Structure and Thermophysical Properties of Molten Calcium-Containing Multi-Component Chlorides by Using Specific BMH Potential Parameters
Abstract
1. Introduction
2. Model and Methodology
2.1. Physical Model
2.2. Force Field
2.3. Simulation Detail
2.4. Experimental
2.4.1. Materials
2.4.2. Specific Heat Capacity Measurements
2.4.3. Density Measurements
3. Properties Evaluation
3.1. Radial Distribution Function
3.2. Coordination Number
3.3. Angular Distribution Function
3.4. Density and Specific Heat Capacity
3.5. Thermal Conductivity
4. Result and Discussion
4.1. Structure and Density Verification of Molten Salt CaCl2
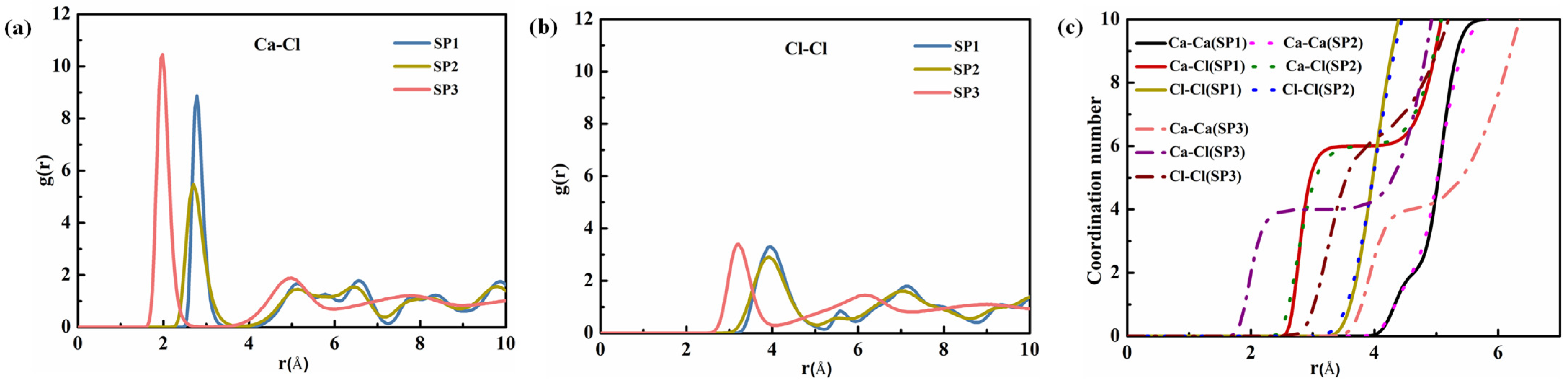
4.1.1. Structure Calculation
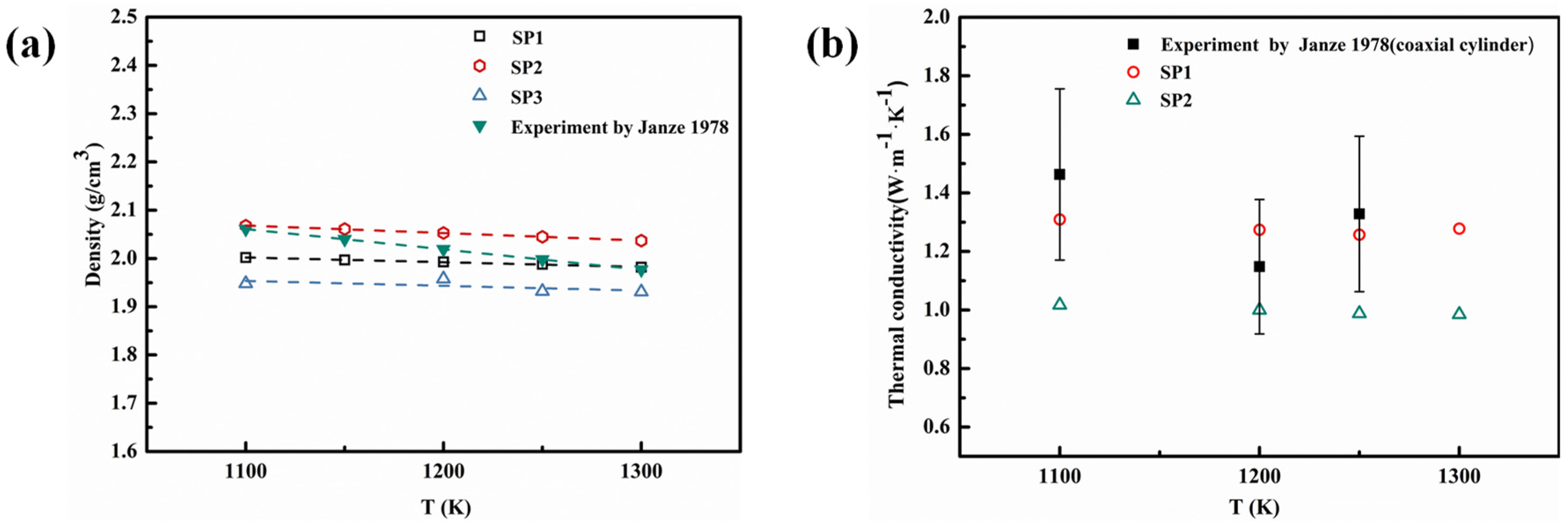
4.1.2. Thermophysical Properties Calculation
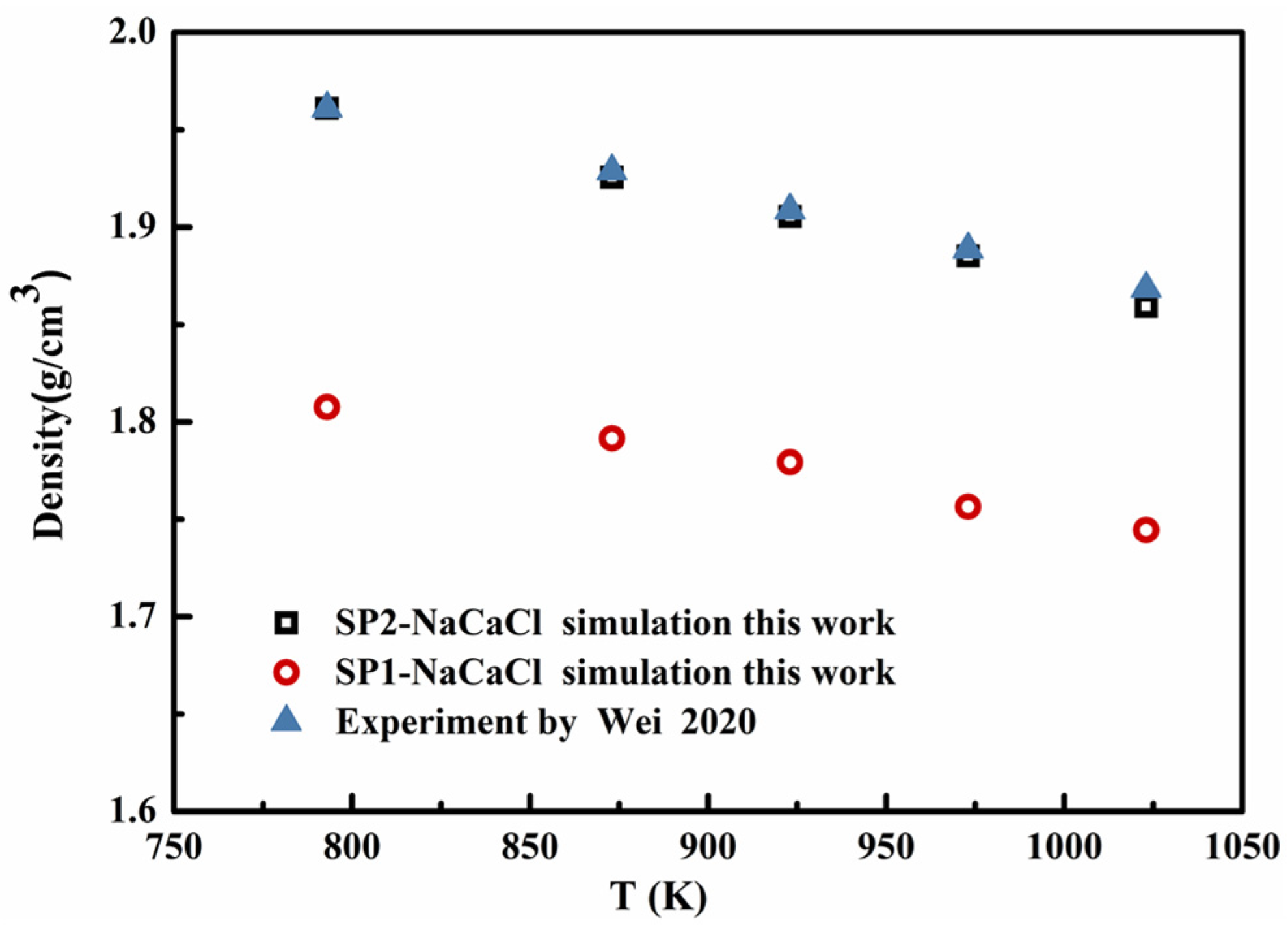
4.2. Verification of Binary Eutectic Salt NaCl-CaCl2 Density
4.3. Thermal Properties of Multi-Chlorine Eutectic Molten Salts
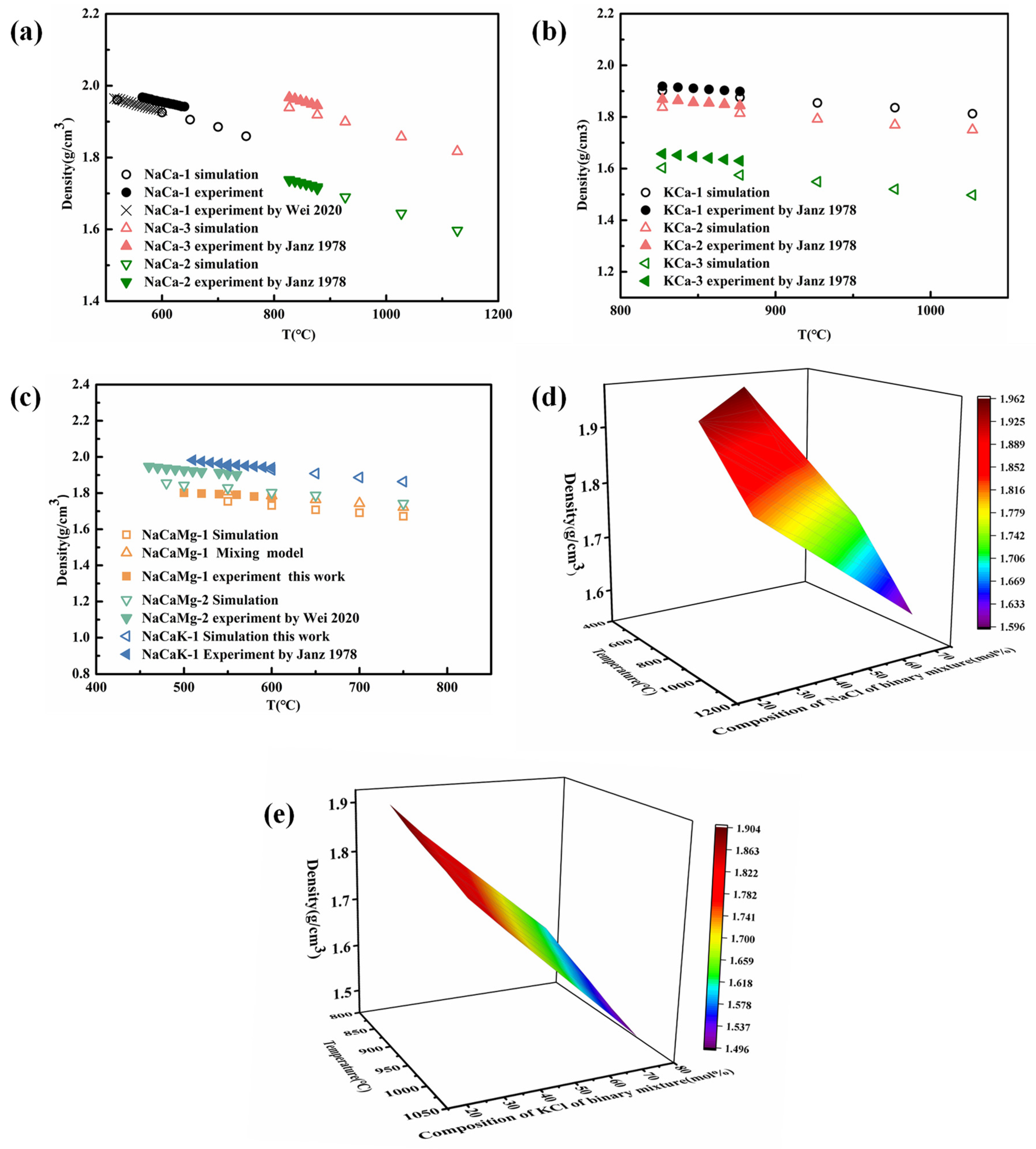
4.3.1. The Density of Chloride Molten Salts
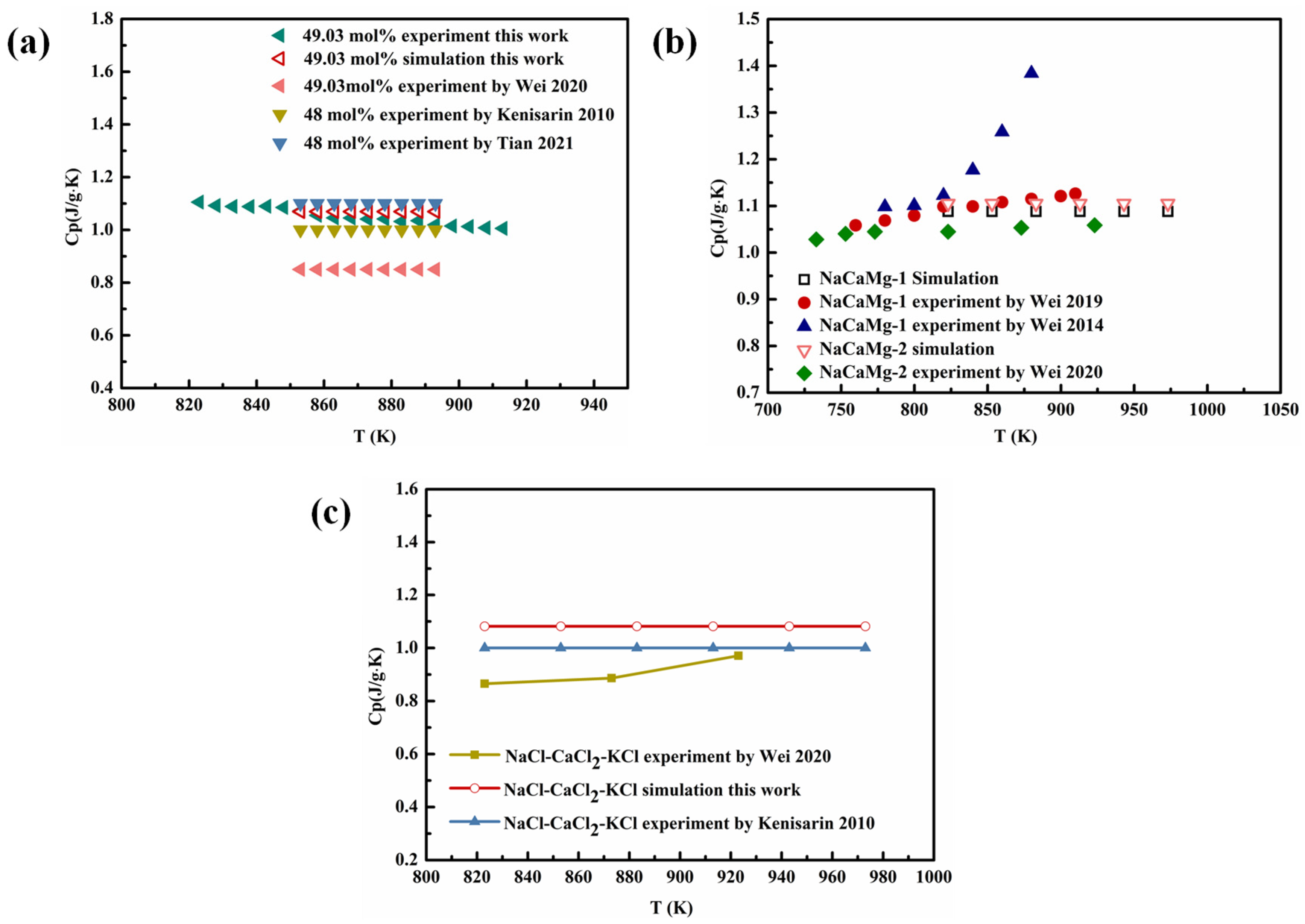
4.3.2. Specific Heat Capacity of Multicomponent Chloride Molten Salts
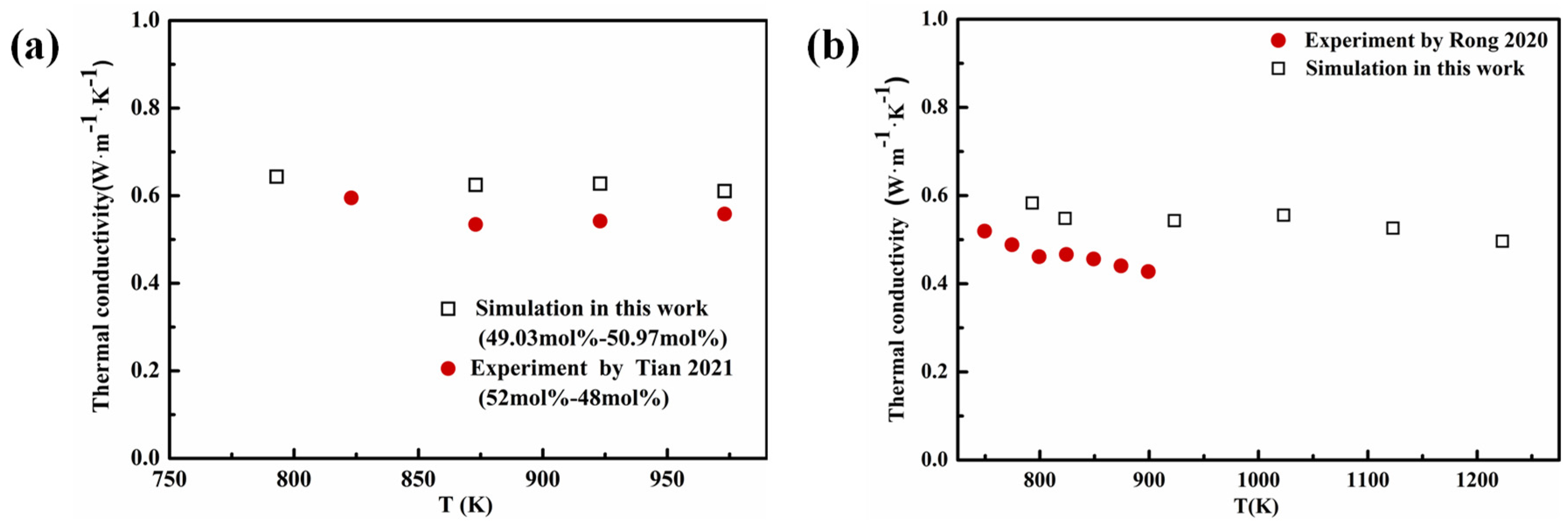
4.3.3. Thermal Conductivity of Multicomponent Chloride Molten Salts
4.4. Structures of Multi-Chlorine Eutectic Molten Salt
4.4.1. Radial Distribution Function and Coordination Number
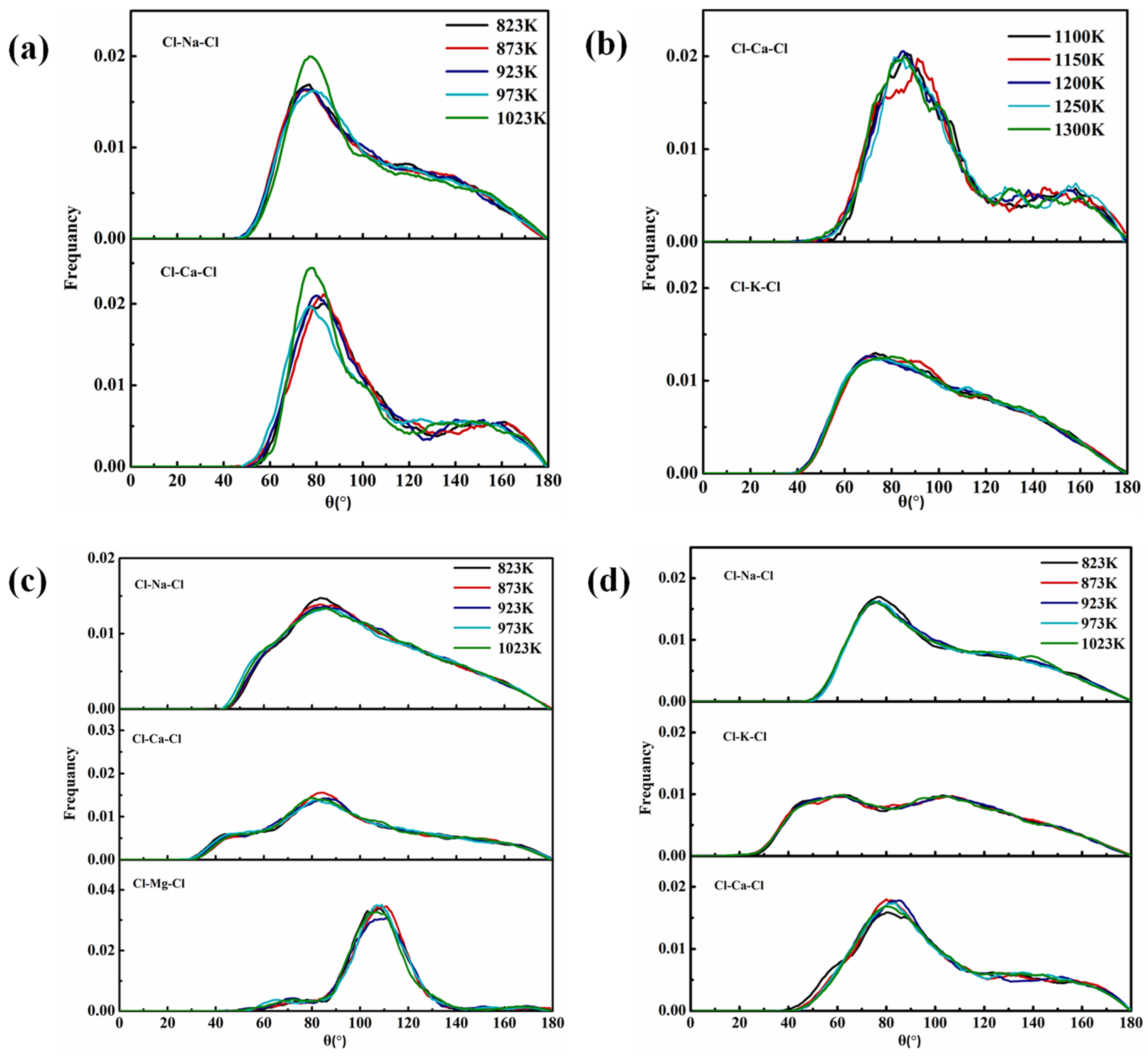
4.4.2. Bond Angle Distribution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roper, R.; Harkema, M.; Sabharwall, P.; Riddle, C.; Chisholm, B.; Day, B.; Marotta, P. Molten salt for advanced energy applications: A review. Ann. Nucl. Energy 2022, 169, 108924. [Google Scholar] [CrossRef]
- Rice, S.A.; Klemperer, W. Spectra of the Alkali Halides. II. The Infrared Spectra of the Sodium and Potassium Halides, RbCl, and CsCl. J. Chem. Phys. 1957, 27, 573–579. [Google Scholar] [CrossRef]
- Dunn, R.I.; Hearps, P.J.; Wright, M.N. Molten-Salt Power Towers: Newly Commercial Concentrating Solar Storage. Proc. IEEE 2012, 100, 504–515. [Google Scholar] [CrossRef]
- Wei, X.; Xie, P.; Zhang, X.; Wang, W.; Lu, J.; Ding, J. on preparation and thermodynamic properties of chloride molten salt materials. CIESC J. 2020, 71, 2423–2431. [Google Scholar] [CrossRef]
- Tian, H.; Wang, W.; Ding, J.; Wei, X. Thermal performance and economic evaluation of NaCl–CaCl2 eutectic salt for high-temperature thermal energy storage. Energy 2021, 227, 120412. [Google Scholar] [CrossRef]
- Pan, G.-C.; Ding, J.; Wang, W.; Lu, J.; Li, J.; Wei, X. Molecular simulations of the thermal and transport properties of alkali chloride salts for high-temperature thermal energy storage. Int. J. Heat Mass Transf. 2016, 103, 417–427. [Google Scholar] [CrossRef]
- Xie, W.; Ding, J.; Pan, G.; Fu, Q.; Wei, X.; Lu, J.; Wang, W. Heat and mass transportation properties of binary chloride salt as a high-temperature heat storage and transfer media. Sol. Energy Mater. Sol. Cells 2020, 209, 110415. [Google Scholar] [CrossRef]
- Bu, M.; Liang, W.; Lu, G.; Yu, J. Static and dynamic ionic structure of molten CaCl2 via first-principles molecular dynamics simulations. Ionics 2020, 27, 771–779. [Google Scholar] [CrossRef]
- Rong, Z.; Ding, J.; Wang, W.; Pan, G.; Liu, S. Ab-initio molecular dynamics calculation on microstructures and thermophysical properties of NaCl–CaCl2–MgCl2 for concentrating solar power. Sol. Energy Mater. Sol. Cells 2020, 216, 110696. [Google Scholar] [CrossRef]
- Rong, Z.; Pan, G.; Lu, J.; Liu, S.; Ding, J.; Wang, W.; Lee, D.-J. Ab-initio molecular dynamics study on thermal property of NaCl–CaCl2 molten salt for high-temperature heat transfer and storage. Renew. Energy 2021, 163, 579–588. [Google Scholar] [CrossRef]
- Igarashi, K.; Tajiri, K.; Asahina, T.; Kosaka, M. Structural Study of Molten CaCl2-KCl System. Mater. Sci. Forum 1991, 73–75, 79–84. [Google Scholar] [CrossRef]
- Igarashi, K.; Okamoto, Y.; Mochinaga, J. X-ray Diffraction Study of Molten CaCl2-KCl System. ECS Proc. Vol. 1987, 7, 175–184. [Google Scholar] [CrossRef]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Jiang, W.; Xu, Y.; Guo, J. Interionic potentials and their parameters in the alkali halides. J. Guangxi Univ. 2004, 3, 202–206. [Google Scholar] [CrossRef]
- Baughan, E.C. The repulsion energies in ionic compounds. Trans. Faraday Soc. 1959, 55, 736–752. [Google Scholar] [CrossRef]
- Woodcock, L.V.; Angell, C.A.; Cheeseman, P. Molecular dynamics studies of the vitreous state: Simple ionic systems and silica. J. Chem. Phys. 1976, 65, 1565–1577. [Google Scholar] [CrossRef]
- Yuen, P.S.; Murfitt, R.M.; Collin, R.L. Interionic forces and ionic polarization in alkaline earth halide crystals. J. Chem. Phys. 1974, 61, 2383–2393. [Google Scholar] [CrossRef]
- Tosi, M.; Fumi, F. Ionic sizes and born repulsive parameters in the NaCl-type alkali halides—II: The generalized Huggins-Mayer form. J. Phys. Chem. Solids 1964, 25, 45–52. [Google Scholar] [CrossRef]
- Lu, J.; Yang, S.; Rong, Z.; Pan, G.; Ding, J.; Liu, S.; Wei, X.; Wang, W. Thermal properties of KCl–MgCl2 eutectic salt for high-temperature heat transfer and thermal storage system. Sol. Energy Mater. Sol. Cells 2021, 228, 111130. [Google Scholar] [CrossRef]
- Smith, F.T. Atomic Distortion and the Combining Rule for Repulsive Potentials. Phys. Rev. A 1972, 5, 1708–1713. [Google Scholar] [CrossRef]
- Ping, H.S.; Yoshida, F. Ionic Properties of the Metal-Salt Solution Cax(CaCl2)1−x. J. Phys. Soc. Jpn. 1997, 66, 392–395. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Scott, R.; Allen, M.P.; Tildesley, D.J. Computer Simulation of Liquids. Math. Comput. 1991, 57, 442–444. [Google Scholar] [CrossRef]
- Müller-Plathe, F. A simple nonequilibrium molecular dynamics method for calculating the thermal conductivity. J. Chem. Phys. 1997, 106, 6082–6085. [Google Scholar] [CrossRef]
- Jayaraman, S.; Thompson, A.P.; von Lilienfeld, O.A.; Maginn, E. Molecular Simulation of the Thermal and Transport Properties of Three Alkali Nitrate Salts. Ind. Eng. Chem. Res. 2010, 49, 559–571. [Google Scholar] [CrossRef]
- Biggin, S.; Enderby, J.E. The structure of molten calcium chloride. J. Phys. C Solid State Phys. 1981, 14, 3577–3583. [Google Scholar] [CrossRef]
- David, R. Handbook Chemistry and Physics, 84th ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Janz, G.J.; Allen, C.B.; Bansal, N.P.; Murphy, R.M.; Tomkins, R.P.T. Physical properties data compilations relevant to energy storage. II. Molten Salts: Data on Single and Multi-Component Salt Systems. In Physical Properties Data Compilations Relevant to Energy Storage; National Bureau of Standards: Gaithersburg, MD, USA, 1978. [Google Scholar]
- Grjotheim, K.; Holm, J.L.; Lillebuen, B.; Øye, H.A. Densities and excess molar volumes of binary and ternary melts of MgCl2, CaCl2 and AlkCl. Trans. Faraday Soc. 1971, 67, 640–648. [Google Scholar] [CrossRef]
- Kenisarin, M.M. High-temperature phase change materials for thermal energy storage. Renew. Sustain. Energy Rev. 2010, 14, 955–970. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, X.; Ding, J.; Wang, W.; Lu, J. The effect of nano-MgO on thermal properties of ternary chloride fluid. Innov. Solut. Energy Transit. 2019, 158, 773–778. [Google Scholar] [CrossRef]
- Wei, X.; Song, M.; Peng, Q.; Ding, J.; Yang, J. A new ternary chloride eutectic mixture and its thermo-physical properties for solar thermal energy storage. Energy Procedia 2014, 61, 1314–1317. [Google Scholar] [CrossRef][Green Version]

| Parameters | Aij (kcal/mol) | σij (Å) | ρij (Å) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ca-Ca | Ca-Cl | Cl-Cl | Ca-Ca | Ca-Cl | Cl-Cl | Ca-Ca | Ca-Cl | Cl-Cl | |
| SP1 [11] | 0.1597 | 0.1749 | 0.1889 | 2.814 | 3.357 | 3.900 | 0.160 | 0.170 | 0.190 |
| SP2 [17,21] | 4.0995 | 3.0746 | 2.0489 | 2.880 [11] | 3.118 | 3.356 [16] | 0.324 [17] | 0.324 | 0.324 |
| SP3 [6,16,17] | 4.0995 | 3.0746 | 2.0489 | 2.180 [17] | 2.675 | 3.170 [6] | 0.324 [17] | 0.324 | 0.324 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Chen, D.; Liu, S.; Wang, W.; Ding, J.; Lu, J. Structure and Thermophysical Properties of Molten Calcium-Containing Multi-Component Chlorides by Using Specific BMH Potential Parameters. Energies 2022, 15, 8878. https://doi.org/10.3390/en15238878
Wei X, Chen D, Liu S, Wang W, Ding J, Lu J. Structure and Thermophysical Properties of Molten Calcium-Containing Multi-Component Chlorides by Using Specific BMH Potential Parameters. Energies. 2022; 15(23):8878. https://doi.org/10.3390/en15238878
Chicago/Turabian StyleWei, Xiaolan, Dandan Chen, Shule Liu, Weilong Wang, Jing Ding, and Jianfeng Lu. 2022. "Structure and Thermophysical Properties of Molten Calcium-Containing Multi-Component Chlorides by Using Specific BMH Potential Parameters" Energies 15, no. 23: 8878. https://doi.org/10.3390/en15238878
APA StyleWei, X., Chen, D., Liu, S., Wang, W., Ding, J., & Lu, J. (2022). Structure and Thermophysical Properties of Molten Calcium-Containing Multi-Component Chlorides by Using Specific BMH Potential Parameters. Energies, 15(23), 8878. https://doi.org/10.3390/en15238878







