrossRef]
- Rizzarelli, P.; Rapisarda, M.; Perna, S.; Mirabella, E.F.; La Carta, S.; Puglisi, C.; Valenti, G. Determination of polyethylene in biodegradable polymer blends and in compostable carrier bags by Py-GC/MS and TGA. J. Anal. Appl. Pyrolysis 2016, 117, 72–81. [Google Scholar] [CrossRef]
- De Stefanis, A.; Cafarelli, P.; Gallese, F.; Borsella, E.; Nana, A.; Perez, G. Catalytic pyrolysis of polyethylene: A comparison between pillared and restructured clays. J. Anal. Appl. Pyrolysis 2013, 104, 479–484. [Google Scholar] [CrossRef]
- Leclerc, P.; Doucet, J.; Chaoukia, J. Development of a microwave thermogravimetric analyzer and its application on polystyrene microwave pyrolysis kinetics. J. Anal. Appl. Pyrolysis 2018, 130, 209–215. [Google Scholar] [CrossRef]
- Ballice, L.; Reimert, R. Classification of volatile products from the temperature-programmed pyrolysis of polypropylene (PP) atactic-polypropyelen (APP) and thermogravimetrically derived kinetics of pyrolysis. Chem. Eng. Process. Process Intensif. 2002, 41, 289–296. [Google Scholar] [CrossRef]
- Audisio, G.; Silvani, A. Catalytic thermal degradation of polymers degradation of polypropylene. J. Anal. Appl. Pyrolysis 1984, 7, 83–90. [Google Scholar] [CrossRef]
- Ojha, D.K.; Vinu, R. Resource recovery via catalytic fast pyrolysis of polystyrene using zeolite. J. Anal. Appl. Pyrolysis 2015, 113, 349–359. [Google Scholar] [CrossRef]
- Zhou, X.; Broadbelt, L.J.; Vinu, R. Mechanistic understanding of thermochemical conversion of Polymers and lignocellulosic biomass. Adv. Chem. Eng. 2016, 49, 95–198. [Google Scholar] [CrossRef]
- Fabbri, D.; Trombini, C.; Vassura, I. Analysis of polystyrene in polluted sediments by pyrolysis-gas chromatography-mass spectroscopy. J. Chromatogr. Sci. 1998, 36, 600–604. [Google Scholar] [CrossRef]
- Lin, R.; White, R.L. Acid-catalyzed cracking of polystyrene. J. Appl. Polym. Sci. 1997, 63, 1287–1298. [Google Scholar] [CrossRef]
- Lattimer, R.P.; Polce, M.J.; Wesdemiotis, C. MALDI-MS analysis of pyrolysis products from a segmented polyurethane. J. Anal. Appl. Pyrolysis 1998, 48, 1–15. [Google Scholar] [CrossRef]
- Hileman, F.D.; Voorhees, K.J.; Wojcik, L.H. Pyrolysis of a flexible urethane foam. J. Polym. Sci. Polym. Chem. Ed. 1975, 13, 571–584. [Google Scholar] [CrossRef]
- Font, R.; Fullana, A.; Caballero, J.A.; Candela, J.; Garcia, A. Pyrolysis study of polyurethane. J. Anal. Appl. Pyrolysis 2001, 58, 63–77. [Google Scholar] [CrossRef]
- Jiao, L.; Xiao, H.; Wang, Q.; Sun, J. Thermal degradation characteristics of rigid polyurethane foam and volatile products analysis with TG-FTIR-MS. Polym. Degrad. Stab. 2013, 98, 2687–2696. [Google Scholar] [CrossRef]
- Dyer, E.; Wright, G.C. Thermal degradation of alkyl N-phenylcarbamates. J. Am. Chem. Soc. 1959, 81, 2138–2143. [Google Scholar] [CrossRef]
- Williams, P.T.; Williams, E.A. Interaction of plastics in mixed-plastics pyrolysis. Energy Fuels 1999, 13, 188–196. [Google Scholar] [CrossRef]
- Pinto, F.; Costa, P.; Gulyurtlu, I.; Cabrita, I. Pyrolysis of plastic wastes. 1. Effect of plastic waste composition of product yield. J. Anal. Appl. Pyrolysis 1999, 51, 39–55. [Google Scholar] [CrossRef]
- Kizza, R.; Bonadda, N.; Seay, J. Qualitative and energy recovery potential analysis: Plastic-derived fuel oil versus conventional diesel oil. Clean Technol. Environ. Policy 2022, 24, 789–800. [Google Scholar] [CrossRef]
- Marcilla, A.; Gomez, A.; Garcia, A.N.; Olaya, M.M. Kinetic study of the catalytic decomposition of different commercial polyethylenes over an MCM-41 catalyst. J. Anal. Appl. Pyrolysis 2002, 64, 85–101. [Google Scholar] [CrossRef]
- de la Puente, G.; Sedran, U. Recycling polystyrene into fuels by means of FCC: Performance of various acidic catalyst. Appl. Catal. B Environ. 1998, 19, 305–311. [Google Scholar] [CrossRef]
- Mertinkat, J.; Predel, L.; Kaminsky, W. Cracking catalysts used as fluidized bed material in Hamburg pyrolysis process. J. Anal. Appl. Pyrolysis 1999, 49, 87–95. [Google Scholar] [CrossRef]
- Faravelli, T.; Bozzano, G.; Colombo, M.; Ranzi, E.; Dente, M. Kinetic modeling of thermal degradation of polyethylene and polystyrene mixtures. J. Anal. Appl. Pyrolysis 2003, 70, 761–777. [Google Scholar] [CrossRef]




| Plastic Waste Material | Catalyst Used | Designation Used |
|---|---|---|
| Polyolefinic plastic waste | ||
| Landfill liners | Y-zeolite | LL |
| Industrial plastic waste 1 | Y-zeolite | IPW1 |
| Medicine bottles | Y-zeolite | MB |
| Polystyrene plastic waste | ||
| Packaging material | FCC spent catalyst | PM |
| Polyurethane plastic waste | ||
| Industrial plastic waste 2 | Sulfated zirconia | IPW2 |
| Compound Name | Formula (Molecular Weight, g/mol) | Area, % | ||
|---|---|---|---|---|
| Without Catalyst | With Y-Zeolite Catalyst | |||
| at 600 °C | at 600 °C | at 500 °C | ||
| Cyclopropane-1,1-dimethyl- | C5H10 (70) | - | - | 1.23 |
| 4-undecene-5-methyl | C12H24 (168) | 3.14 | 11.94 | - |
| Azulene | C10H8 (128) | - | - | 4.99 |
| Tritetracontane | C43H88 (604) | 17.02 | - | - |
| Benzene-1,3-bis(1,1-dimethylethyl)- | C14H22 (190) | 9.36 | 4.09 | 1.12 |
| 2-tridecenal | C13H24O (196) | - | 1.39 | - |
| Decane-2,3,5,8-tetramethyl- | C14H30 (198) | - | - | 0.36 |
| Naphthalene-1-methyl- | C11H10 (142) | - | - | 33.06 |
| Cyclopentane-1-pentyl, 2-propyl- | C13H26 (182) | 6.03 | - | - |
| Pent-1-yn-3-ene, 4-methyl-3-phenyl- | C12H12 (156) | - | 2.24 | 1.30 |
| Biphenyl | C12H10 (154) | 1.80 | 2.87 | 7.28 |
| Acenaphthalene | C12H10 (154) | - | - | 11.27 |
| Diphenylmethane | C13H12 (168) | 13.07 | 3.43 | - |
| Biphenylene | C12H8 (152) | 7.12 | 20.48 | |
| Cyclohexane-1,2,4,5-tetraethyl | C14H28 (196) | 2.16 | 1.38 | - |
| 4-nonene-5-butyl- | C13H26 (182) | - | 1.45 | |
| Bibenzyl | C14H14 (182) | 34.34 | - | - |
| Stilbene | C14H12 (180) | - | 2.27 | 2.46 |
| Fluorene | C13H10 (166) | - | - | 0.77 |
| Heptane-1,1-dicyclohexyl- | C19H36 (264) | - | - | 0.20 |
| 3,5-dodecadiene-2-methyl- | C13H24 (180) | - | 1.56 | - |
| Diphenylacetylene | C14H10 (178) | - | 8.54 | 0.28 |
| 2-methyltetracosane | C25H52 (352) | - | - | 9.36 |
| Nonadecane | C19H40 (269) | - | 22.92 | - |
| 3-butynylbenzene | C10H10 (130) | 8.32 | 20.37 | 3.21 |
| 4-isopropyldicyclohexylmethane | C16H30 (222) | - | 1.77 | - |
| Phenanthrene-1-methyl- | C15H12 (192) | - | 4.20 | 1.55 |
| 1-nonadecene | C19H38 (266) | 4.78 | - | - |
| Anthracene | - | 2.47 | 1.07 | |
| Compound Name | Formula (Molecular Weight, g/mol) | Area, % | ||
|---|---|---|---|---|
| Without Catalyst | With Y-Zeolite Catalyst | |||
| at 600 °C | at 600 °C | at 500 °C | ||
| 2-hexene-5-methyl- | C7H14 (98) | 4.17 | - | - |
| Cyclopentane-1,2-dimethyl | C7H15 (98) | 2.94 | - | - |
| Tritetracontane | C43H88 (604) | 6.64 | 0.97 | 2.59 |
| 1-decene | C10H20 (140) | 3.25 | - | - |
| 7-tetradecene | C14H28 (196) | 4.66 | 3.96 | 4.30 |
| 1-nonene-4,6,8-trimethyl- | C12H24 (168) | 0.97 | - | - |
| 1,6-heptadiene-3,3-dimethyl | C9H16 (124) | - | 0.57 | - |
| 4-decyne | C10H18 (138) | 0.50 | - | - |
| Decane-2,3,5,8-tetramethyl- | C14H30 (198) | 11.78 | 6.86 | 2.34 |
| 6-dodecyne | C12H22 (166) | 3.83 | - | 1.92 |
| 9-octadecene | C18H36 (252) | 9.09 | - | 1.62 |
| Cyclododecane | C12H24 (168) | 7.73 | - | 0.61 |
| Azulene | C10H8 (128) | - | - | 5.73 |
| Cyclotetradecane | C14H28 (196) | 3.58 | - | - |
| 7-hexadecyne | C16H30 (222) | 1.43 | - | - |
| 1-nonadecene | C19H38 (266) | 13.26 | - | - |
| Cyclopentane-1-pentyl-2-propyl- | C13H26 (182) | - | - | 7.30 |
| Benzocycloheptatriene | C11H10 (142) | - | 17.76 | 14.32 |
| Cyclooctene-3-methyl- | C9H16 (124) | 1.37 | - | - |
| Hexane-1-(isopropylidenecyclopropyl) | C12H22 (166) | - | - | 1.24 |
| Biphenyl | C12H10 (154) | - | 13.07 | 4.70 |
| 4,4-dipropylheptane | C13H28 (184) | - | 2.06 | |
| Naphthalene-2,3-dimethyl- | C12H12 (156) | - | 7.93 | 14.17 |
| Acenaphthalene | C12H10 (154) | - | 2.38 | 1.20 |
| Diphenylmethane | C13H12 (168) | - | 9.94 | - |
| Biphenylene | C12H8 (152) | - | 13.84 | 6.21 |
| 2-octene-2,3,7-trimethyl- | C11H22 (154) | - | - | 1.66 |
| Cyclopentane-1,1,3,4-tetramethyl | C9H18 (126) | - | 1.52 | - |
| cis stilbene | C14H12 (180) | - | - | 4.03 |
| Bibenzyl | C14H14 (182) | - | 1.56 | - |
| 2-undecene-4,5-dimethyl- | C13H26 (182) | - | - | 0.76 |
| 9-octadecyne | C18H34 (250) | 5.59 | 3.28 | - |
| 2-methyltetracosane | C25H52 (352) | - | - | 2.09 |
| Anthracene-1,2-dihydro | C14H12 (180) | - | 4.88 | 1.67 |
| 3-butynylbenzene | C10H10 (130) | - | 5.96 | 4.63 |
| Nonadecane | C19H40 (268) | 2.60 | - | 2.46 |
| Phenanthrene-1-methyl- | C15H12 (152) | - | 5.52 | 10.23 |
| Cyclohexane-tricosyl- | C29H58 (406) | - | - | 2.16 |
| 2-methyltetracosane | C25H52 (352) | 1.71 | - | - |
| 1-hexacosene | C26H52 (364) | 10.02 | - | - |
| 1,7-hexadecadiene | C16H30 (222) | 4.87 | - | - |
| Compound Name | Formula (Molecular Weight, g/mol) | Area, % | ||
|---|---|---|---|---|
| Without Catalyst | With Y-Zeolite Catalyst | |||
| at 600 °C | at 600 °C | at 500 °C | ||
| Cyclopropane-butyl- | C7H14 (98) | 12.33 | - | - |
| 1-octene-3,7-dimethyl- | C10H22 (140) | 7.94 | - | - |
| Pentane-2-cyclopropyl- | C8H16 (112) | 3.91 | - | - |
| Cyclopentane-1,3-dimethyl- | C7H14 (98) | 1.35 | - | - |
| 1,5-hexadie-3-yne | C6H6 (78) | 0.70 | - | - |
| 3-hexene | C6H12 (84) | 2.28 | 2.88 | - |
| 4-undecene, 5-methyl- | C12H24 (168) | - | 0.74 | 9.65 |
| 1,5-decadiyne | C10H14 (134) | 0.82 | - | - |
| 2-nonyne | C9H16 (124) | 0.90 | - | - |
| 1,1,4-trimethylcyclohexane | C9H18 (126) | 0.77 | - | - |
| Cyclopentane-1,1,3,4-tetramethyl- | C9H18 (126) | 4.17 | - | - |
| 3-octyne-2-methyl- | C9H16 (124) | 0.86 | - | - |
| 1-hexene-3,3-dimethyl- | C8H16 (112) | 1.14 | - | - |
| Octadecane-5-methyl- | C19H40 (268) | - | 2.66 | - |
| Tritetracontane | C43H88 (604) | - | 1.33 | 21.37 |
| 1-nonene-4,6,8-trimethyl- | C12H24 (168) | - | 1.19 | - |
| Benzene (1-methylpropyl)- | C10H14 (134) | - | - | 1.32 |
| 3-octadecene | C18H36 (252) | 48.57 | - | - |
| 1-octene-3,7-dimethyl- | C10H20 (140) | - | 1.15 | - |
| Cyclopentane hexyl- | C11H22 (154) | 5.52 | 0.74 | - |
| 1,4-undecadiene | C11H20 (152) | 1.15 | - | - |
| Benzene (1-cyclopropyl-1-methylethyl)- | C12H16 (160) | - | 1.06 | - |
| Naphthalene | C10H8 (128) | - | 2.52 | 10.37 |
| 1,3-cyclopentadiene-5-(1,3-dimethylbutylidene)- | C11H16 (148) | - | - | 4.86 |
| 3-tetradecene | C14H28 (196) | 5.11 | - | - |
| Decane-2,3,5,8-tetramethyl- | C14H30 (198) | - | 6.80 | - |
| 1,13-tetradecadiene | C14H26 (194) | 1.54 | - | - |
| Biphenyl | C12H10 (154) | - | 39.15 | 6.27 |
| 2-allylnaphtahlene | C13H12 (1680 | - | 1.27 | - |
| Acenaphathalene | C12H10 (154) | - | 0.42 | - |
| Diphenylmethane | C13H12 (168) | - | 20.98 | - |
| Benzene-1,1′-(1-methyl-1,2-ethanediyl) bis- | C15H16 (196) | - | 1.75 | - |
| 1,19-eicosadiene | C20H28 (278) | 0.94 | - | - |
| 1,1”-biphenyl-4-ethenyl- | C14H12 (180) | - | 7.51 | - |
| Benzene-1,1′-(1,3-propanediyl) bis- | C15H16 (196) | - | 0.49 | - |
| Diphenylacetylene | C14H10 (178) | - | 0.96 | - |
| 1,2-diphenylcyclopropane | C15H14 (194) | - | 0.16 | - |
| Nonadecane | C19H40 (268) | - | - | 12.27 |
| 3-butyynylbenzene | C10H10 (130) | - | 2.92 | 4.87 |
| 1,3,5-cycloheptatriene-7,7-dimethyl- | C9H12 (120) | - | 0.61 | - |
| Benzene-1,1″-(2-cyclopropen-1-ylidene) bis- | C15H12 (192) | - | 0.24 | 5.45 |
| Anthracene | C14H10 (178) | - | 0.84 | 16.25 |
| 1,3-butadiene-1,4-diphenyl- | C16H14 (206) | - | 1.62 | 1.79 |
| (4-methyl-1-methylenepent-4-enyl) benzene | C13H16 (172) | - | - | 5.52 |
| Compound Name | Formula (Molecular Weight, g/mol) | Area, % | ||
|---|---|---|---|---|
| Without Catalyst | With FCC Spent Catalyst | |||
| at 600 °C | at 600 °C | at 500 °C | ||
| n-hexane | C6H14 (80) | - | - | 1.15 |
| Pentane-2,3-dimethyl- | C7H16 (100) | - | 14.31 | 9.01 |
| Toluene | C7H9 (92) | 21.45 | - | - |
| Styrene | C8H8 (104) | 48.35 | 22.47 | 36.48 |
| α-methylstyrene | C9H10 (118) | - | - | 18.58 |
| 2,4-heptadiene | C7H12 (96) | - | 15.30 | - |
| 1,6-heptadiyne | C7H8 (92) | - | - | 1.44 |
| 3-undecen-1-yne | C11H18 (150) | - | 1.10 | - |
| Cyclopentane-1-ethyl-2-methyl-cis | C8H16 (112) | - | 6.62 | - |
| 1-decyne | C10H18 (138) | - | 5.77 | - |
| 3-hexene-2,2,5,5-tetramethyl- | C10H20 (140) | - | 4.98 | - |
| Cyclohexane-1,2,4-trimethyl- | C9H18 (126) | - | 2.13 | - |
| Octane-2,7-dimethyl- | C10H22 (142) | - | 3.33 | - |
| Cyclopentane-1,2-dipropyl- | C11H22 (154) | - | 0.83 | - |
| 4-tetradecene | C14H28 (196) | - | 5.66 | - |
| Biphenyl | C12H10 (154) | - | - | 2.26 |
| 2-undecene | C11H22 (154) | - | - | 0.63 |
| 2,3,5,8-tetramethyldecane | C14H30 (198) | - | - | 0.61 |
| Acenaphthalene | C12H10 (154) | - | - | 0.64 |
| Biphenylene | C12H8 (152) | - | - | 2.38 |
| Tritetracontane | C43H88 (604) | - | 1.92 | - |
| Cyclotetradecane | C14H28 (196) | - | - | 2.32 |
| Decane-3-methyl- | C11H24 (156) | - | - | 6.05 |
| Bibenzyl | C14H14 (182) | 14.09 | - | - |
| Decane-2,6,10-trimethyl | C15H32 (212) | - | - | 17.73 |
| Decane-2,3,5,8-tetamethyl- | C14H30 (198) | - | 6.70 | - |
| Dodecane-2-phenyl- | C18H30 (246) | - | - | 0.73 |
| Naphthalene-1,2,3,4-tetrahydro-2-phenyl- | C16H16 (208) | 16.11 | - | - |
| Bicyclo [10.1.0] tridec-1-ene | C13H22 (178) | - | 8.88 | - |
| Compound Name | Formula (Molecular Weight, g/mol) | Area, % | ||
|---|---|---|---|---|
| Without Catalyst | With Sulfated Zirconia Catalyst | |||
| at 600 °C | at 600 °C | at 500 °C | ||
| Cyclopentane-butyl- | C9H18 (126) | - | 11.62 | 7.99 |
| 1-octene-6-methyl- | C9H18 (126) | 4.13 | 14.73 | 8.23 |
| 4-nonene | C9H18 (126) | - | - | 2.36 |
| 5-undecene-4-methyl- | C12H24 (168) | 0.98 | 5.12 | 2.05 |
| Cyclooctane-methyl- | C9H18 (126) | - | 4.73 | - |
| Cyclohexane-1,3,5-trimethyl- | C9H18 (126) | - | - | 3.16 |
| 3-heptene-2-methyl- | C8H16 (112) | - | 2.86 | 2.23 |
| 3-heptene-4-propyl- | C10H20 (140) | - | 18.17 | 22.86 |
| 2-decene | C10H20 (140) | 5.81 | 9.03 | 3.93 |
| Cyclooctane-butyl- | C9H18 (126) | - | 3.01 | 0.79 |
| 2-octenal | C8H14O (126) | - | 0.83 | - |
| 1-tetradecene | C14H28 (182) | - | 1.62 | - |
| 1-tridecene | C13H26 (182) | 2.04 | - | 1.35 |
| Phthalic anhydride | C8H4O3 (148) | - | 4.24 | 2.70 |
| n-heptyl isocyanate | C8H15NO (141) | - | 2.02 | 1.22 |
| 1-methylpentyl cyclopropane | C9H18 (126) | 2.76 | - | - |
| Biphenyl | C12H10 (152) | - | - | 0.58 |
| Cyclododecane | C12H24 (168) | - | - | 0.30 |
| Decane-2,3,5,8-tetramethyl- | C14H30 (198) | - | 0.22 | 0.32 |
| Biphenylene | C12H8 (152) | - | - | 0.70 |
| Tritetracontane | C43H88 (604) | 0.30 | - | - |
| Bibenzyl | C14H14 (182) | 0.21 | - | - |
| Cyclotetradecane | C14H28 (196) | 0.18 | 0.13 | - |
| Decane-2,3,5,8-tetramethyl- | C14H30 (198) | 0.42 | - | - |
| 3-butnylbenzyl | C10H10 (130) | 0.23 | - | - |
| Anthracene | C14H10 (178) | 0.09 | - | |
| 3-eicosene | C20H40 (280) | 0.09 | - | - |
| Tetradecanoic acid-12-methylmethylester | C16H32O2 (256) | - | - | |
| 1-tetracosene | C24H48 (336) | 0.16 | 0.13 | 0.19 |
| Naphthalene-2-phenyl- | C16H12 (204) | - | 0.19 | |
| 1-nonadecene | C19H38 (266) | 0.50 | 0.09 | |
| 1,9-eicosadiene | C20H38 (278) | 0.27 | - | - |
| 1-benzylindole | C15H13N (207) | - | - | |
| 2-heptenoic acid tetradecyl ester | C22H42O2 (324) | 42.18 | 11.51 | |
| Dimethyl-4-methylphthalate | C26H24O4 (418) | 39.72 | 27.08 | |
| Samples | Elemental Properties | Calorific Value | |||
|---|---|---|---|---|---|
| Carbon, wt.% | Hydrogen, wt.% | Nitrogen, wt.% | Oxygen, wt.% | HHV, MJ/kg | |
| Landfill liner (LL) | 84.2 | 14.0 | 0.4 | 1.40 | 48.3 |
| Plastic crude oil (PCO) | 85.9 | 13.9 | 0.2 | - | 48.8 |
| Distillate Fractions | |||||
| Gasoline range (<150 °C) | 82.0 | 13.3 | 0.1 | 4.60 | 46.1 |
| Aviation range fuel (150–275 °C) | 85.8 | 14.3 | 0.2 | - | 49.4 |
| Gas oil plus range (>275 °C) | 86.1 | 14.1 | 0.3 | - | 49.2 |
| Commercial jet fuel | 86.0 | 13.9 | 0.2 | - | 48.9 |
| Distillate Fractions | Hydrocarbon Composition, wt.% | ||
|---|---|---|---|
| Paraffinic Hydrogen | Olefinic Hydrogen | Aromatic Hydrogen | |
| Gasoline range (<150 °C) | 90.9 | 8.7 | 0.4 |
| Aviation range fuel (150–275 °C) | 95.5 | 4.2 | 0.3 |
| Gas oil plus range (>275 °C) | 98.6 | 1 | 0.4 |
| Commercial jet fuel | 95.2 | 1.3 | 3.5 |
| Compound Name | Area, % |
|---|---|
| Gasoline range fraction (<150 °C) | |
| 1-hexene | 7.7 |
| 1-heptene | 10.3 |
| Heptane | 11.0 |
| cis-1-butyl-2-methylcyclopropane/1-cotene | 12.6 |
| Heptane, 2,4-dimethyl- | 13.1 |
| 1-nonene | 13.2 |
| Nonane | 13.0 |
| 1-decene | 11.2 |
| Decane | 8.0 |
| Aviation range fuel fraction (150–275 °C) | |
| 1-undecene | 4.9 |
| Undecane | 5.1 |
| 1-dodecene | 7.3 |
| Dodecane | 7.2 |
| 1-tridecene | 7.5 |
| Tridecane | 7.8 |
| 1-tetradecene | 8.0 |
| Tetradecane | 9.1 |
| 1-pentadecene | 7.3 |
| Pentadecane | 11.3 |
| 1-hexadecene | 5.6 |
| Hexadecane | 11.5 |
| Gas oil plus range fraction (>275 °C) | |
| Heptadecane | 7.2 |
| Heptadecane | 8.4 |
| Octadecane | 15.5 |
| Nonadecane | 18.9 |
| Eicosane | 17.7 |
| Heneicosane | 15.2 |
| Octacosane | 11.0 |
| Hentriacontane | 8.1 |
| Tetracosane | 5.0 |
| Properties | Method Used | Aviation Fuel Range Fraction | Commercial Jet Fuel |
|---|---|---|---|
| Cloud point, °C | ASTM D5773 | −17.2 ± 0.7 | −54.8 ± 0.2 |
| Pour point, °C | ASTM D5949 | −15 | −65 |
| Cold filter plugging point, °C | ASTM D6371 | −19 | >51 |
| Induction period, h | EN 15751 | 16.3 ± 0.1 | >90 |
| Kinematic viscosity at 40 °C, cSt | ASTM D445 | 1.88 | 1.31 |
| Kinematic viscosity at −20 °C, cSt | ASTM D445 | - | 4.16 ± 0.01 |
| Oxidative stability, °C | 201.5 ± 0.6 | 217 ± 0.3 | |
| Specific gravity, 15 °C | ASTM D 4052 | 0.7933 | 0.8126 |
| Density at 15 °C, kg/m3 | ASTM D4052 | 0.7925 | 0.8118 |
| Acid value, mg KOH/g | AOCS Cd 3d-63 | 0.24 ± 0.02 | 0.02 ± 0.01 |
| Gross Heat of Combustion (higher heating value, MJ/kg) | ASTM D4809 | 45.757 ± 0.255 | 45.17 ± 0.114 |
| Surface tension at 25 °C, mN/m | 25.6 ± 0.0 | 24.8 ± 0.1 | |
| Surface tension at 40 °C, mN/m | 24.2 ± 0.1 | 23.2 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohli, K.; Chandrasekaran, S.R.; Prajapati, R.; Kunwar, B.; Al-Salem, S.; Moser, B.R.; Sharma, B.K. Pyrolytic Depolymerization Mechanisms for Post-Consumer Plastic Wastes. Energies 2022, 15, 8821. https://doi.org/10.3390/en15238821
Kohli K, Chandrasekaran SR, Prajapati R, Kunwar B, Al-Salem S, Moser BR, Sharma BK. Pyrolytic Depolymerization Mechanisms for Post-Consumer Plastic Wastes. Energies. 2022; 15(23):8821. https://doi.org/10.3390/en15238821
Chicago/Turabian StyleKohli, Kirtika, Sriraam R. Chandrasekaran, Ravindra Prajapati, Bidhya Kunwar, Sultan Al-Salem, Bryan R. Moser, and Brajendra K. Sharma. 2022. "Pyrolytic Depolymerization Mechanisms for Post-Consumer Plastic Wastes" Energies 15, no. 23: 8821. https://doi.org/10.3390/en15238821
APA StyleKohli, K., Chandrasekaran, S. R., Prajapati, R., Kunwar, B., Al-Salem, S., Moser, B. R., & Sharma, B. K. (2022). Pyrolytic Depolymerization Mechanisms for Post-Consumer Plastic Wastes. Energies, 15(23), 8821. https://doi.org/10.3390/en15238821







